Performance of Wild Non-Conventional Yeasts in Fermentation of Wort Based on Different Malt Extracts to Select Novel Starters for Low-Alcohol Beers
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Wort Preparation
- -
- ME for Pilsner beer: original specific gravity (SG) 1036, pH 5.92;
- -
- ME for Weizen beer: SG 1036, pH 5.94;
- -
- ME for Amber beer: SG1038, pH 5.91.
2.3. Microfermentation Trials
2.4. Analytical Determination
2.5. Statistical Analysis
3. Results and Discussion
3.1. Mixed Fermentation Trials in Three Worts
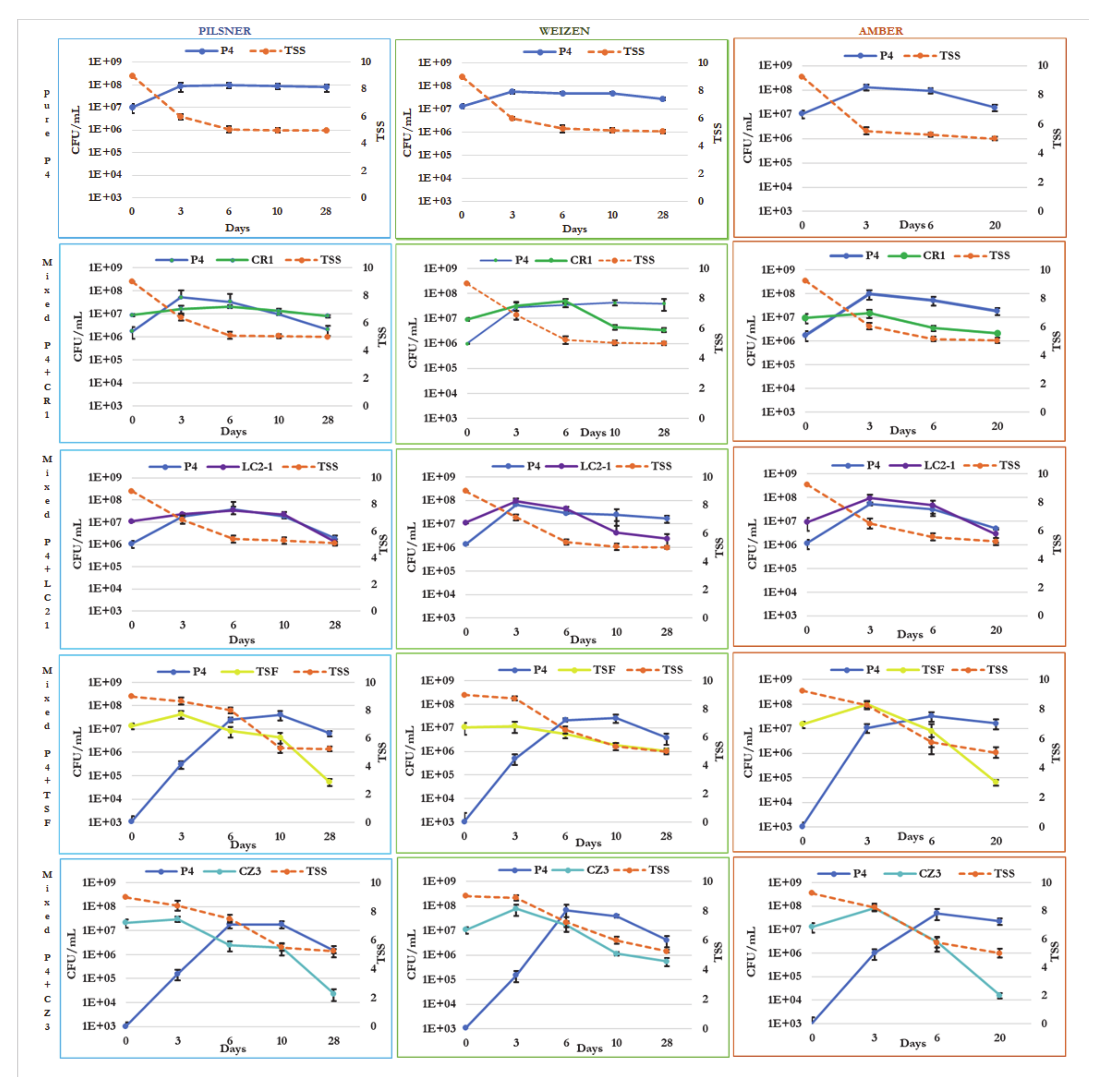
3.2. Cell Growth Dynamics of the Strains and Evolution of Total Soluble Solids during Mixed Fermentation
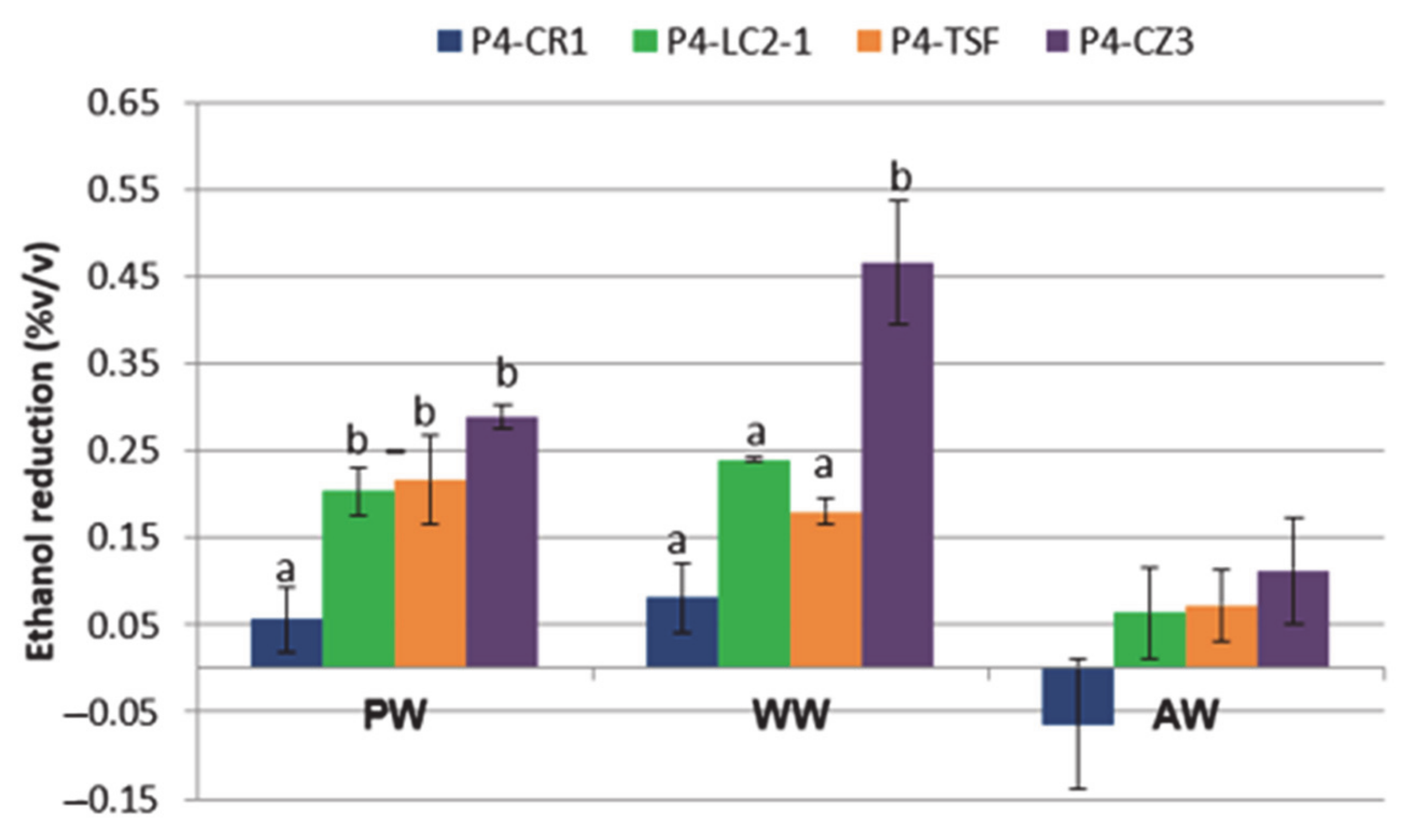
3.3. Analytical Parameters of the Experimental Beers
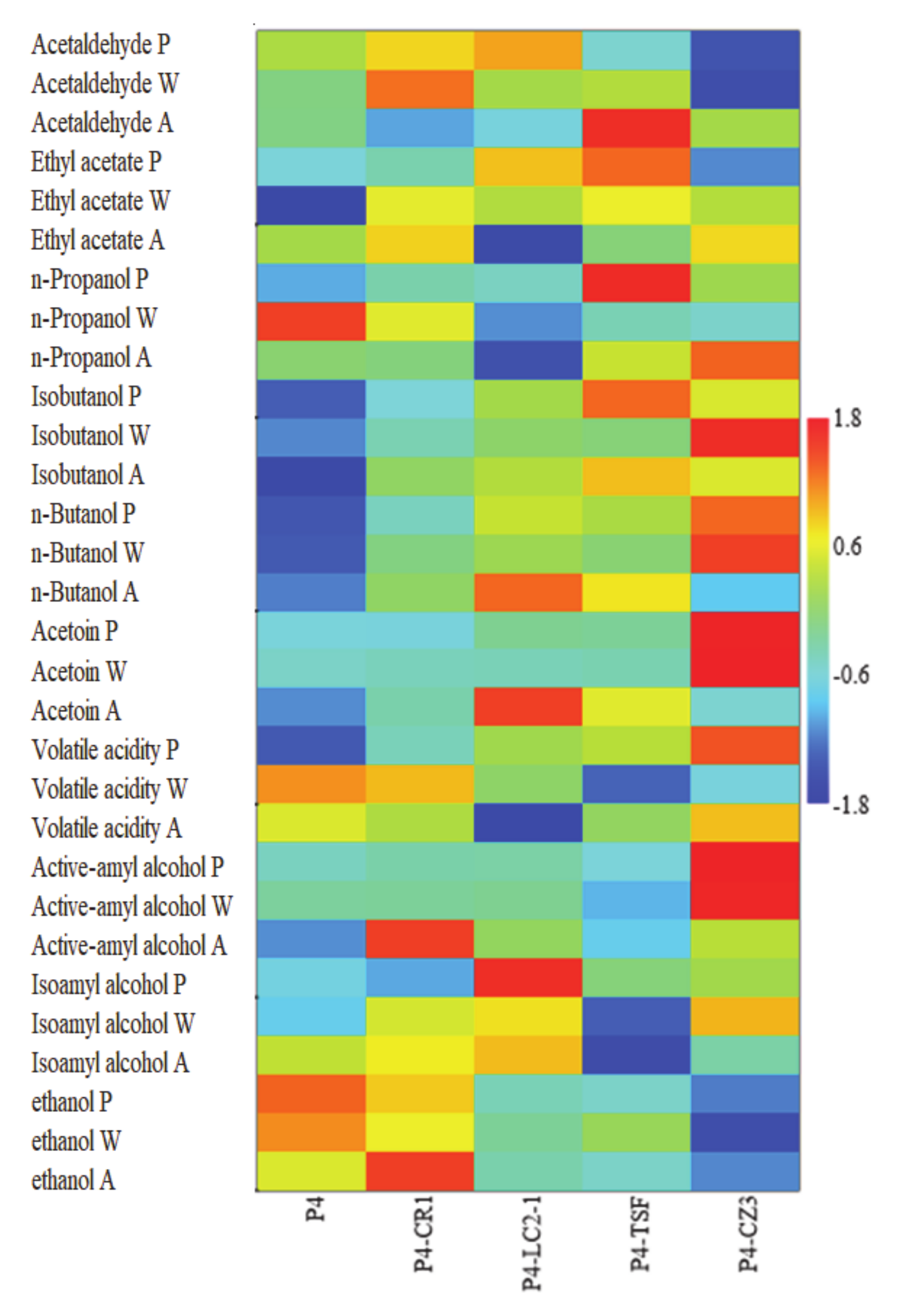
3.4. Main Volatile Compounds in the Different Experimental Beers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verstrepen, K.J.; Derdelinckx, G.; Dufour, J.P.; Winderickx, J.; Thevelein, J.M.; Pretorius, I.S.; Delvaux, F.R. Flavor-active esters: Adding fruitiness to beer. J. Biosci. Bioeng. 2003, 96, 110–118. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma-a review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Dzialo, M.C.; Park, R.; Steensels, J.; Lievens, B.; Verstrepen, K.J. Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiol. Rev. 2017, 41, S95–S128. [Google Scholar] [CrossRef] [PubMed]
- Steensels, J.; Daenen, L.; Malcorps, P.; Derdelinckx, G.; Verachtert, H.; Verstrepen, K.J. Brettanomyces yeasts-From spoilage organisms to valuable contributors to industrial fermentations. Int. J. Food Microbiol. 2015, 206, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Varela, C. The impact of non-Saccharomyces yeasts in the production of alcoholic beverages. Appl. Microbiol. Biotechnol. 2016, 100, 9861–9874. [Google Scholar] [CrossRef] [PubMed]
- Gamero, A.; Quintilla, R.; Groenewal, M.; Alkema, W.; Boekhout, T.; Hazelwood, L. High-throughput screening of a large collection of non-conventional yeasts reveals their potential for aroma formation in food fermentation. Food Microbiol. 2016, 60, 147–159. [Google Scholar] [CrossRef]
