AFM/XPS Analysis of the Growth and Architecture of Oriented Molecular Monolayer by Spin Cast Process and Its Cross-Linking Induced by Hyperthermal Hydrogen
Abstract
:1. Introduction
2. Materials and Methods
3. Results
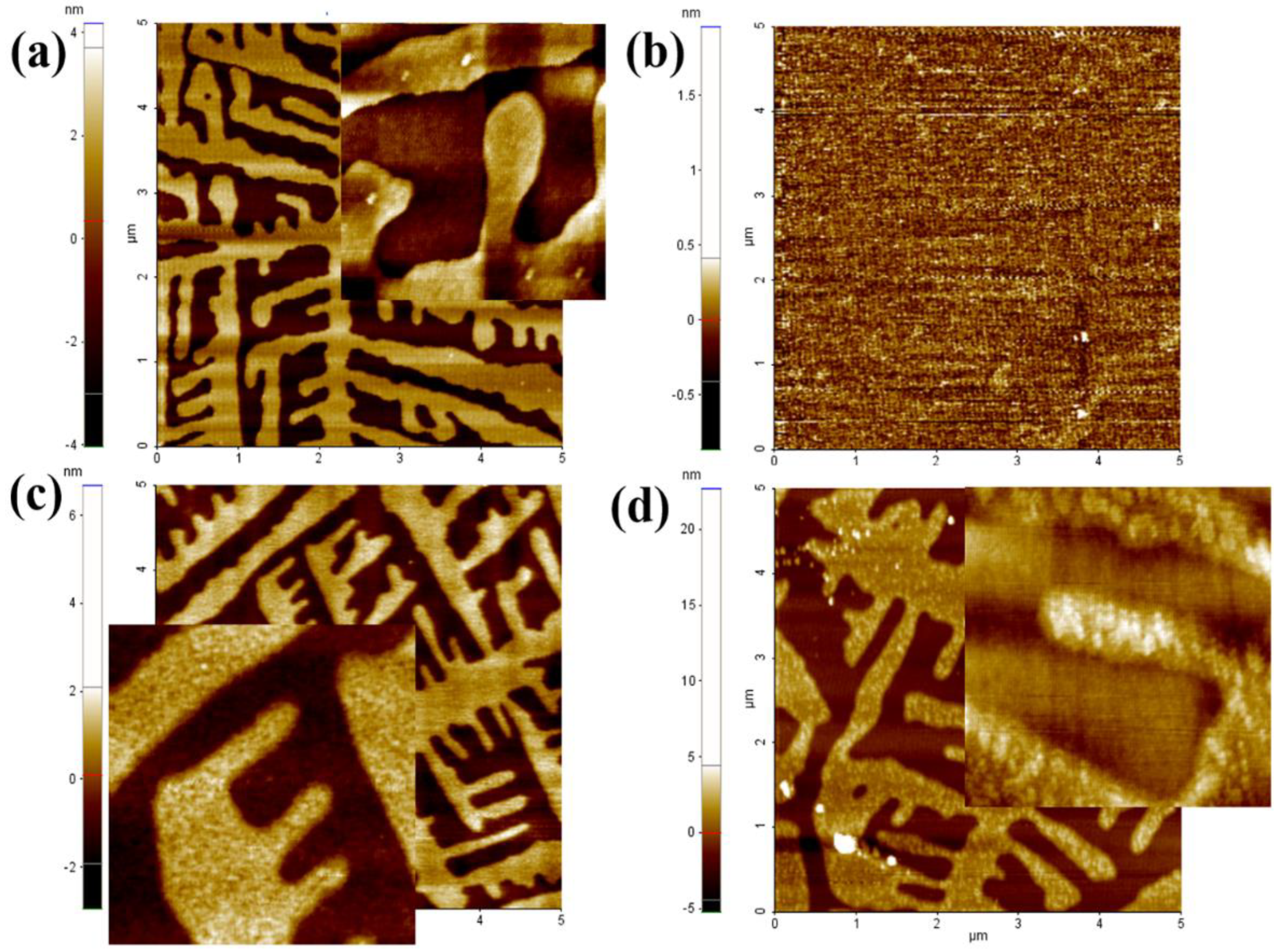
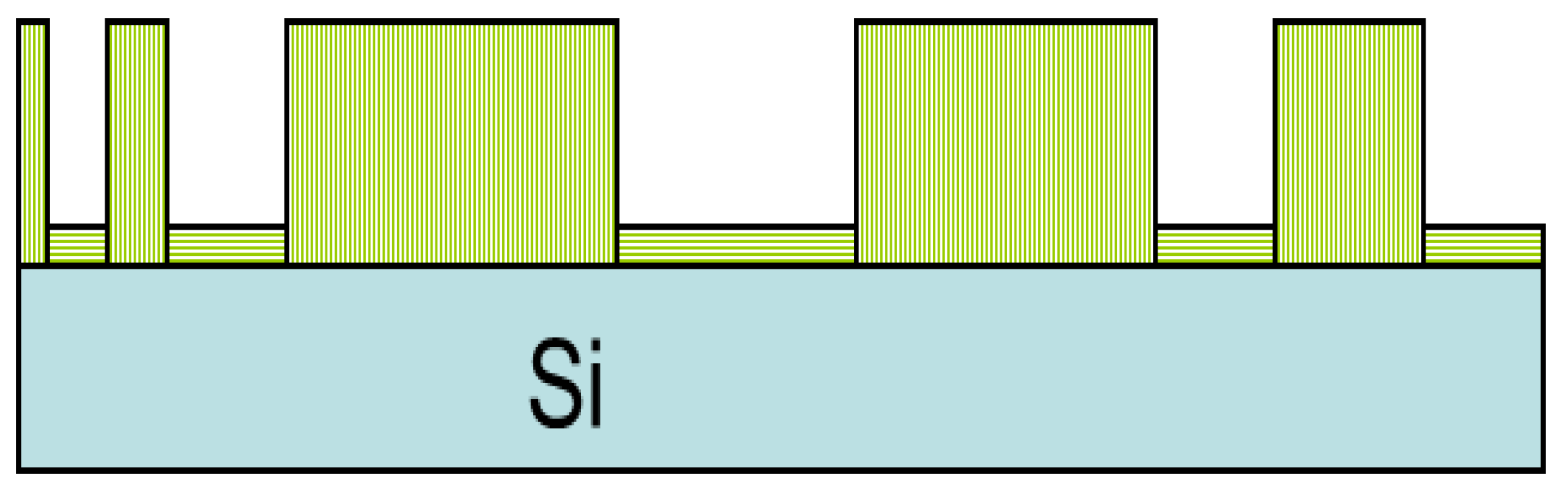
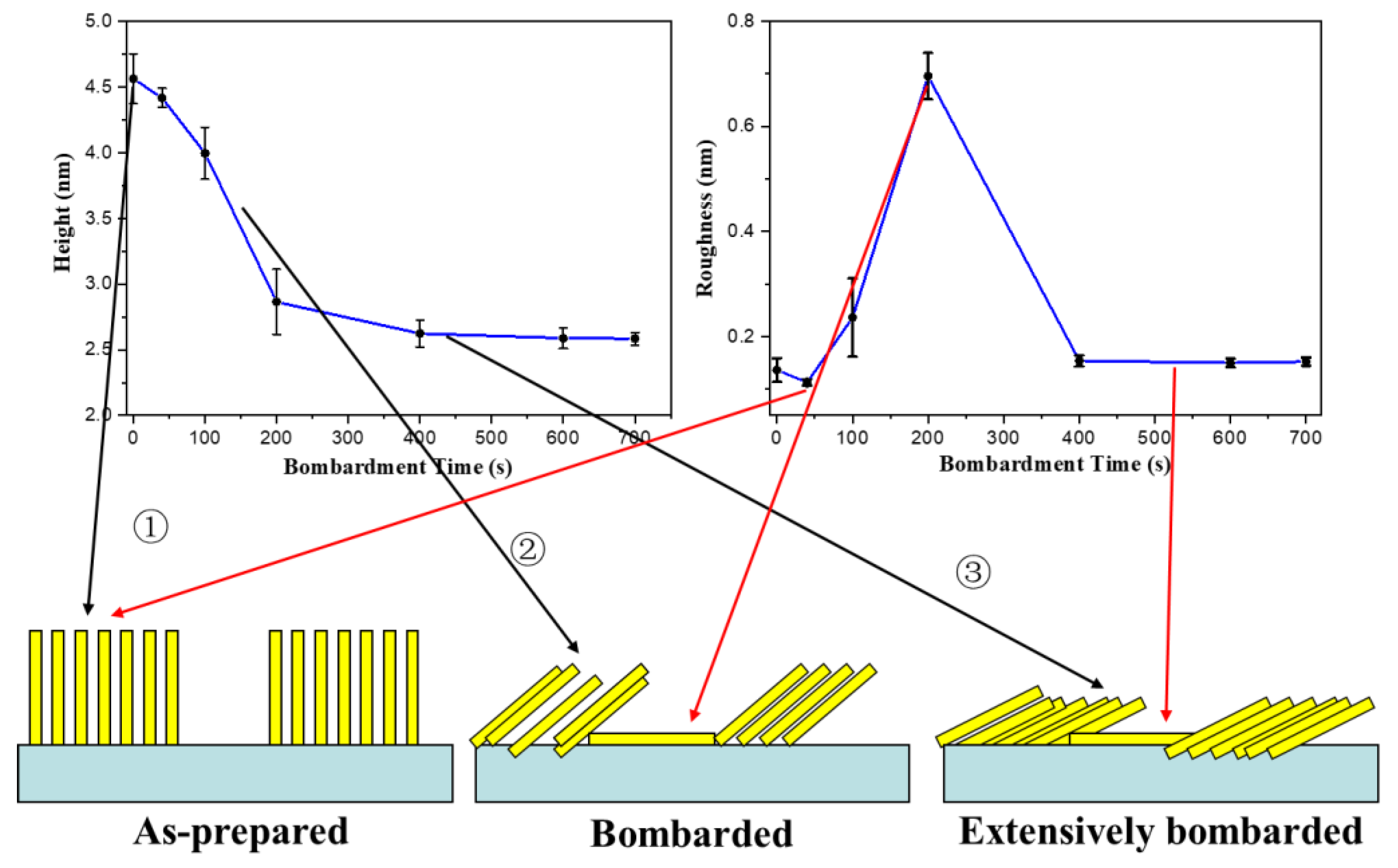
3.1. Surface Morphology of the C32H66 Thin Films by AFM
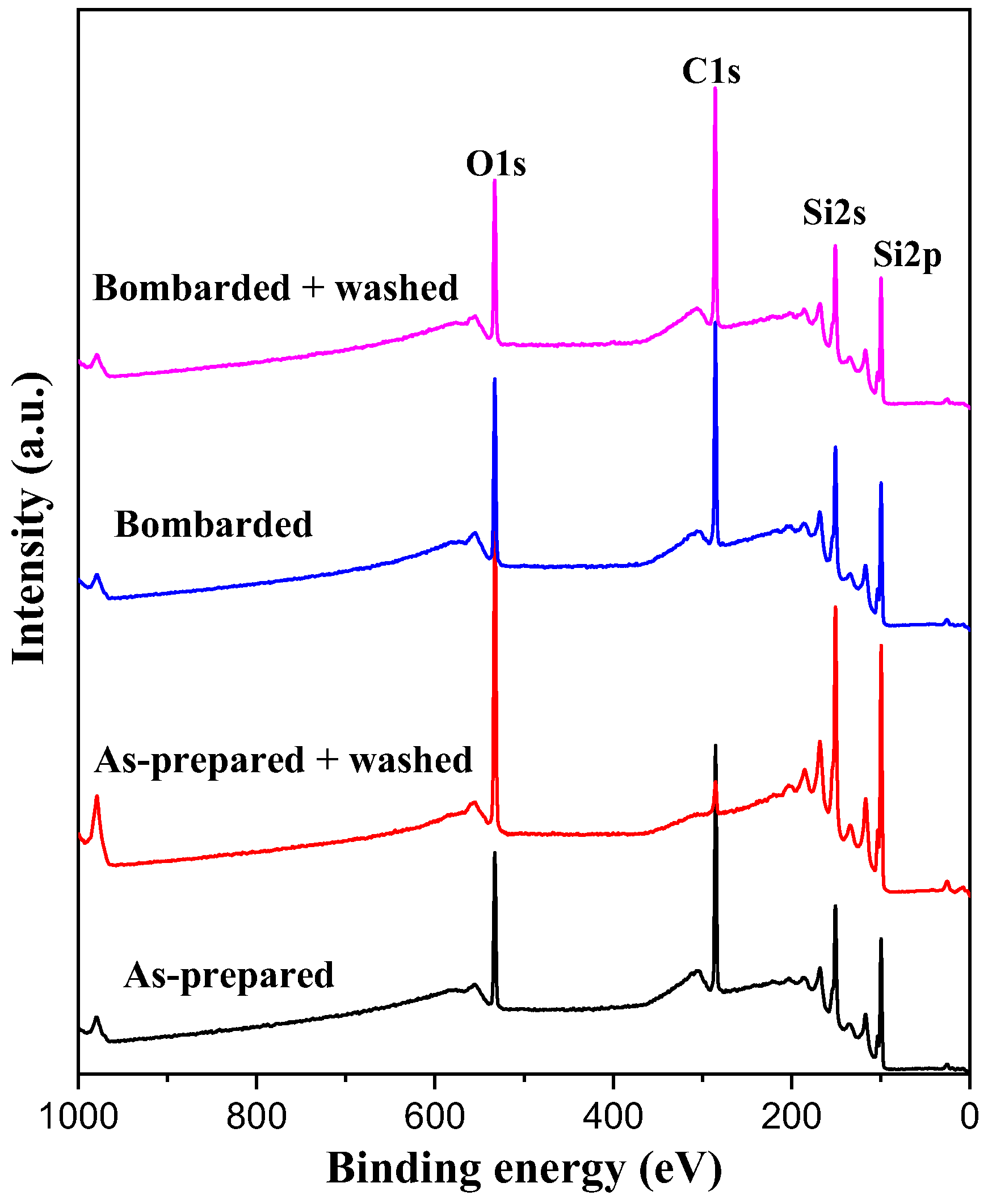
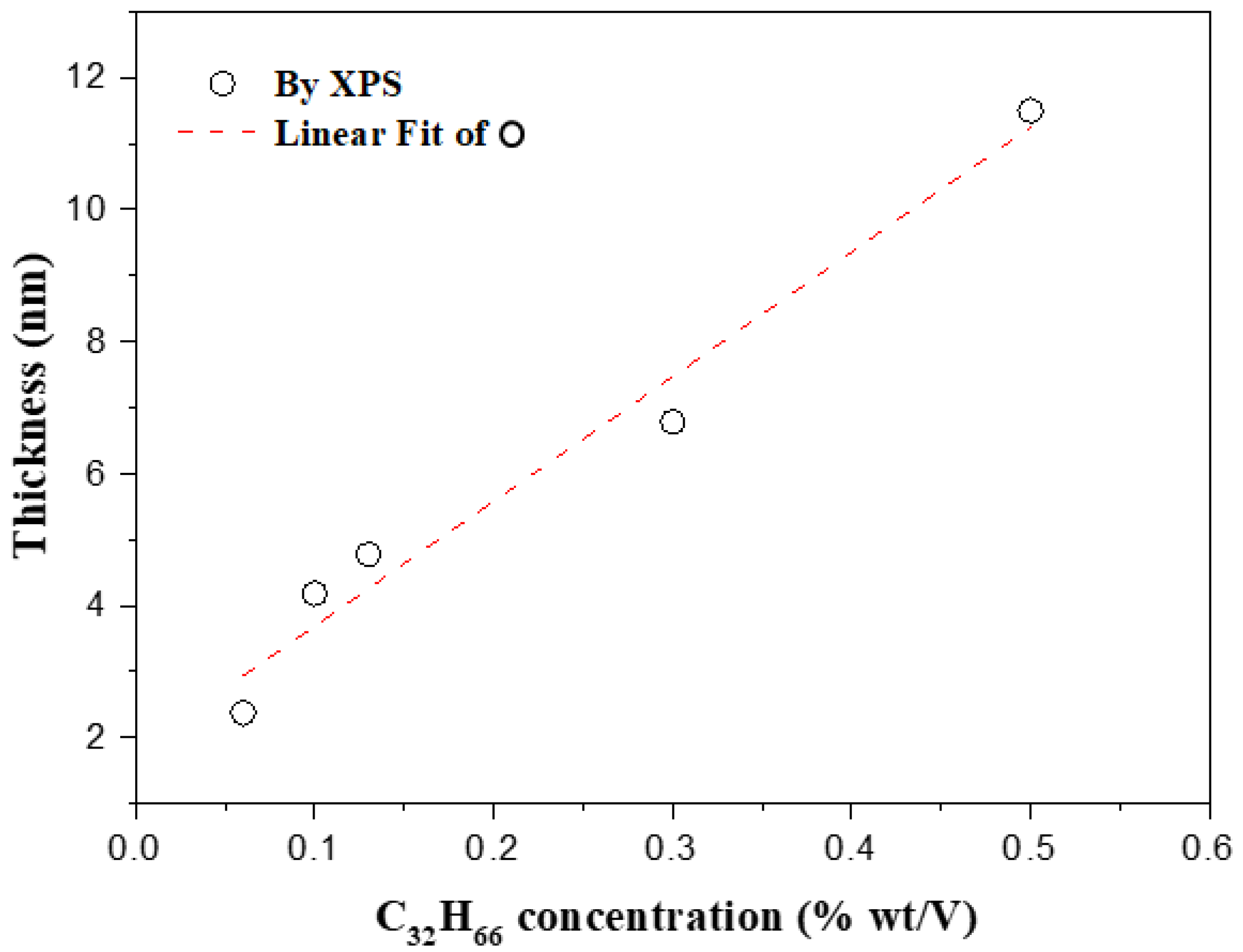
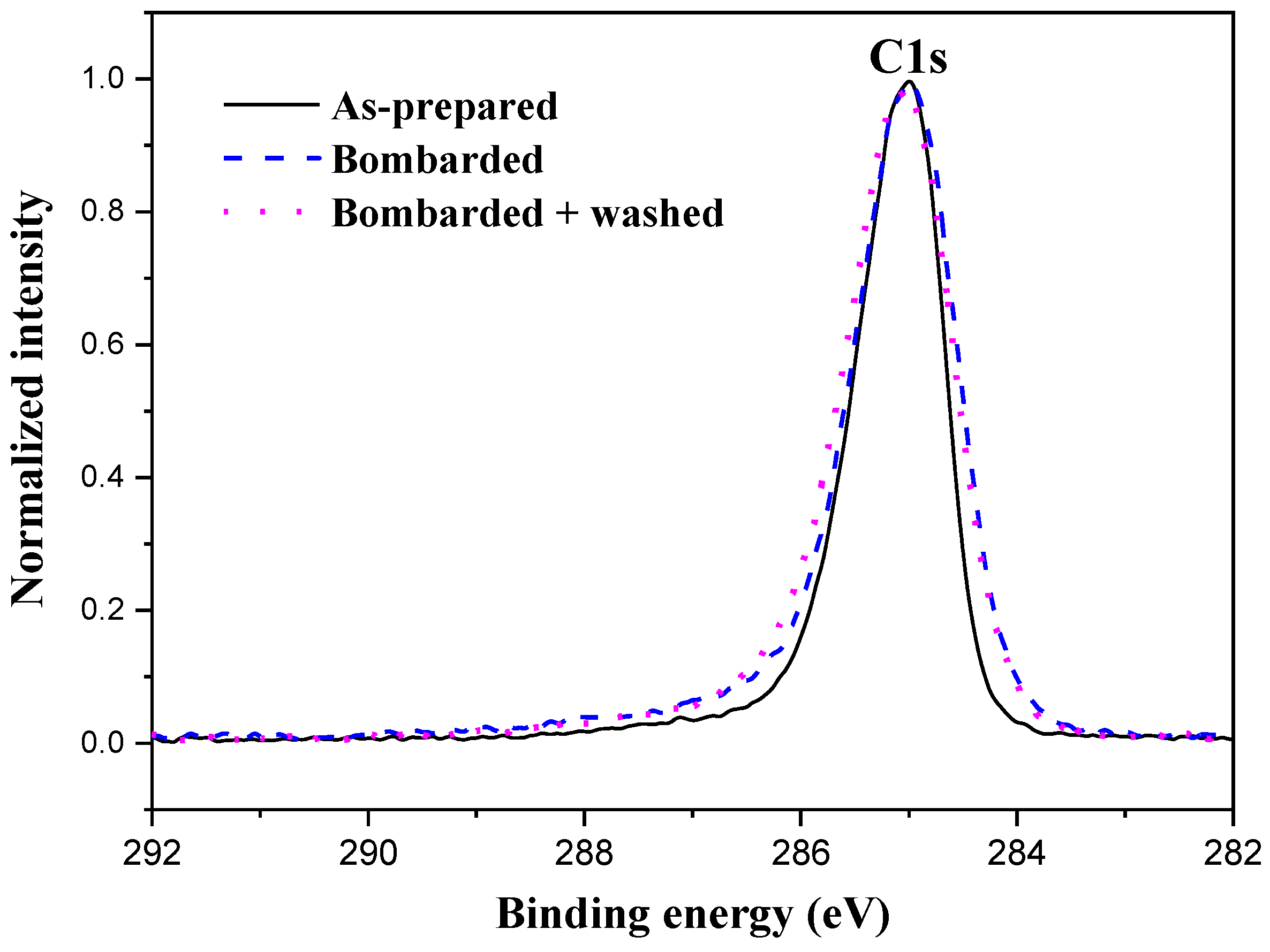
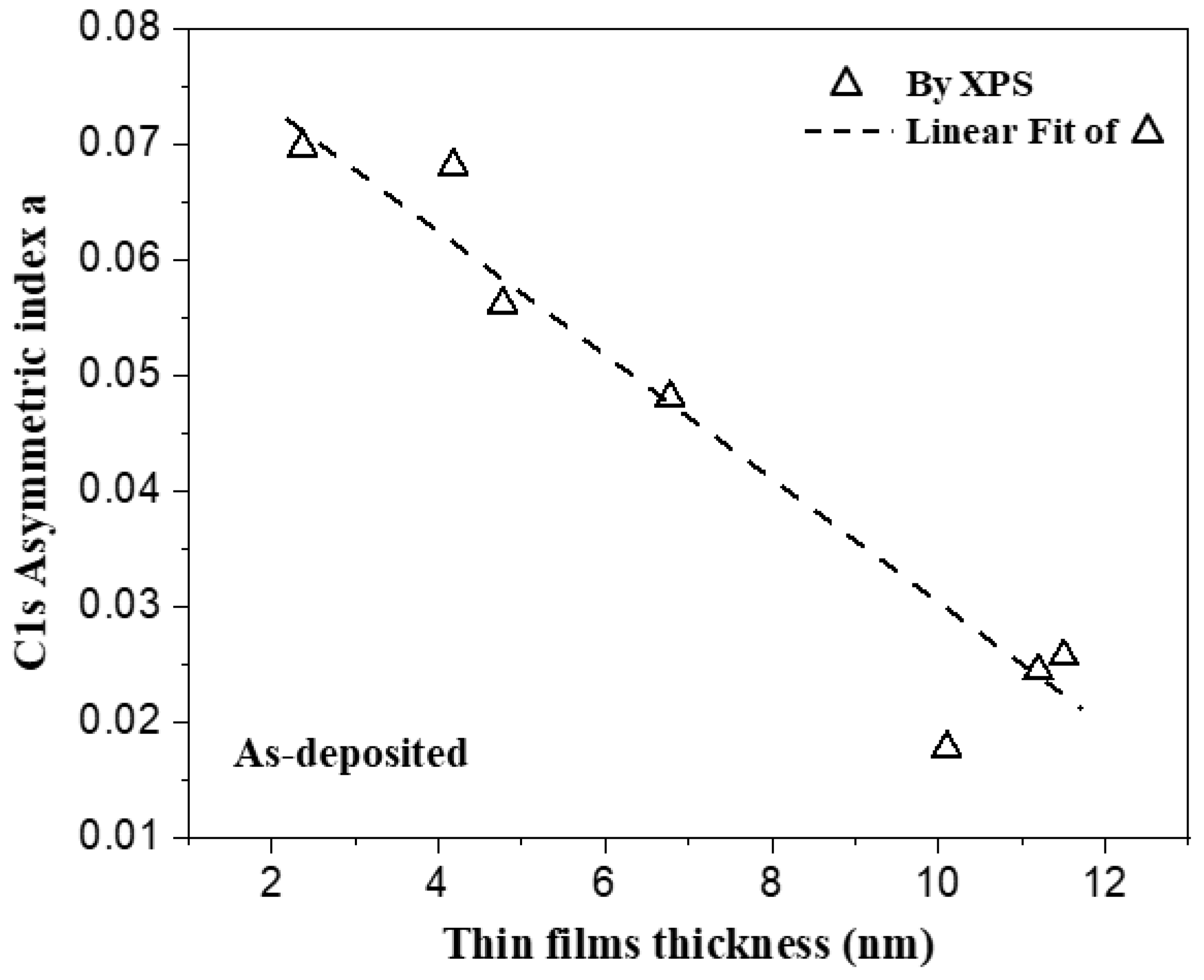
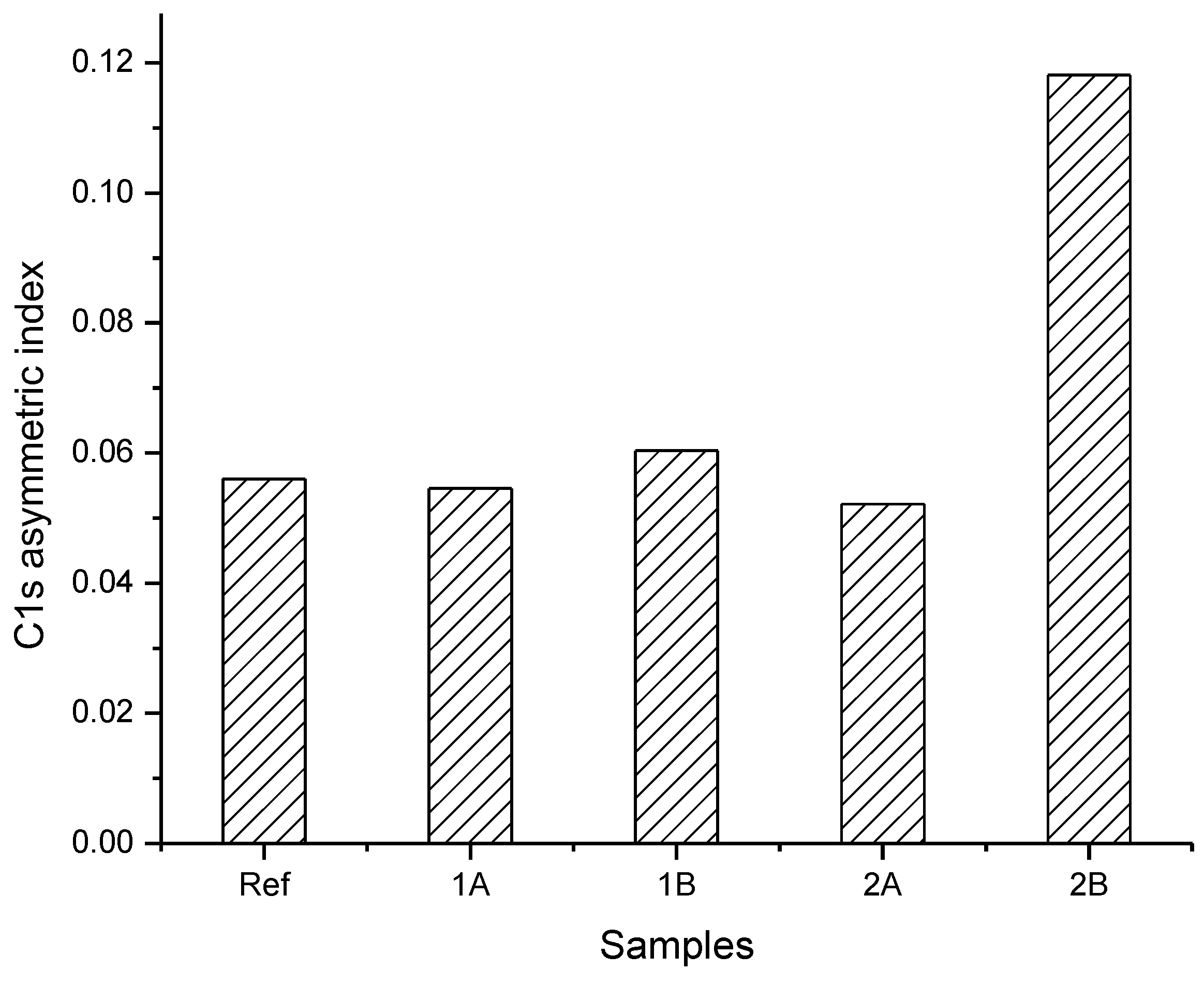
3.2. Surface Chemical Composition of the C32H66 Thin Films
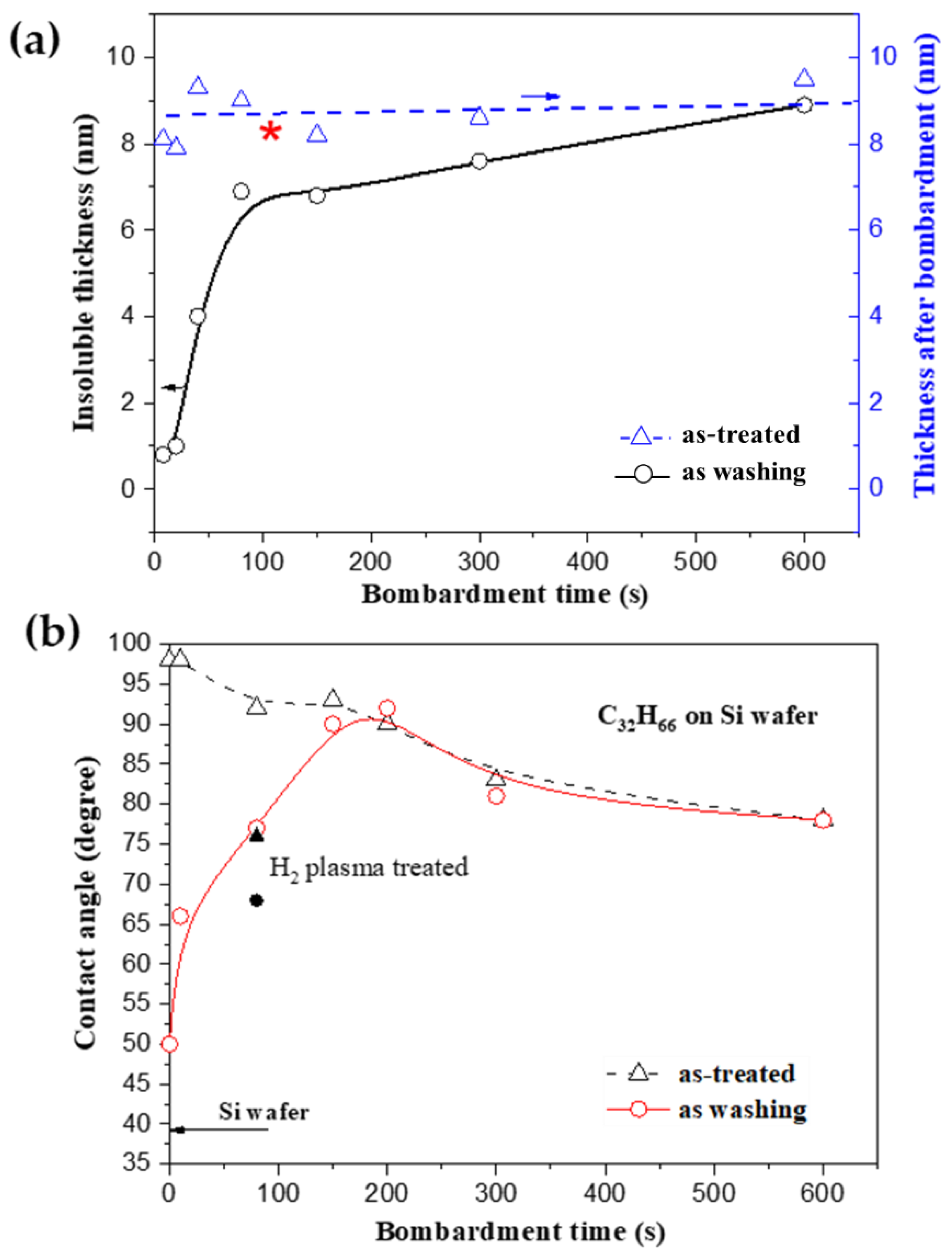
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fazekas, T.J.; Alty, J.W.; Neidhart, E.K.; Miller, A.S.; Leibfarth, F.A.; Alexanian, E.J. Diversification of aliphatic C–H bonds in small molecules and polyolefins through radical chain transfer. Science 2022, 375, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, D.Q.; Nie, H.Y.; Lau, W.M.; Yang, J. Study of a hydrogen-bombardment process for molecular cross-linking within thin films. J. Chem. Phys. 2011, 134, 074704. [Google Scholar] [CrossRef] [PubMed]
