Oil Bodies Cream from Olive Paste: Extraction of a Functional Ingredient for Developing a Stable Food Emulsion
Abstract
:1. Introduction
2. Materials and Methods
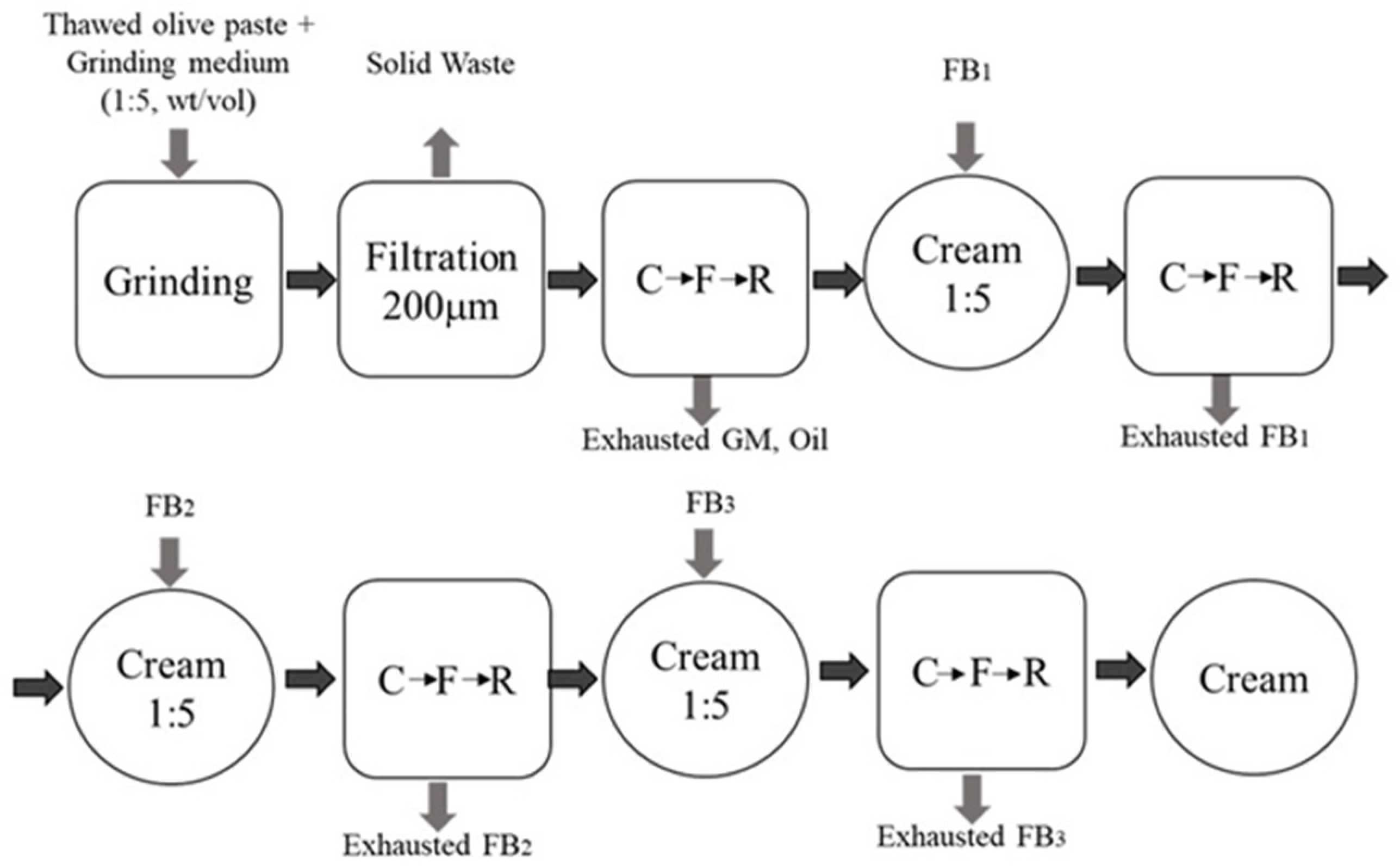
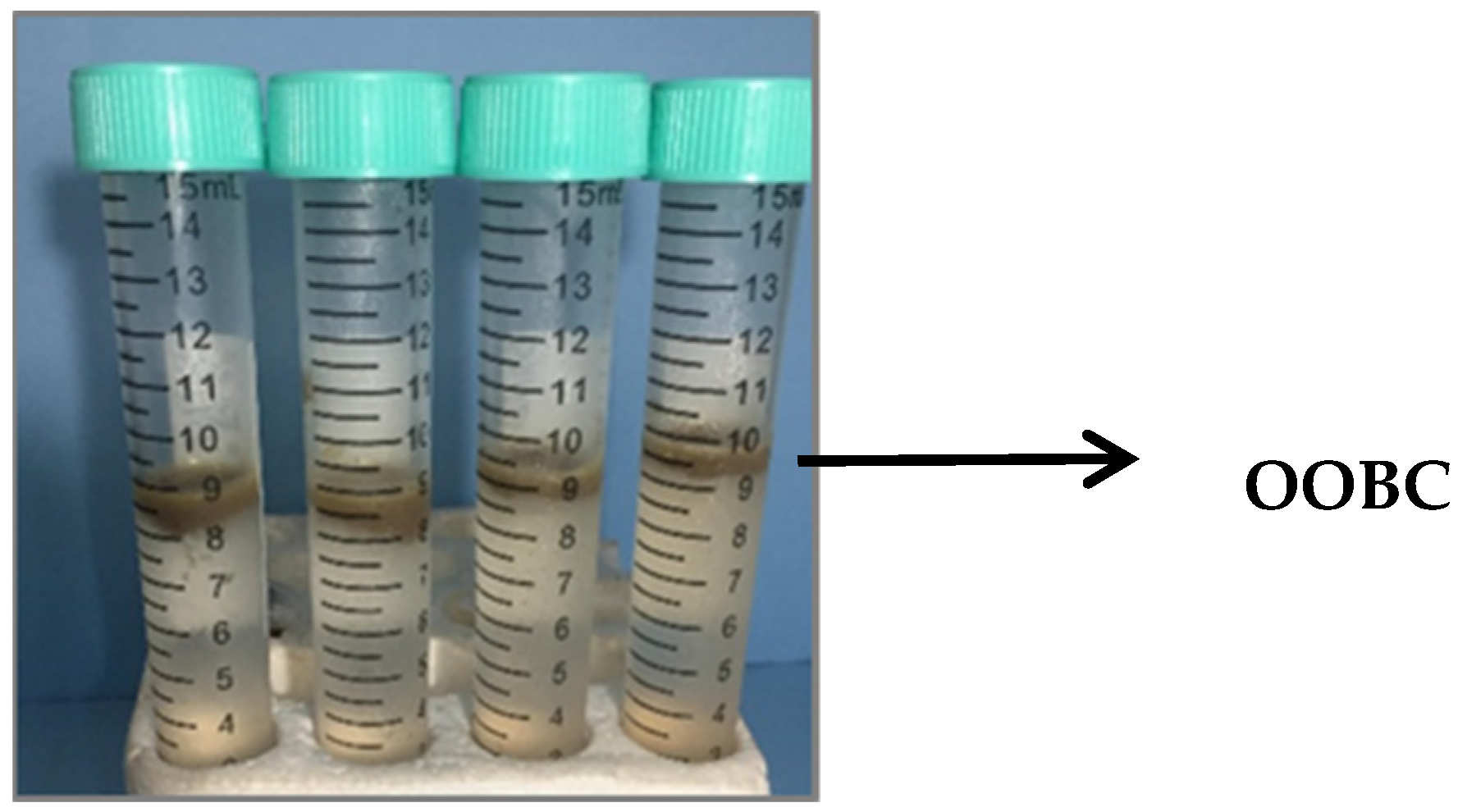
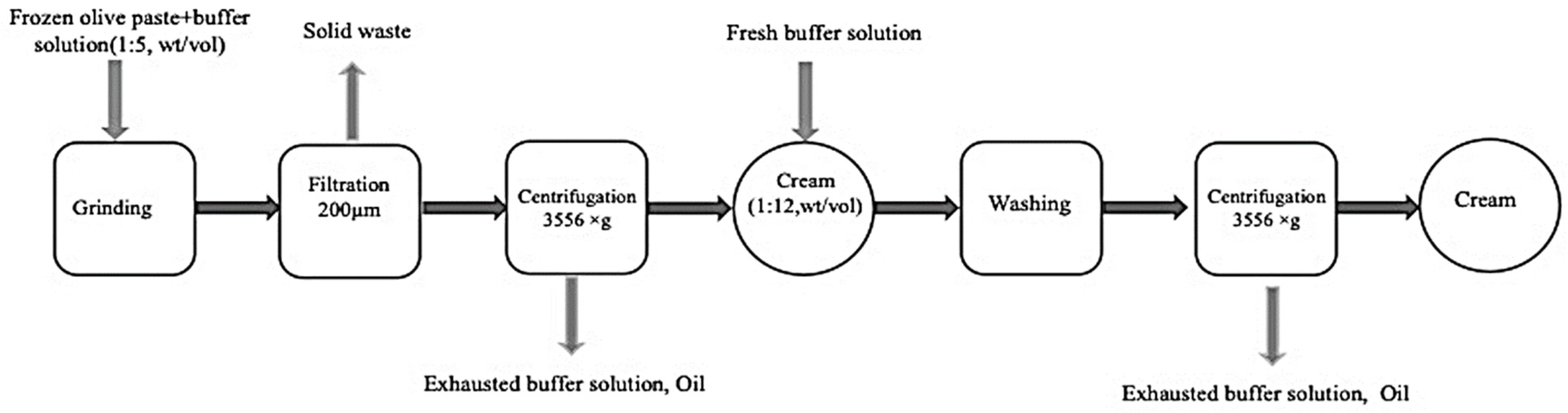
2.1. Extraction of Olive Oil Bodies
2.2. OOBC Characterisations
2.2.1. Proximate Composition
2.2.2. Concentration of Polyphenols
2.2.3. Concentration of Flavonoids
2.2.4. Antioxidant Power
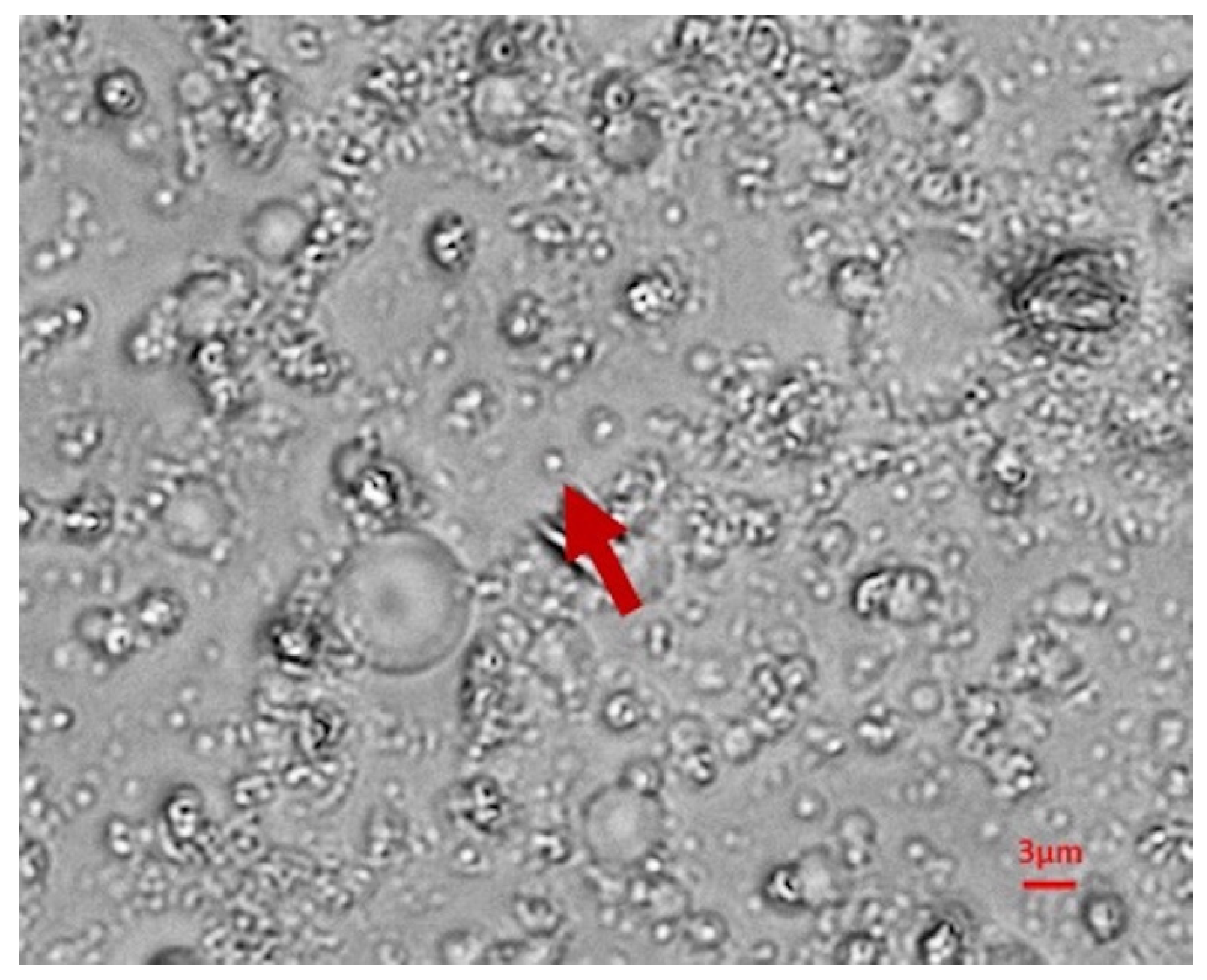
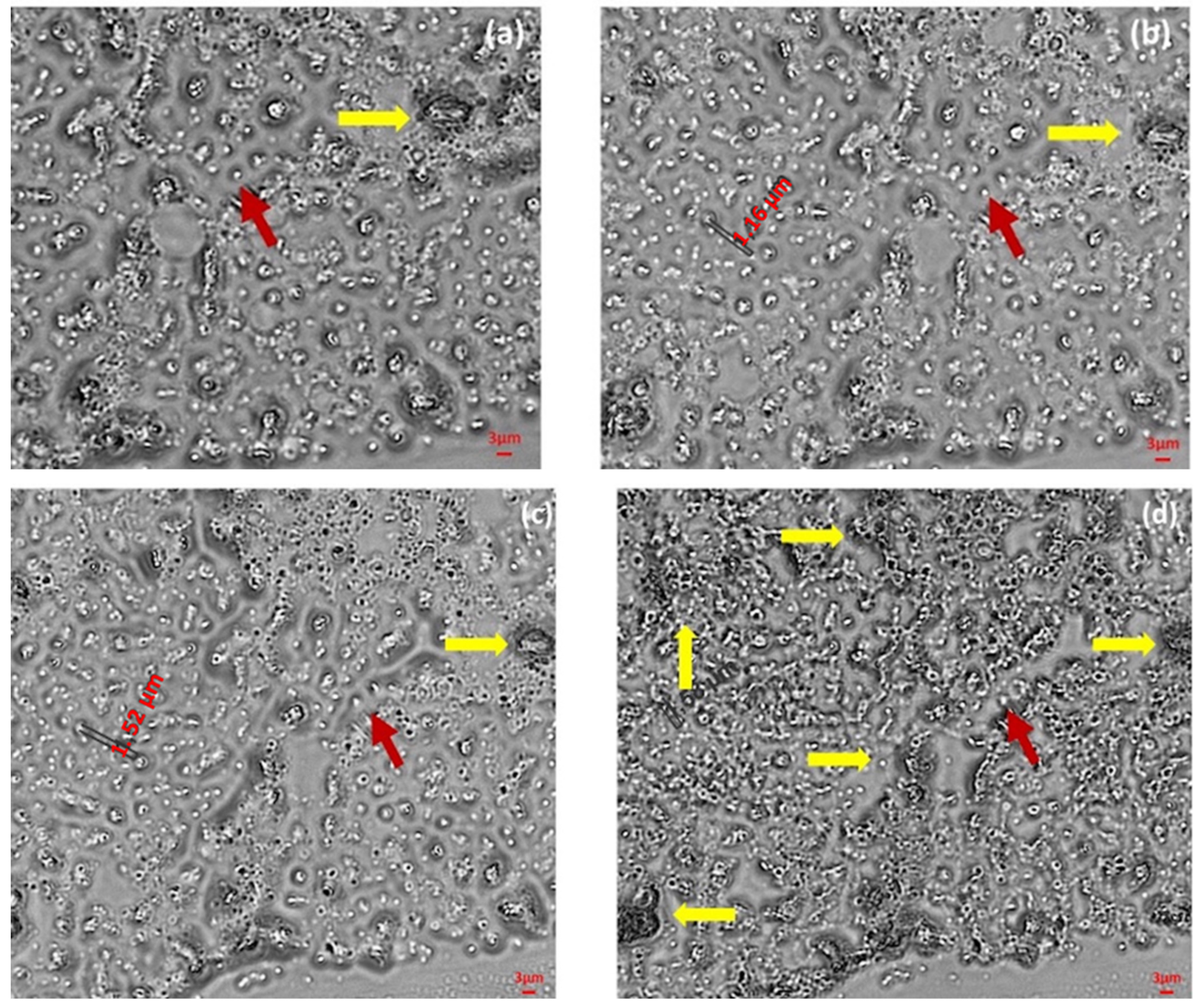
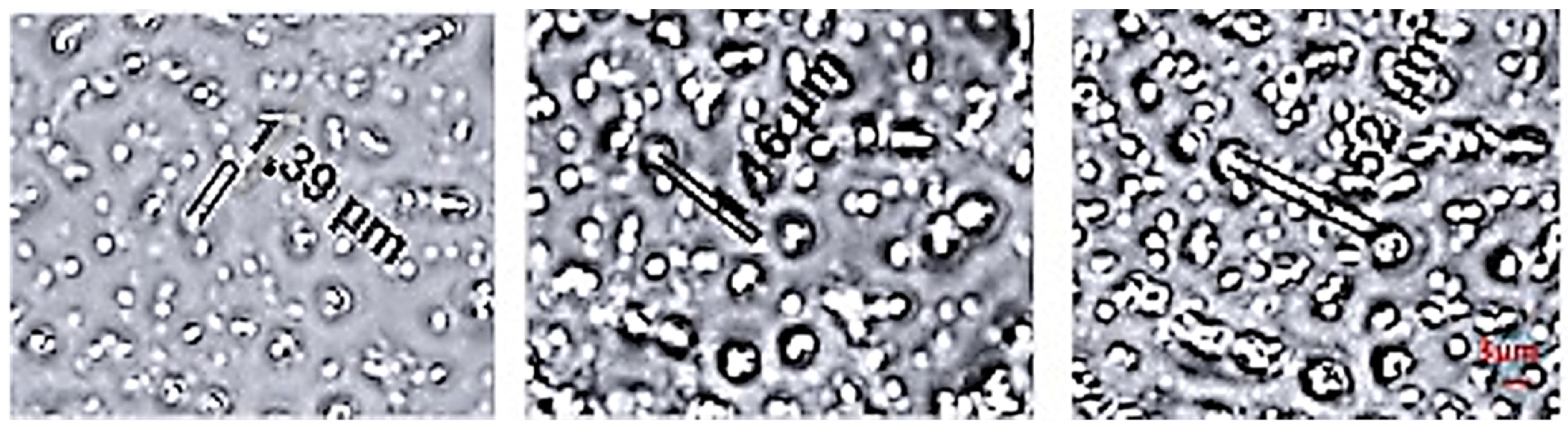
2.2.5. Size Determination and Morphological Analysis