- Van Rijswijck, I.M.H.; Wolkers-Rooijackers, J.C.M.; Abee, T.; Smid, E.J. Performance of non-conventional yeasts in co-culture with brewers’ yeast for steering ethanol and aroma production. Microbiol. Biotechnol. 2017, 10, 1591–1602. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Chua, J.Y.; Liu, S.Q. Impact of simultaneous fermentation with Saccharomyces cerevisiae and Torulaspora delbrueckii on volatile and non-volatile constituents in beer. LWT-Food Sci. Technol. 2018, 91, 26–33. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F. Non-Saccharomyces wine yeasts have a promising role in biotechnological approaches to winemaking. Ann. Microbiol. 2010, 61, 25–32. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.; Mukherjee, V.; Lievens, B.; Verstrepen, K.J.; Thevelein, J.M. Bioflavoring by non-conventional yeasts in sequential beer fermentations. Food Microbiol. 2017, 72, 55–66. [Google Scholar] [CrossRef]
- Canonico, L.; Galli, E.; Ciani, E.; Comitini, F.; Ciani, M. Exploitation of three non-conventional yeast species in the brewing process. Microorganisms 2019, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, G.; Turchetti, B.; Sileoni, V.; Marconi, O.; Perretti, G. Screening of new strains of Saccharomycodes ludwigii and Zygosaccharomyces rouxii to produce low-alcohol beer. J. Inst. Brew. 2015, 121, 113–121. [Google Scholar] [CrossRef]
- Iattici, F.; Catallo, M.; Solieri, L. Designing New Yeasts for Craft Brewing: When Natural Biodiversity Meets Biotechnology. Beverages 2020, 6, 3. [Google Scholar] [CrossRef]
- Haslbeck, K.; Jerebic, S.; Zarnkow, M. Characterization of the unfertilized and fertilized hop varieties progress and hallertauer tradition-Analysis of free and glycosidic-bound flavor compounds and β-glucosidase activity. Brew. Sci. 2017, 70, 148–158. [Google Scholar]
- Meier-Dörnberg, T.; Hutzler, M.; Jacob, F.; Schneiderbanger, H. Geschmacklich ansprechend. Brauindustrie 2015, 7, 12–15. [Google Scholar]
- Saerens, S.; Swiegers, J.H. Production of Low-Alcohol or Alcohol-Free Beer with Pichia kluyveri Yeast Strains. Patent No. WO2014135673A2, 12 September 2014. [Google Scholar]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulaspora delbrueckii in the brewing process: A new approach to enhance bioflavour and to reduce ethanol content. Food Microbiol. 2016, 56, 45–51. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Torulaspora delbrueckii contribution in mixed brewing fermentations with different Saccharomyces cerevisiae strains. Int. J. Food Microbiol. 2017, 259, 7–13. [Google Scholar] [CrossRef]