- Schollmeyer, H.; Struth, B.; Riegler, H. Long Chain n-Alkanes at SiO2/Air Interfaces: Molecular Ordering, Annealing, and Surface Freezing of Triacontane in the Case of Excess and Submonolayer Coverage. Langmuir 2003, 19, 5042–5051. [Google Scholar] [CrossRef]
- Lazar, P.; Schollmeyer, H.; Riegler, H. Spreading and Two-Dimensional Mobility of Long-Chain Alkanes at Solid/Gas Interfaces. Phys. Rev. Lett. 2005, 94, 116101. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, U.G.; Pino, M.; Altamirano, L.; Taub, H.; Hansen, F.Y. High-resolution ellipsometric study of an n-alkane film, dotriacontane, adsorbed on a SiO2 surface. J. Chem. Phys. 2002, 116, 2107–2115. [Google Scholar] [CrossRef] [Green Version]
- Mo, H.; Taub, H.; Volkmann, U.; Pino, M.; Ehrlich, S.; Hansen, F.Y.; Lu, E.; Miceli, P. A novel growth mode of alkane films on a SiO2 surface. Chem. Phys. Lett. 2003, 377, 99–105. [Google Scholar] [CrossRef]
- Mo, H.; Trogisch, S.; Taub, H.; Ehrlich, S.; Volkmann, U.; Hansen, F.Y.; Pino, M. Structure and growth of dotriacontane films on SiO2 and Ag(111) surfaces: Synchrotron X-ray scattering and molecular dynamics simulations. Phys. Status Solidi A 2004, 201, 2375–2380. [Google Scholar] [CrossRef]
