Influence of Yeast Strain on Odor-Active Compounds in Fiano Wine
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Wines and Yeast Strains
2.2. Extraction and Analysis of Volatile Compounds
2.3. Data Analysis
3. Results and Discussion
3.1. Impact of In-Barrique Fermentation Using Selected Yeast Strains on Wine Volatile Compound Composition
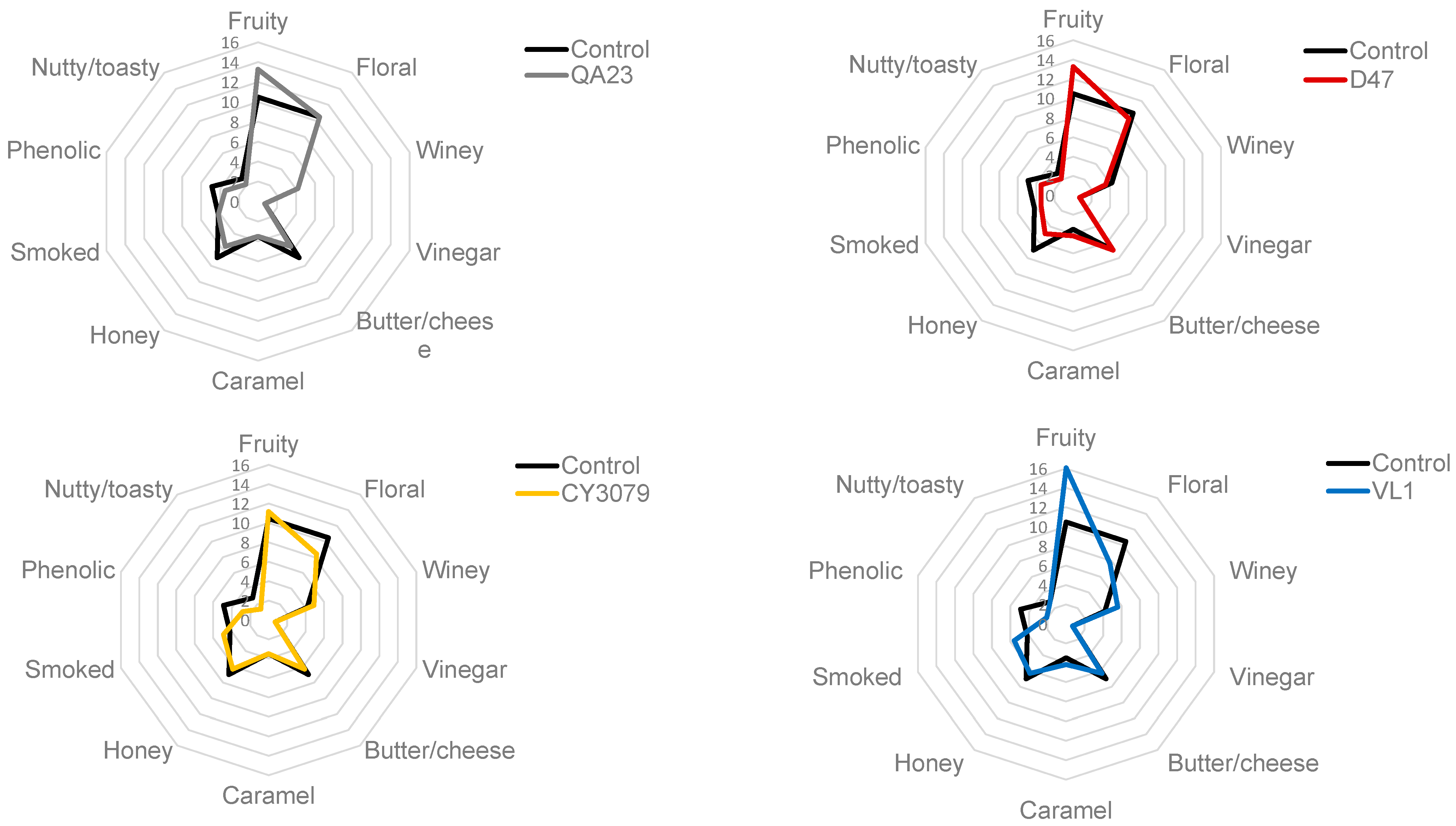
3.2. Gas Chromatography–Olfactometry Analysis
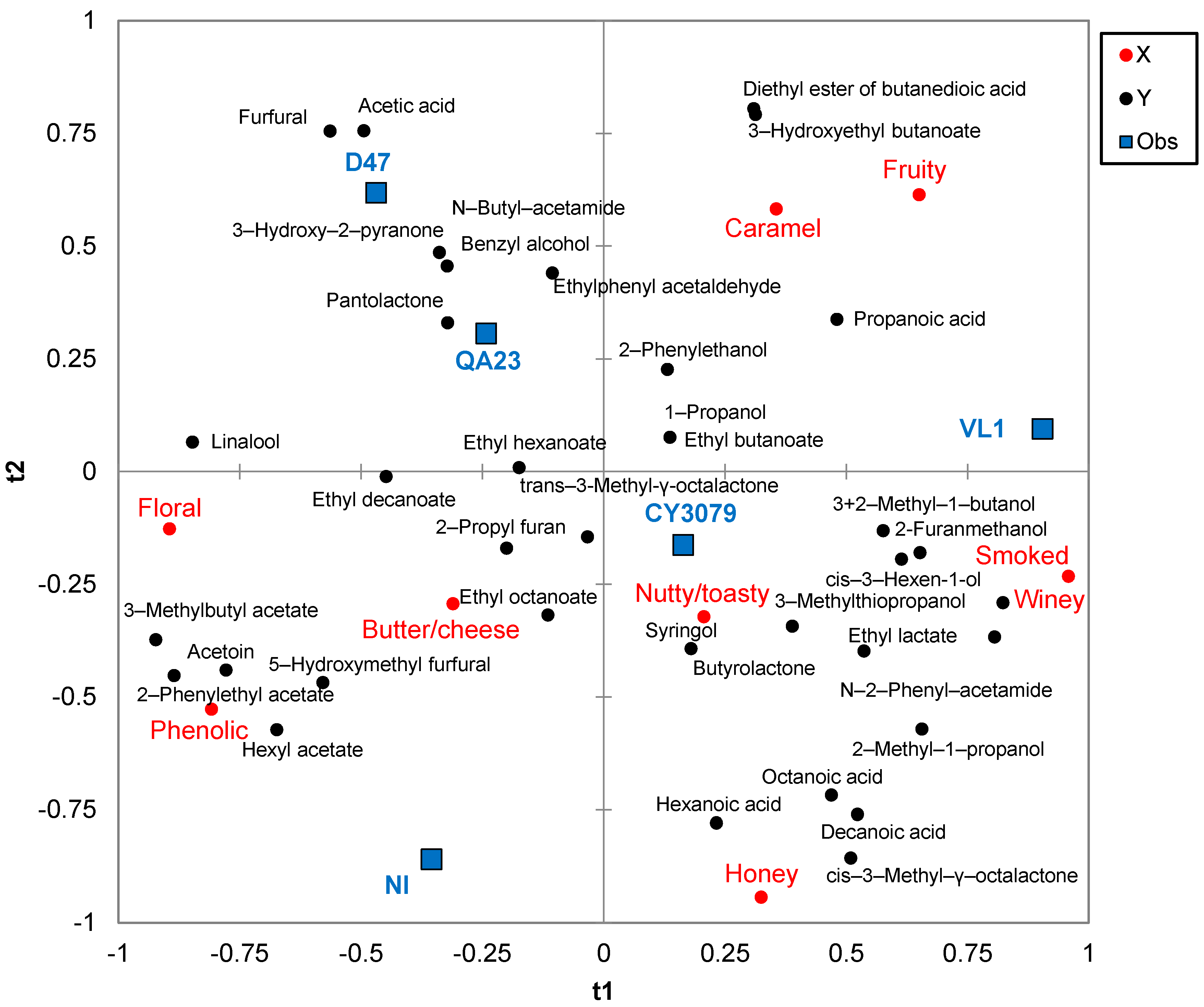
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lambrechts, M.; Pretorius, I. Yeast and its importance to wine aroma-a review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef] [Green Version]
- Romano, P.; Fiore, C.; Paraggio, M.; Caruso, M.; Capece, A. Function of yeast species and strains in wine flavour. Int. J. Food Microbiol. 2003, 86, 169–180. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, J.; Bartowsky, E.; Henschke, P.; Pretorius, I. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Siesto, G.; Romano, P. Diversity of Saccharomyces cerevisiae yeasts associated to spontaneously fermenting grapes from an Italian “heroic vine-growing area”. Food Microbiol. 2012, 31, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Cotea, V.V.; Focea, M.C.; Luchian, C.E.; Colibaba, L.C.; Scutarașu, E.C.; Marius, N.; Zamfir, C.I.; Popîrdă, A. Influence of different commercial yeasts on volatile fraction of sparkling wines. Foods 2021, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Fraile, P.; Garrido, J.; Ancín, C. Influence of a Saccharomyces cerevisiae selected strain in the volatile composition of rose wines. Evolution during fermentation. J. Agric. Food Chem. 2000, 48, 1789–1798. [Google Scholar] [CrossRef]
- Francesca, N.; Chiurazzi, M.; Romano, R.; Aponte, M.; Settanni, L.; Moschetti, G. Indigenous yeast communities in the environment of “Rovello bianco” grape variety and their use in commercial white wine fermentation. World J. Microbiol. Biotechnol. 2010, 26, 337–351. [Google Scholar] [CrossRef] [Green Version]