2.2.6. OBs Thermal Stability
2.3. Statistical Analysis
2.4. OOBC Stability Assessment
2.5. Rheological Measurements
2.5.1. Viscosity
2.5.2. Thermal Flow Curve
2.5.3. Amplitude and Frequency Sweep
2.6. Tribological Measurements—Stribeck Curve
3. Results and Discussion
3.1. Olive Oil Bodies Extraction
3.2. Proximate Composition
3.3. Polyphenols, Flavonoid Contents and Antioxidant Power
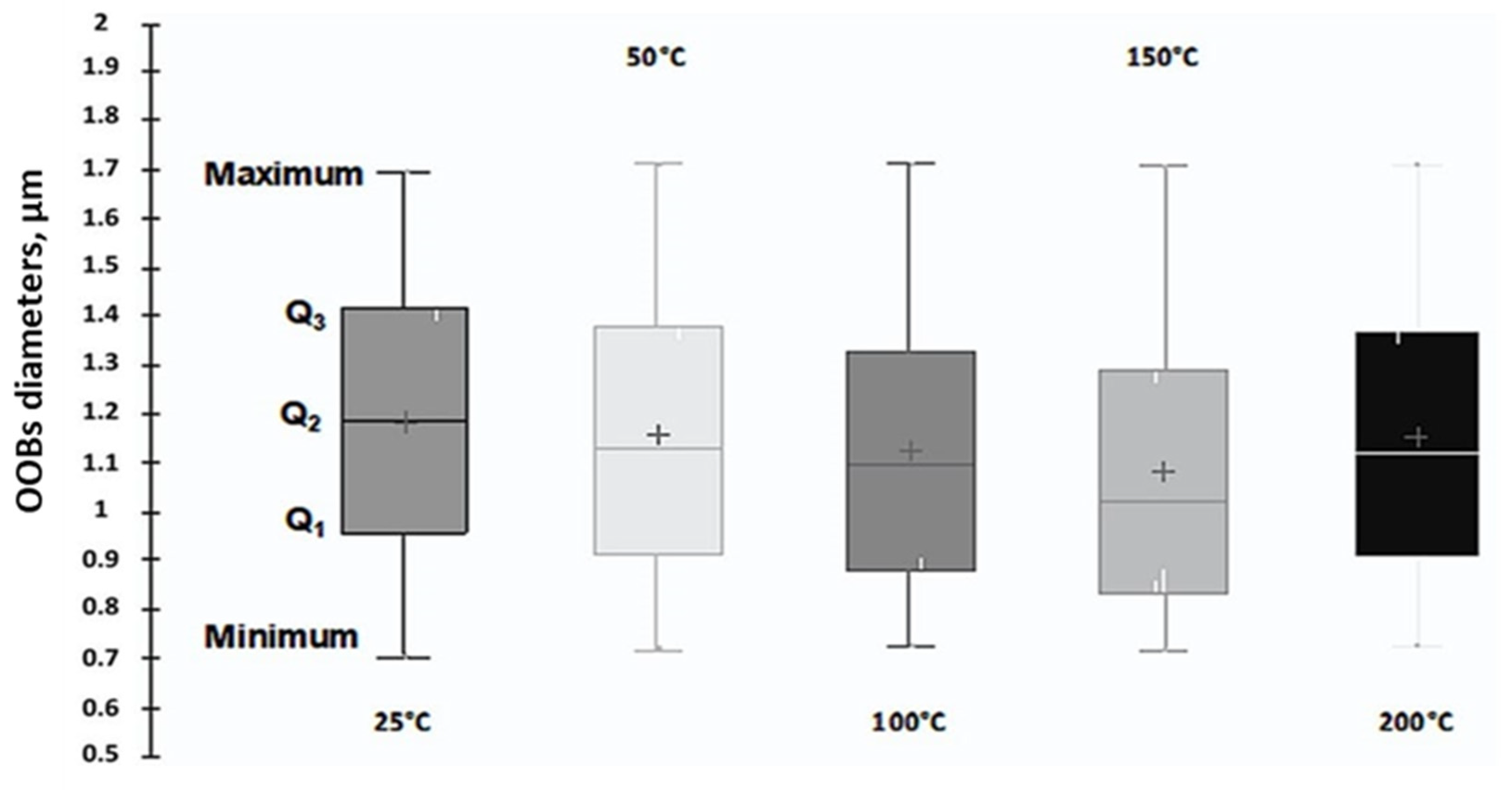
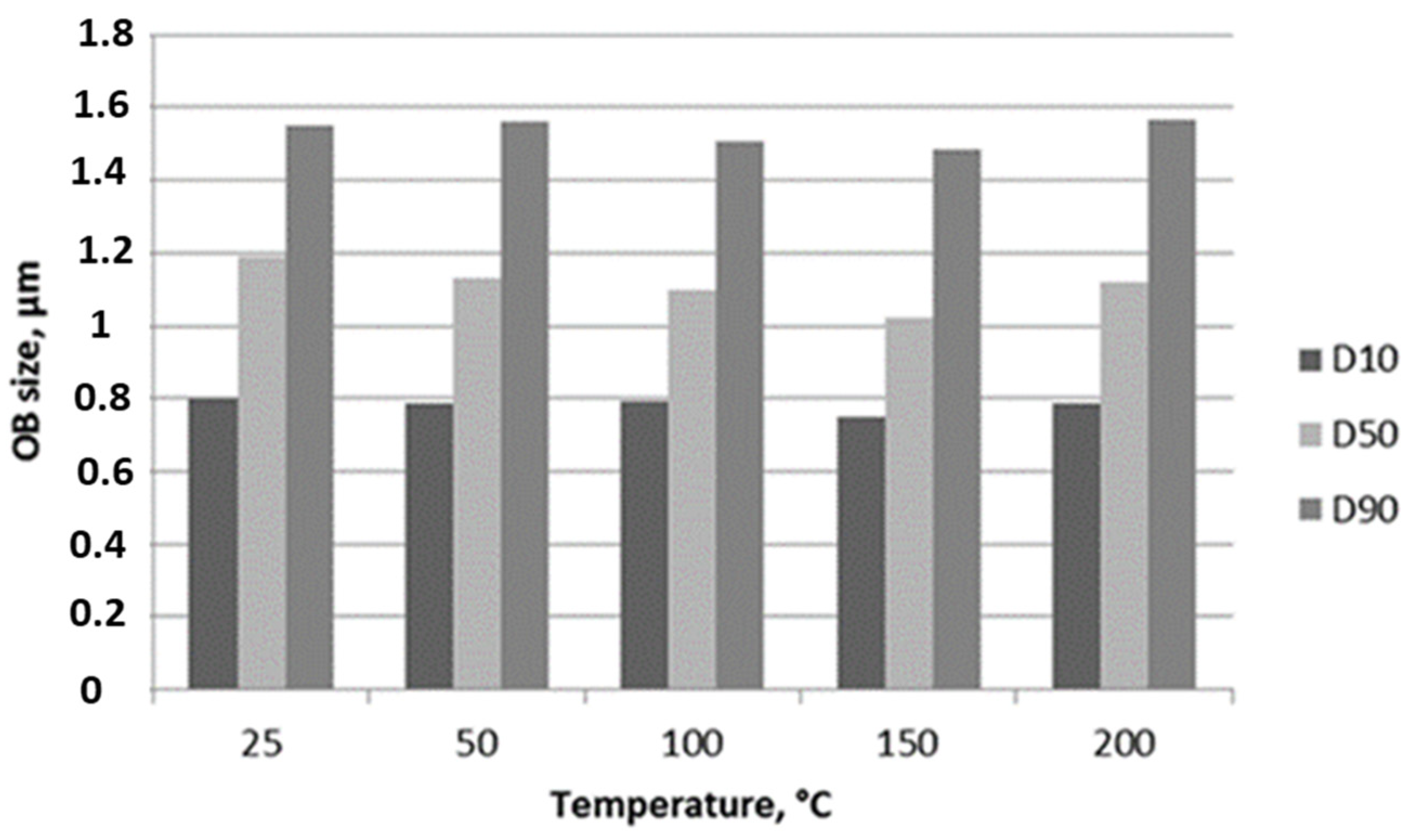
3.4. OBs Morphological Analysis and Thermal Stability
3.5. OOBC Stability Assessment
3.6. Rheological Measurements
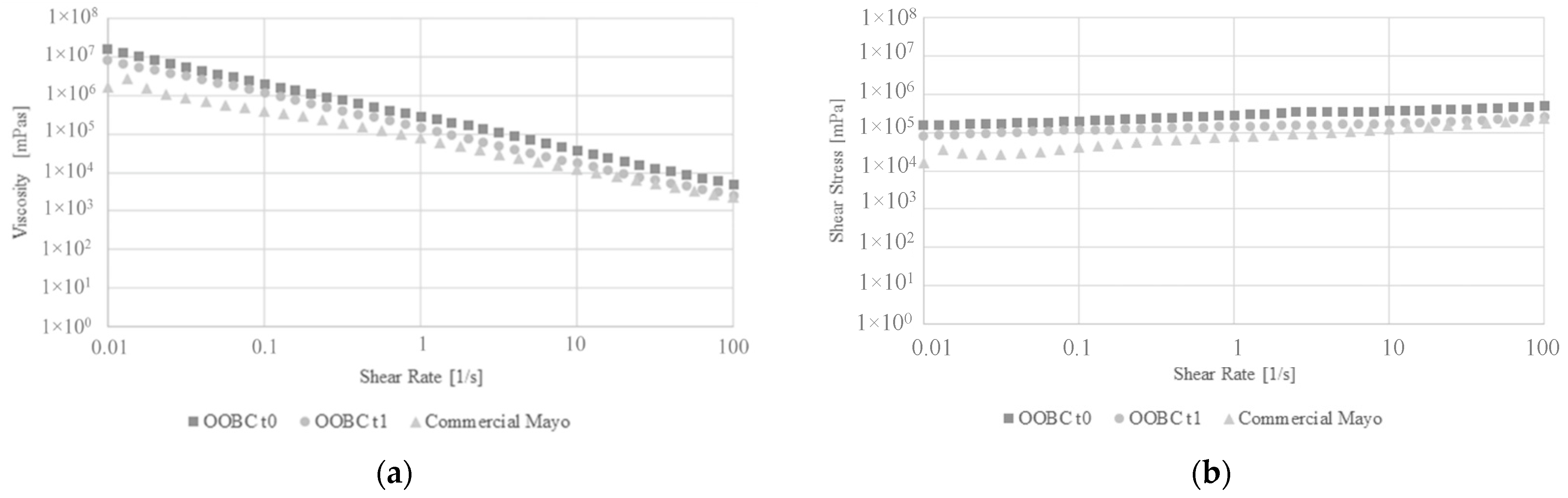