- Michel, M.; Kopecká, J.; Meier-Dörnberg, T.; Zarnkow, M.; Jacob, F.; Hutzler, M. Screening for new brewing yeasts in the non-Saccharomyces sector with Torulaspora delbrueckii as model. Yeast 2016, 33, 129–144. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Dominance and influence of selected Saccharomyces cerevisiae strains on the analytical profile of craft beer refermentation. J. Inst. Brew. 2014, 120, 262–267. [Google Scholar] [CrossRef]
- Tataridis, P.; Kanelis, A.; Logotetis, S.; Nerancis, E. Use of non-Saccharomyces Torulaspora delbrueckii yeast strains in winemaking and brewing. Zb. Matice Srp. Prir. Nauke 2013, 124, 415–426. [Google Scholar] [CrossRef]
- Petruzzi, L.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Brewer’s yeast in controlled and uncontrolled fermentations, with a focus on novel, nonconventional, and superior strains. Food Rev. Int. 2016, 32, 341–363. [Google Scholar] [CrossRef]
- Domizio, P.; House, J.F.; Joseph, C.M.L.; Bisson, L.F.; Bamforth, C.W. Lachancea thermotolerans as an alternative yeast for the production of beer. J. Inst. Brew. 2016, 122, 599–604. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Zambuto, M.; Romano, P. Use of Saccharomyces cerevisiae var boulardii in co-fermentations with S. cerevisiae for the production of craft beers with potential healthy value added. Int. J. Food Microbiol. 2018, 284, 22–30. [Google Scholar]
- Granchi, L.; Bosco, M.; Messini, A.; Vincenzini, M. Rapid detection and quantification of yeast species during spontaneous wine fermentation by PCR-RFLP analysis of the rDNA ITS region. J. Appl. Microbiol. 1999, 87, 949–956. [Google Scholar] [CrossRef]
- Kurtzman, C.; Robnett, C.J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie van Leeuwenhoek 1998, 73, 331–371. [Google Scholar] [CrossRef]