- Nurgel, C.; Erten, H.; Canbaş, A.; Cabaroğlu, T.; Selli, S. Influence of Saccharomyces cerevisiae strains on fermentation and flavor compounds of white wines made from cv. Emir grown in Central Anatolia, Turkey. J. Ind. Microbiol. Biotechnol. 2002, 29, 28–33. [Google Scholar] [CrossRef]
- Orlic, S.; Redzepovic, S.; Jeromel, A.; Herjavec, S.; Iacumin, L. Influence of indigenous Saccharomyces paradoxus strains on Chardonnay wine fermentation aroma. Int. J. Food Sci. Technol. 2007, 42, 95–101. [Google Scholar] [CrossRef]
- Puertas, B.; Jimenez-Hierro, M.; Cantos-Villar, E.; Marrufo-Curtido, A.; Carbú, M.; Cuevas, F.; Moreno-Rojas, J.; González-Rodríguez, V.; Cantoral, J.; Ruiz-Moreno, M. The influence of yeast on chemical composition and sensory properties of dry white wines. Food Chem. 2018, 253, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Singh, P.N.; Sawant, S. Evaluation of fermentation efficiency of yeast strains and their effect on quality of young wines. Indian J. Microbiol. 2012, 52, 495–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzzi, G.; Arfelli, G.; Schirone, M.; Corsetti, A.; Perpetuini, G.; Tofalo, R. Effect of grape indigenous Saccharomyces cerevisiae strains on Montepulciano d’Abruzzo red wine quality. Food Res. Int. 2012, 46, 22–29. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Kievit, R.L.; Siebert, T.; Lattey, K.A.; Bramley, B.R.; Francis, I.L.; King, E.S.; Pretorius, I.S. The influence of yeast on the aroma of Sauvignon Blanc wine. Food Microbiol. 2009, 26, 204–211. [Google Scholar] [CrossRef]
- Torrens, J.; Urpí, P.; Riu-Aumatell, M.; Vichi, S.; López-Tamames, E.; Buxaderas, S. Different commercial yeast strains affecting the volatile and sensory profile of cava base wine. Int. J. Food Microbiol. 2008, 124, 48–57. [Google Scholar] [CrossRef]