- Trogisch, S.; Simpson, M.; Taub, H.; Volkmann, U.G.; Pino, M.; Hansen, F.Y. Atomic force microscopy measurements of topography and friction on dotriacontane films adsorbed on a SiO2 surface. J. Chem. Phys. 2005, 123, 154703. [Google Scholar] [CrossRef] [Green Version]
- Kruchten, F.; Knorr, K.; Volkmann, U.G.; Taub, H.; Hansen, F.Y.; Matthies, B.; Herwig, K.W. Ellipsometric and neutron diffraction study of pentane physisorbed on graphite. Langmuir 2005, 21, 7507–7512. [Google Scholar] [CrossRef]
- Nozaki, K.; Saihara, R.; Ishikawa, K.; Yamamoto, T. Structure of Normal Alkane Evaporated Films: Molecular Orientation. Jpn. J. Appl. Phys. 2007, 46, 761–769. [Google Scholar] [CrossRef] [Green Version]
- Jandt, K.; Miles, M.; Petermann, J.; Thomson, N. STM investigations of an alkane-metal-system (C32H66/In). Polym. Bull. 1994, 33, 687–691. [Google Scholar] [CrossRef]
- Espeau, P.; Reynolds, P.A.; Dowling, T.; Cookson, D.; White, J.W. X-Ray diffraction from layers of n-alkanes adsorbed on graphite. J. Chem. Soc. Faraday Trans. 1997, 93, 3201–3208. [Google Scholar] [CrossRef]
- Basu, S.; Satija, S. In-situ X-ray reflectivity study of alkane films grown from the vapor phase. Langmuir 2007, 23, 8331–8335. [Google Scholar] [CrossRef]