3.6.1. Flow Curve Tests and Thermal Flow Curve Ramp
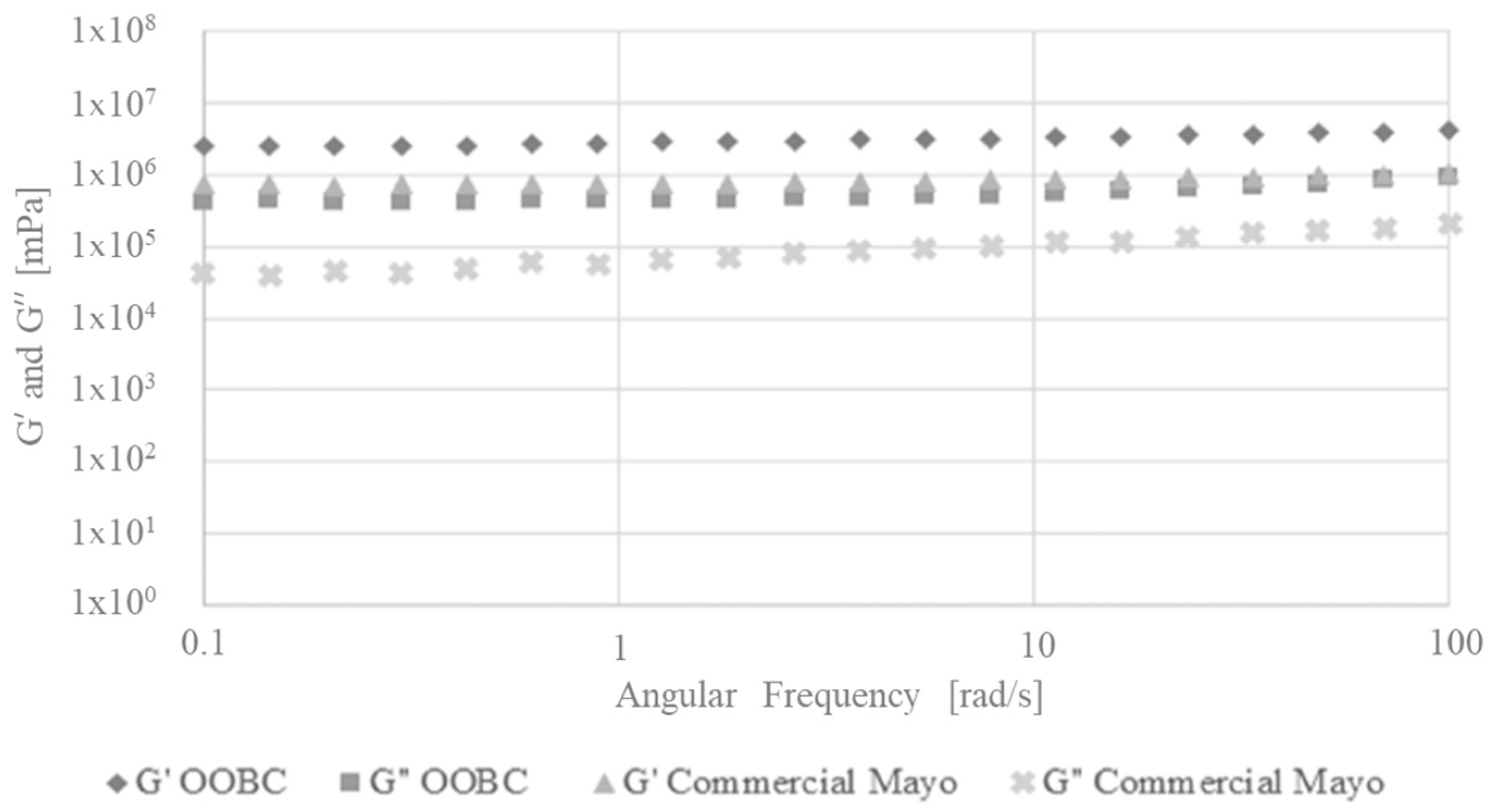
3.6.2. Amplitude and Frequency Sweep
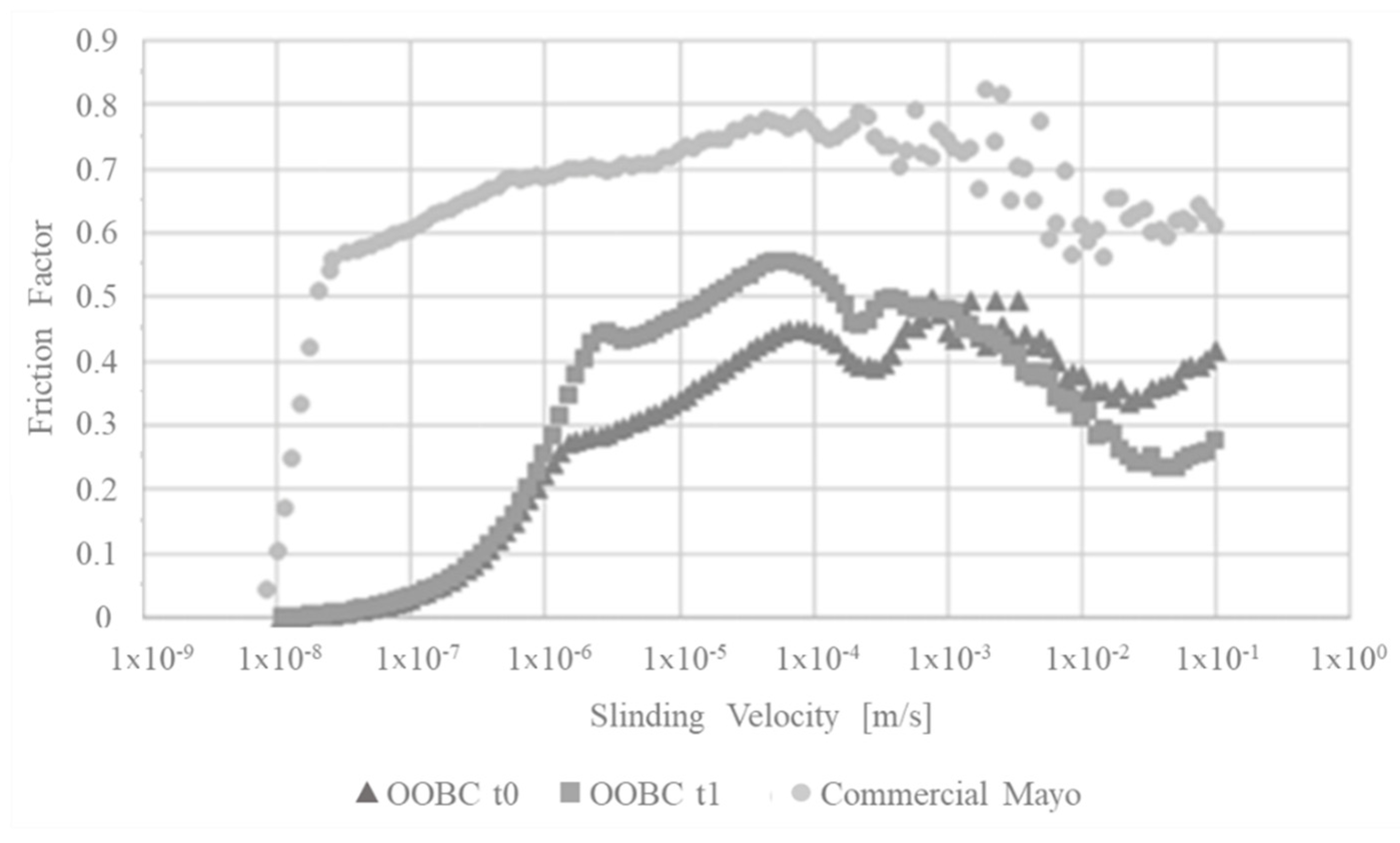
3.7. Tribological Measurements—Stribeck Curve
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.C.F.; Tsai, C.C.Y.; Tzen, J.T.C. Cloning and Secondary Structure Analysis of Caleosin, a Unique Calcium-Binding Protein in Oil Bodies of Plant Seeds. Plant Cell Physiol. 1999, 40, 1079–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.J.; Tai, S.S.K.; Peng, C.C.; Tzen, J.T.C. Correction. Plant Physiol. 2002, 129, 1930. [Google Scholar] [CrossRef] [Green Version]