- Vidgren, V.; Huuskonen, A.; Virtanen, H.; Ruohonen, L.; Londesborough, J. Improved fermentation performance of a lager yeast after repair of its AGT1 maltose and maltotriose transporter genes. Appl. Environ. Microbiol. 2009, 75, 2333–2345. [Google Scholar] [CrossRef][Green Version]
- Gutiérrez, A.; Boekhout, T.; Gojkovic, Z.; Katz, M. Evaluation of non-Saccharomyces yeasts in the fermentation of wine, beer and cider for the development of new beverages. J. Inst. Brew. 2018, 124, 389–402. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Cubillos, F.A.; Gibson, B.; Grijalva-Vallejos, N.; Krogerus, K.; Nikulin, J. Bioprospecting for brewers: Exploiting natural diversity for naturally diverse beers. Yeast 2019, 36, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Turchetti, B.; Sileoni, V.; Marconi, O.; Perretti, G. Evaluation of Saccharomyces cerevisiae strains isolated from non-brewing environments in beer production. J. Inst. Brew. 2018, 124, 381–388. [Google Scholar] [CrossRef]
- Marongiu, A.; Zara, G.; Legras, J.-L.; Del Caro, A.; Mascia, I.; Fadda, C.; Budroni, M. Novel starters for old processes: Use of Saccharomyces cerevisiae strains isolated from artisanal sourdough for craft beer production at a brewery scale. J. Ind. Microbiol. Biotechnol. 2015, 42, 85–92. [Google Scholar] [CrossRef]
- Mascia, I.; Fadda, C.; Dostálek, P.; Kerabín, M.; Zara, G.; Budroni, M.; Del Caro, A. Is it possible to create an innovative craft durum wheat beer with sourdough yeasts? A case study. J. Inst. Brew. 2015, 121, 283–286. [Google Scholar] [CrossRef]
- Mascia, I.; Fadda, C.; Kerabín, M.; Dostálek, P.; Del Caro, A. Aging of craft durum wheat beer fermented with sourdough yeasts. LWT-Food Sci. Technol. 2016, 65, 487–494. [Google Scholar] [CrossRef]
- King, A.; Dickinson, J.R. Biotransformation of monoterpene alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluvveromyces lactis. Yeast 2000, 16, 499–506. [Google Scholar] [CrossRef]
- Estela-Escalante, W.D.; Rosales-Mendoza, S.; Moscosa-Santillán, M.; González-Ramírez, J.E. Evaluation of the fermentative potential of Candida zemplinina yeasts for craft beer fermentation. J. Inst. Brew. 2016, 122, 530–535. [Google Scholar] [CrossRef]
- Michel, M.; Meier-Dörnberg, T.; Jacob, F.; Methner, F.-J.; Zarnkow, M.; Wagner, R.S.; Hutzler, M. Review: Pure non-Saccharomyces starter cultures for beer fermentation with a focus on secondary metabolites and practical applications. J. Inst. Brew. 2016, 122, 569–587. [Google Scholar] [CrossRef]