- Knüfing, L.; Schollmeyer, H.; Riegler, H.; Mecke, K. Fractal analysis methods for solid alkane monolayer domains at SiO2/air interfaces. Langmuir 2005, 21, 992–1000. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nozaki, K.; Yamaguchi, A.; Urakami, N. Molecular simulation of crystallization in n-alkane ultrathin films: Effects of film thickness and substrate attraction. J. Chem. Phys. 2007, 127, 154704. [Google Scholar] [CrossRef] [PubMed]
- Hess, G.B. Phase Transitions in Surface Films 2. NATO Adv. Study Inst. N. Y. 1991, 267, 357–389. [Google Scholar]
- Li, Z.; Gorp, H.V.; Walke, P.; Phan, T.H.; Fujita, Y.; Greenwood, J.; Ivasenko, O.; Tahara, K.; Tobe, Y.; Uji-I, H.; et al. Area-selective passivation of sp2 carbon surfaces by supramolecular self-assembly. Nanoscale 2017, 9, 5188–5193. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Trogisch, S.; Magonov, S.; Taub, H. Explanation and correction of false step heights in amplitude modulation atomic force microscopy measurements on alkane films. Ultramicroscopy 2008, 108, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Trogisch, S.; Taub, H.; Ehrlich, S.; Volkmann, U.G.; Hansen, F.Y.; Pino, M. Studies