- Næsted, H.; Frandsen, G.I.; Jauh, G.Y.; Hernandez-Pinzon, I.; Nielsen, H.B.; Murphy, D.J.; Rogers, J.C.; Mundy, J. Caleosins: Ca2+-binding proteins associated with lipid bodies. Plant Molecoular Biol. 2000, 44, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Tzen, J.; Cao, Y.; Laurent, P.; Ratnayake, C.; Huang, A. Lipids, Proteins, and Structure of Seed Oil Bodies from Diverse Species. Plant Physiol. 1993, 101, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzen, J.; Huang, A. Surface structure and properties of plant seed oil bodies. J. Cell Biol. 1992, 117, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Fisk, I.D.; White, D.A.; Carvalho, A.; Gray, D.A. Tocopherol-An intrinsic component of sunflower seed oil bodies. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 341–344. [Google Scholar] [CrossRef]
- Tzen, J.T.C.; Lie, G.C.; Huang, A.H.C. Characterization of the charged components and their topology on the surface of plant seed oil bodies. J. Biol. Chem. 1992, 267, 15626–15634. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Israelachvili, J. Intermolecular and Surface Forces; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Demetriades, K.; Coupland, J.N.; Mcclements, D.J. Physical properties of whey protein stabilized emulsions as related to pH and NACl. J. Food Sci. 1997, 62, 342–347. [Google Scholar] [CrossRef]