- Lodolo, E.J.; Kock, J.L.F.; Axcell, B.C.; Brooks, M. The yeast Saccharomyces cerevisiae: The main character in beer brewing. FEMS Yeast Res. 2008, 8, 1018–1036. [Google Scholar] [CrossRef]
- Pratt, P.L.; Bryce, J.H.; Stewart, G.G. The effect of osmotic pressure and ethanol on yeast viability and morphology. J. Inst. Brew. 2003, 109, 218–228. [Google Scholar] [CrossRef]
- Fugelsang, K.C.; Edwards, C.G. Wine Microbiology: Practical Applications and Procedures; Springer Science: New York, NY, USA, 2007. [Google Scholar]
- Zuehlke, J.M.; Childs, B.C.; Edwards, C.G. Evaluation of Zygosaccharomyces bailii to Metabolize Residual Sugar Present in Partially-Fermented Red Wines. Fermentation 2015, 1, 3–12. [Google Scholar] [CrossRef]
- Estela-Escalante, W.D.; Moscosa-Santillán, M.; González-Ramírez, J.E.; Rosales-Mendoza, S. Evaluation of the Potential Production of ethanol by Candida zemplinina Yeast with Regard to Beer Fermentation. J. Am. Soc. Brew. Chem. 2017, 75, 130–135. [Google Scholar]
- Domizio, P.; Romani, C.; Comitini, F.; Gobbi, M.; Lencioni, L.; Mannazzu, I.; Ciani, M. Potential spoilage non-Saccharomyces yeasts in mixed cultures with Saccharomyces erevisiae. Ann. Microbiol. 2011, 61, 137–144. [Google Scholar] [CrossRef]
- Garavaglia, J.; de Souza Schneider, R.D.C.; Camargo Mendes, S.D.; Welke, J.E.; Zini, C.A.; Caramão, E.B.; Valente, P. Evaluation of Zygosaccharomyces bailii BCV 08 as a co-starter in wine fermentation for the improvement of ethyl esters production. Microbiol. Res. 2015, 173, 59–65. [Google Scholar] [CrossRef]
- Sohrabvandi, S.; Razavi, S.H.; Mousavi, S.M.; Mortazavian, A.; Rezaei, K. Application of Saccharomyces rouxii for the production of non-alcoholic beer. Food Sci. Biotechnol. 2009, 18, 1132–1137. [Google Scholar]
- Bellut, K.; Michel, M.; Zarnkow, M.; Hutzler, M.; Jacob, F.; DeSchutter, D.P.; Daenen, L.; Lynch, K.M.; Zannini, E.; Arendt, E.K. Application of Non-Saccharomyces Yeasts Isolated from Kombucha in the Production of Alcohol-Free Beer. Fermentation 2018, 4, 66. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G.; Mortimer, R.; Polsinelli, M. Production of high levels of acetoin in Saccharomyces cerevisiae wine yeasts is a recessive trait. J. Appl. Bacteriol. 1995, 78, 169–174. [Google Scholar] [CrossRef]