of the structure and growth mode of dotriacontane films by synchrotron x-ray scattering and molecular dynamics simulations. J. Phys. Condens. Matter 2004, 16, S2905. [Google Scholar] [CrossRef] [Green Version]
- Trebicky, T.; Crewdson, P.; Paliy, M.; Bello, I.; Nie, H.Y.; Zheng, Z.; Fan, X.L.; Yang, J.; Gillies, E.R.; Tang, C.Y.; et al. Cleaving C–H bonds with hyperthermal H2: Facile chemistry to cross-link organic molecules under low chemical- and energy-loads. Green Chem. 2014, 16, 1316–1325. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, X.D.; Fan, X.L.; Lau, W.-M.; Kwok, R.W.M. Ultrathin polymer film formation by collision-induced cross-linking of adsorbed organic molecules with hyperthermal protons. J. Am. Chem. Soc. 2004, 126, 12336–12342. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Kwok, W.M.; Lau, W.M. A new cross-linking route via the unusual collision kinematics of hyperthermal protons in unsaturated hydrocarbons: The case of poly(trans-isoprene). Chem. Commun. 2006, 29, 3122–3124. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wong, K.W.; Lau, W.C.; Kwok, R.W.M.; Lau, W.M. Unusual Kinematics-Driven Chemistry: Cleaving C-H but Not COO-H Bonds with Hyperthermal Protons To Synthesize Tailor-Made Molecular Films. Chem.-A Eur. J. 2007, 13, 3187–3192. [Google Scholar] [CrossRef] [PubMed]