- Demetriades, K.; McClements, D.J. Influence of pH and Heating on Physicochemical Properties of Whey Protein-Stabilized Emulsions Containing a Nonionic Surfactant. J. Agric. Food Chem. 1998, 46, 3936–3942. [Google Scholar] [CrossRef]
- Hunt, J.A.; Dalgleish, D.G. Effect of pH on the stability and surface composition of emulsions made with whey protein isolate. J. Agric. Food Chem. 1994, 42, 2131–2135. [Google Scholar] [CrossRef]
- Kulmyrzaev, A.; Sivestre, M.P.C.; McClements, D.J. Rheology and stability of whey protein stabilized emulsions with high CaCl2 concentrations. Food Res. Int. 2000, 33, 21–25. [Google Scholar] [CrossRef]
- Georgalaki, M.D.; Bachmann, A.; Sotiroudis, T.G.; Xenakis, A.; Porzel, A.; Feussner, I. Characterization of a 13-lipoxygenase from virgin olive oil and oil bodies of olive endosperms. Lipid-Fett 1998, 100, 554–560. [Google Scholar] [CrossRef]
- Gómez-Escalonilla, M.; Vidal-Hernámdez, J. Variedades del Olivar; Hojas Divulgadoras; Ministerio de Agricultura: Madrid, Spain, 2011; p. 32.
- Available online: https://ec.europa.eu/info/food-farming-fisheries/farming/facts-and-figures/markets/prices/price-monitoring-sector/olive-oil_it (accessed on 7 June 2022).