- Meilgaard, M.C. Prediction of flavor differences between beers from their chemical composition. J. Agric. Food Chem. 1982, 30, 1009–1017. [Google Scholar] [CrossRef]
- Hughes, P. Beer flavour. In Beer: A Quality Perspective; Bamforth, C.W., Ed.; Elsevier: New York, NY, USA, 2009; pp. 61–83. [Google Scholar]
- Holt, S.; Miks, M.H.; de Carvalho, B.T.; Foulquié-Moreno, M.R.; Thevelein, J.M. The molecular biology of fruity and floral aromas in beer and other alcoholic beverages. FEMS Microbiol. Rev. 2019, 43, 193–222. [Google Scholar] [CrossRef]
- Meilgaard, M.C. Flavor chemistry of beer: Part II: Flavour and threshold of 239 aroma volatiles. MBAA TQ 1975, 12, 151–168. [Google Scholar]

| STARTER | INOCULUM LEVEL |
|---|---|
| P4-TSF | 1 × 103 (P4) + 1 × 107 (TSF) |
| P4-CZ3 | 1 × 103 (P4) + 1 × 107 (CZ3) |
| P4-CR1 | 1 × 106 (P4) + 9 × 106 (CR1) |
| P4-LC2-1 | 1 × 106 (P4) + 9 × 106 (LC2-1) |
| P4; SP; SW; SA | 1 × 107 |
| P4 | P4-CR1 | P4-LC2-1 | P4-TSF | P4-CZ3 | ||
|---|---|---|---|---|---|---|
| PW | AA | 81.24 ± 6.78 | 81.30 ± 6.70 | 67.70 ± 5.8 | 81.54 ± 4.31 | 81.52 ± 4.8 |
| RA | 66.65 ± 3.90 | 66.70 ± 4.33 | 55.52 ± 3.98 | 66.90 ± 3.54 | 66.88 ± 3.55 | |
| VA | 0.13 ± 0.01 a | 0.23 ± 0.009 b | 0.30 ± 0.013 c | 0.31 ± 0.015 c | 0.41 ± 0.007 d | |
| AC | 3.09 ± 0.02 a | 3.03 ± 0.05 abc | 2.89 ± 0.01 b | 2.87 ± 0.06 b | 2.80 ± 0.03 b | |
| WW | AA | 88.10 ± 4.8 | 74.60 ± 4.78 | 81.22 ± 4.75 | 81.54 ± 4.31 | 81.30 ± 4.88 |
| RA | 72.20 ± 3.96 | 61.10 ± 3.92 | 66.64 ± 3.90 | 66.90 ± 3.54 | 66.69 ± 3.55 | |
| VA | 0.24 ± 0.013 a | 0.35 ± 0.012 b | 0.39 ± 0.01 b | 0.35 ± 0.006 b | 0.60 ± 0.008 c | |
| AC | 3.10 ± 0.02 a | 3.02 ± 0.06 a | 2.86 ± 0.02 b | 2.92 ± 0.01 ab | 2.63 ± 0.05 c | |
| AW | AA | 70.60 ± 6.4 | 70.58 ± 0.08 | 70.27 ± 0.11 | 70.38 ± 0.31 | 70.38 ± 0.13 |
| RA | 57.90 ± 3.73 | 57.88 ± 0.02 | 57.63 ± 0.37 | 57.72 ± 0.74 | 57.68 ± 0.32 | |
| VA | 0.51 ± 0.018 a | 0.44 ± 0.016 bc | 0.56 ± 0.004 a | 0.55 ± 0.015 a | 0.41 ± 0.020 c | |
| AC | 3.19 ± 0.04 | 3.25 ± 0.03 | 3.13 ± 0.07 | 3.12 ± 0.08 | 3.08 ± 0.02 |