- Laibinis, P.E.; Bain, C.D.; Whitesides, G.M. Attenuation of Photoelectrons in Monolayers of n-Alkanethiols Adsorbed on Copper, Silver and Gold. J. Phys. Chem. 1991, 95, 7017–7021. [Google Scholar] [CrossRef]
- Lamont, C.L.; Wilkes, J. Attenuation Length of Electrons in Self-Assembled Monolayers of n-Alkanethiols on Gold. Langmuir 1999, 15, 2037–2042. [Google Scholar] [CrossRef]
- Wallart, X.; Henry de Villeneuve, C.; Allongue, P. Truly Quantitative XPS Characterization of Organic Monolayers on Silicon: Study of Alkyl and Alkoxy Monolayers on H-Si(111). J. Am. Chem. Soc. 2005, 127, 7871–7878. [Google Scholar] [CrossRef]
- Yang, D.-Q.; Sacher, E. Carbon 1s X-ray photoemission line shape analysis of highly oriented pyrolytic graphite: The influence of structural damage on peak asymmetry. Langmuir 2006, 22, 860–862. [Google Scholar] [CrossRef]
- Yang, D.-Q.; Sacher, E. Ar+-induced surface defects on HOPG and their effect on the nucleation, coalescence and growth of evaporated copper. Surf. Sci. 2002, 516, 43–55. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; Wiley: New York, NY, USA, 1992; pp. 125–148. [Google Scholar]