- Conde, C.; Delrot, S.; Gerós, H. Physiological, biochemical and molecular changes occurring during olive development and ripening. J. Plant Physiol. 2008, 165, 1545–1562. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, G.; Lama, A.; Rodríguez, R.; Jiménez, A.; Guillén, R.; Fernández-Bolaños, J. Olive stone an attractive source of bioactive and valuable compounds. Bioresour. Technol. 2008, 99, 5261–5269. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Alaiz, M.; Hidalgo, F.J. Influence of Cultivar and Fruit Ripening on Olive (Olea europaea) Fruit Protein Content, Composition, and Antioxidant Activity. J. Agric. Food Chem. 2001, 49, 4267–4270. [Google Scholar] [CrossRef]
- Nergiz, C.; Ergönül, P.G. Organic acid content and composition of the olive fruits during ripening and its relationship with oil and sugar. Sci. Hortic. 2009, 122, 216–220. [Google Scholar] [CrossRef]
- Pokorný, J. Olive Oil. In Chemistry and Technology; Boskou, D., Ed.; AOCS Press: Champaign, IL, USA, 1997; Volume 41, pp. 119–120. [Google Scholar] [CrossRef]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.-H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.)—A Review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Matsakidou, A.; Kiosseoglou, V. Composition, properties and potential food applications of natural emulsions and cream materials based on oil bodies. RSC Adv. 2014, 4, 25067–25078. [Google Scholar] [CrossRef] [Green Version]
- Beindorff, C.M.; Ghislain, C.Y.; Sergey, L.; Melnikov, M.; Winter, I. Satiety Enhancing Food Products. WO2007115899, 18 October 2007. [Google Scholar]
- White, D.A.; Fisk, I.D.; Mitchell, J.R.; Wolf, B.; Hill, S.E.; Gray, D.A. Sunflower-seed oil body emulsions: Rheology and stability assessment of a natural emulsion. Food Hydrocoll. 2008, 22, 1224–1232. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kim, J.S.; Kang, O.J.; Gweon, O.C. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J. Funct. Foods 2013, 5, 80–86. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man. Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Prakash, S.; Tan, D.D.Y.; Chen, J. Applications of tribology in studying food oral processing and texture perception. Food Res. Int. 2013, 54, 1627–1635. [Google Scholar] [CrossRef]
- Laguna, L.; Farrell, G.; Bryant, M.; Morina, A.; Sarkar, A. Relating rheology and tribology of commercial dairy colloids to sensory perception. Food Funct. 2017, 8, 563–573. [Google Scholar] [CrossRef]
- Pondicherry, K.S.; Rummel, F.; Laeuger, J. Extended stribeck curves for food sample. Biosurface Biotribol. 2018, 4, 34–37. [Google Scholar] [CrossRef]
- Sethupathy, P.; Moses, J.A.; Anandharamakrishnan, C. Food Oral Processing and Tribology: Instrumental Approaches and Emerging Applications. Food Rev. Int. 2021, 37, 538–571. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Alaiz, M.; Zamora, R. Determination of Peptides and Proteins in Fats and Oils. Anal. Chem. 2001, 73, 698–702. [Google Scholar] [CrossRef]
- Esteve, C.; D’Amato, A.; Marina, M.L.; García, M.C.; Righetti, P.G. Analytical Approaches for the Characterization and Identification of Olive (Olea europaea) Oil Proteins. J. Agric. Food Chem. 2013, 61, 10384–10391. [Google Scholar] [CrossRef]
- Ninfali, P.; Aluigi, G.; Bacchiocca, M.; Magnani, M. Antioxidant capacity of extra-virgin olive oils. J. Am. Oil Chem. Soc. 2001, 78, 243–247. [Google Scholar] [CrossRef]
- Sánchez, C.S.; González, A.M.T.; García-Parrilla, M.C.; Granados, J.J.Q.; de la Serrana, H.L.G.; Martínez, M.C.L. Different radical scavenging tests in virgin olive oil and their relation to the total phenol content. Anal. Chim. Acta 2007, 593, 103–107. [Google Scholar] [CrossRef] [PubMed]

| OOBC Proximate Composition, % | |
|---|---|
| Water | 40.00 |
| Lipid | 26.76 |
| Protein | 5.99 |
| Other | 27.25 |
| OOBC TP, TF and Antioxidant Power | |
|---|---|
| Total polyphenols | 1.11 mgGAE/gdry |
| Total flavonoids | 0.50 mgQE/gdry |
| FRAP | 0.08 millimoliFe/gdry |
| 20.04 mgTroloxE/gdry | |
| 25 °C | 50 °C | 100 °C | 150 °C | 200 °C | |
|---|---|---|---|---|---|
| Mean diameters, µm ± STD | 1.183 ± 0.277 | 1.158 ± 0.280 | 1.123 ± 0.270 | 1.078 ± 0.271 | 1.150 ± 0.278 |
| Mean circularities ± STD | 0.921 ± 0.103 | 0.712 ± 0.179 | 0.653 ± 0.202 | 0.686 ± 0.188 | 0.713 ± 0.179 |
| Centrifugation Step Number | T (min) | Separation Grade % OOBC-U | Separation Grade % OOBC-H |
|---|---|---|---|
| 1 | 5 | 1.1 | 0 |
| 2 | 10 | 1.1 | 0 |
| 3 | 15 | 2.2 | 3.8 |
| 4 | 20 | 3.4 | 4.6 |
| 5 | 25 | 4.5 | 5.8 |
| 6 | 30 | 4.5 | 6.9 |
| µ Static | <µ Dynamic> | |
|---|---|---|
| OOBC t0 | 0.2712 | 0.3196 |
| OOBC t1 | 0.4411 | 0.4412 |
| Commercial mayo | 0.7753 | 0.7005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itri, S.; Gallo, M.; Orefice, C.; Garella, I.; Di Domenico, M.; Vitali, S.; Stanzione, V.; Grilli, S.; Ferraro, P.; Nigro, R. Oil Bodies Cream from Olive Paste: Extraction of a Functional Ingredient for Developing a Stable Food Emulsion. Appl. Sci. 2022, 12, 6019. https://doi.org/10.3390/app12126019
Itri S, Gallo M, Orefice C, Garella I, Di Domenico M, Vitali S, Stanzione V, Grilli S, Ferraro P, Nigro R. Oil Bodies Cream from Olive Paste: Extraction of a Functional Ingredient for Developing a Stable Food Emulsion. Applied Sciences. 2022; 12(12):6019. https://doi.org/10.3390/app12126019
Chicago/Turabian StyleItri, Simona, Marianna Gallo, Carlo Orefice, Isidoro Garella, Marica Di Domenico, Serena Vitali, Vitale Stanzione, Simonetta Grilli, Pietro Ferraro, and Roberto Nigro. 2022. "Oil Bodies Cream from Olive Paste: Extraction of a Functional Ingredient for Developing a Stable Food Emulsion" Applied Sciences 12, no. 12: 6019. https://doi.org/10.3390/app12126019
APA StyleItri, S., Gallo, M., Orefice, C., Garella, I., Di Domenico, M., Vitali, S., Stanzione, V., Grilli, S., Ferraro, P., & Nigro, R. (2022). Oil Bodies Cream from Olive Paste: Extraction of a Functional Ingredient for Developing a Stable Food Emulsion. Applied Sciences, 12(12), 6019. https://doi.org/10.3390/app12126019