| Main By-Products (mg/L) | ||||||
|---|---|---|---|---|---|---|
| P4 | P4-CR1 | P4-LC2-1 | P4-TSF | P4-CZ3 | ||
| PW | n-Propanol | 8.47 ± 0.37 a | 9.19 ± 0.41 ac | 9.04 ± 0.36 ac | 11.58 ± 0.01 b | 9.80 ± 0.14 c |
| Isobutanol | 86.50 ± 1.63 a | 98.19 ± 4.06 ab | 110.65 ± 12.22 bc | 127.16 ± 3.31 c | 115.59 ± 3.35 c | |
| n-Butanol | 132.61 ± 5.04 a | 147.39 ± 0.18 b | 160.75 ± 5.60 bc | 157.97 ± 0.14 bc | 173.43 ± 4.80 c | |
| Active amyl alcohol | 21.13 ± 2.69 a | 21.82 ± 0.81 a | 21.87 ± 0.61 a | 20.35 ± 2.31 a | 33.09 ± 1.14 b | |
| Isoamyl alcohol | 59.17 ± 1.02 a | 57.82 ± 3.42 ab | 70.07 ± 0.37 ac | 61.90 ± 4.61 a | 63.27 ± 2.65 a | |
| Ethyl acetate | 9.45 ± 0.52 a | 10.42 ± 4.44 a | 15.42 ± 1.72 b | 16.94 ± 0.37 b | 7.55 ± 0.35 a | |
| Acetaldehyde | 52.46 ± 1.91 a | 57.00 ± 2.12 a | 58.81 ± 0.39 a | 45.74 ± 3.46 ab | 38.2 ± 5.44 b | |
| Acetoin | 12.86 ± 2.42 a | 12.82 ± 1.46 a | 15.11 ± 2.28 a | 14.95 ± 1.41 a | 26.31 ± 0.10 b | |
| WW | n-Propanol | 14.43 ± 0.47 a | 12.32 ± 0.67 b | 8.54 ± 0.11 c | 10.08 ± 0.16 c | 9.80 ± 0.55 c |
| Isobutanol | 53.68 ± 2.55 a | 69.73 ± 2.94 b | 78.51 ± 3.83 b | 76.63 ± 1.56 b | 115.79 ± 3.14 c | |
| n-Butanol | 65.87 ± 3.44 a | 112.44 ± 2.19 b | 123.21 ± 4.91 b | 115.55 ± 5.29 b | 173.42 ± 4.64 c | |
| Active amyl alcohol | 20.50 ± 1.07 a | 22.95 ± 1.09 ab | 20.03 ± 1.14 a | 20.04 ± 0.38 a | 28.10 ± 3.30 b | |
| Isoamyl alcohol | 58.25 ± 2.13 | 62.05 ± 2.48 | 62.87 ± 2.66 | 56.54 ± 1.26 | 63.33 ± 3.86 | |
| Ethyl acetate | 6.28 ± 0.08 | 7.87 ± 0.18 | 7.67 ± 0.27 | 7.90 ± 0.18 | 7.69 ± 0.0.82 | |
| Acetaldehyde | 49.41 ± 6.91 a | 60.23 ± 4.20 ab | 52.35 ± 5.50 a | 52.97 ± 3.65 a | 38.29 ± 2.29 ac | |
| Acetoin | 13.22 ± 2.07 a | 13.75 ± 1.17 a | 14.01 ± 0.06 a | 14.28 ± 1.10 a | 33.83 ± 2.79 b | |
| AW | n-Propanol | 13.68 ± 1.14 a | 13.51 ± 0.79 a | 9.71 ± 0.36 ab | 15.01 ± 1.91 ac | 17.34 ± 0.44 ac |
| Isobutanol | 64.85 ± 1.71 a | 89.63 ± 3.49 b | 93.60 ± 1.94 bc | 101.88 ± 1.74 c | 96.84 ± 4.22 bc | |
| n-Butanol | 117.55 ± 9.07 a | 131.63 ± 8.26 ab | 146.78 ± 0.97 b | 140.28 ± 7.70 ab | 121.33 ± 5.51 ab | |
| Active amyl alcohol | 32.90 ± 0.17 a | 23.75 ± 2.06 b | 22.75 ± 1.01 b | 26.06 ± 0.38 ab | 23.76 ± 1.22 b | |