- Yang, D.-Q.; Sacher, E. s–p Hybridization in highly oriented pyrolytic graphite and its change on surface modification, as studied by X-ray photoelectron and Raman spectroscopies. Surf. Sci. 2002, 504, 125–137. [Google Scholar] [CrossRef]
- Yang, D.-Q.; Rochette, J.-F.; Sacher, E. Controlled Chemical Functionalization of Multiwalled Carbon Nanotubes by Kiloelectronvolt Argon Ion Treatment and Air Exposure. Langmuir 2005, 21, 8539–8545. [Google Scholar] [CrossRef]
- Speranza, G.; Minati, L.; Anderle, M. The C1s core line in irradiated graphite. J. Appl. Phys. 2007, 102, 043504. [Google Scholar] [CrossRef]
- Xi, L.; Zheng, Z.; Lam, N.-S.; Grizzi, O.; Lau, W.-M. Effects of hyperthermal proton bombardment on alkanethiol self-assembled monolayer on Au(1 1 1). Appl. Surf. Sci. 2007, 254, 113–115. [Google Scholar] [CrossRef]
- Xi, L.; Zheng, Z.; Lam, N.-S.; Nie, H.-Y.; Grizzi, O.; Lau, W.-M. Study of the Hyperthermal Proton Bombardment Effects on Self-Assembled Monolayers of Dodecanethiol on Au(111). J. Phys. Chem. C 2008, 112, 12111–12115. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Xu, R.; Zhu, Y.; Yang, D.