| Isoamyl alcohol | 89.07 ± 3.16 a | 63.95 ± 3.49 b | 64.98 ± 3.72 b | 59.99 ± 0.91 b | 62.06 ± 2.34 b | |
| Ethyl acetate | 8.58 ± 0.95 | 8.89 ± 0.85 | 7.57 ± 0.65 | 8.42 ± 0.49 | 8.88 ± 0.68 | |
| Acetaldehyde | 39.19 ± 1.76 a | 33.41 ± 0.16 b | 35.68 ± 0.46 ab | 51.66 ± 2.23 c | 41.76 ± 0.66 ad | |
| Acetoin | 8.04 ± 1.66 a | 11.61 ± 1.29 ac | 20.53 ± 0.07 bc | 16.10 ± 1.96 c | 10.62 ± 0.77 a | |
| F-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FD | Ethanol | Volatile | n-Propanol | Isobutanol | n-Butanol | Active amyl | Isoamyl | Ethyl | Acetaldehyde | Acetoin | |
| Acidity | Alcohol | Alcohol | Acetate | ||||||||
| S | 4 | 43.30 ** | 180.70 *** | 23.4 ** | 143.7 *** | 77.97 *** | 18.56 ** | 10.68 * | 21.51 ** | 12.83 * | 52.59 ** |
| B | 2 | 93.49 *** | 743.10 *** | 96.95 *** | 189.40 *** | 123.10 *** | 12.99 * | 19.01 ** | 111.40 *** | 36.85 ** | 19.87 ** |
| S × B | 8 | 4.68 * | 108.00 *** | 18.66 ** | 20.08 ** | 28.50 ** | 14.21 * | 16.79 ** | 23.44 ** | 12.61 * | 26.55 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capece, A.; De Fusco, D.; Pietrafesa, R.; Siesto, G.; Romano, P. Performance of Wild Non-Conventional Yeasts in Fermentation of Wort Based on Different Malt Extracts to Select Novel Starters for Low-Alcohol Beers. Appl. Sci. 2021, 11, 801. https://doi.org/10.3390/app11020801
Capece A, De Fusco D, Pietrafesa R, Siesto G, Romano P. Performance of Wild Non-Conventional Yeasts in Fermentation of Wort Based on Different Malt Extracts to Select Novel Starters for Low-Alcohol Beers. Applied Sciences. 2021; 11(2):801. https://doi.org/10.3390/app11020801
Chicago/Turabian StyleCapece, Angela, Deborah De Fusco, Rocchina Pietrafesa, Gabriella Siesto, and Patrizia Romano. 2021. "Performance of Wild Non-Conventional Yeasts in Fermentation of Wort Based on Different Malt Extracts to Select Novel Starters for Low-Alcohol Beers" Applied Sciences 11, no. 2: 801. https://doi.org/10.3390/app11020801
APA StyleCapece, A., De Fusco, D., Pietrafesa, R., Siesto, G., & Romano, P. (2021). Performance of Wild Non-Conventional Yeasts in Fermentation of Wort Based on Different Malt Extracts to Select Novel Starters for Low-Alcohol Beers. Applied Sciences, 11(2), 801. https://doi.org/10.3390/app11020801