-Q.; Nie, H.-Y.; Lau, W.M. AFM/XPS Analysis of the Growth and Architecture of Oriented Molecular Monolayer by Spin Cast Process and Its Cross-Linking Induced by Hyperthermal Hydrogen. Appl. Sci. 2022, 12, 6233. https://doi.org/10.3390/app12126233
Liu J, Xu R, Zhu Y, Yang D-Q, Nie H-Y, Lau WM. AFM/XPS Analysis of the Growth and Architecture of Oriented Molecular Monolayer by Spin Cast Process and Its Cross-Linking Induced by Hyperthermal Hydrogen. Applied Sciences. 2022; 12(12):6233. https://doi.org/10.3390/app12126233
Chicago/Turabian StyleLiu, Jinkun, Run Xu, Yan Zhu, De-Quan Yang, Heng-Yong Nie, and Woon Ming Lau. 2022. "AFM/XPS Analysis of the Growth and Architecture of Oriented Molecular Monolayer by Spin Cast Process and Its Cross-Linking Induced by Hyperthermal Hydrogen" Applied Sciences 12, no. 12: 6233. https://doi.org/10.3390/app12126233
APA StyleLiu, J., Xu, R., Zhu, Y., Yang, D.-Q., Nie, H.-Y., & Lau, W. M. (2022). AFM/XPS Analysis of the Growth and Architecture of Oriented Molecular Monolayer by Spin Cast Process and Its Cross-Linking Induced by Hyperthermal Hydrogen. Applied Sciences, 12(12), 6233. https://doi.org/10.3390/app12126233