- Mauriello, G.; Capece, A.; D’Auria, M.; Garde-Cerdán, T.; Romano, P. SPME–GC method as a tool to differentiate VOC profiles in Saccharomyces cerevisiae wine yeasts. Food Microbiol. 2009, 26, 246–252. [Google Scholar] [CrossRef]
- Tufariello, M.; Fragasso, M.; Pico, J.; Panighel, A.; Castellarin, S.D.; Flamini, R.; Grieco, F. Influence of non-Saccharomyces on wine chemistry: A focus on aroma-related compounds. Molecules 2021, 26, 644. [Google Scholar] [CrossRef]
- Varela, C. The impact of non-Saccharomyces yeasts in the production of alcoholic beverages. Appl. Microbiol. Biotechnol. 2016, 100, 9861–9874. [Google Scholar] [CrossRef] [PubMed]
- Alañón, M.E.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. New strategies to improve sensorial quality of white wines by wood contact. Beverages 2018, 4, 91. [Google Scholar] [CrossRef] [Green Version]
- Cadahía, E.; Fernández de Simón, B.; Jalocha, J. Volatile compounds in Spanish, French, and American oak woods after natural seasoning and toasting. J. Agric. Food Chem. 2003, 51, 5923–5932. [Google Scholar] [CrossRef]
- Koussissi, E.; Dourtoglou, V.; Ageloussis, G.; Paraskevopoulos, Y.; Dourtoglou, T.; Paterson, A.; Chatzilazarou, A. Influence of toasting of oak chips on red wine maturation from sensory and gas chromatographic headspace analysis. Food Chem. 2009, 114, 1503–1509. [Google Scholar] [CrossRef]
- Gambetta, J.M.; Bastian, S.E.; Cozzolino, D.; Jeffery, D.W. Factors influencing the aroma composition of Chardonnay wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef] [PubMed]
- Liberatore, M.T.; Pati, S.; Del Nobile, M.A.; La Notte, E. Aroma quality improvement of Chardonnay white wine by fermentation and ageing in barrique on lees. Food Res. Int. 2010, 43, 996–1002. [Google Scholar] [CrossRef]
- Obradović, V.; Mesić, J.; Ravančić, M.E.; Mijowska, K.; Svitlica, B. Chemical and sensory properties of Chardonnay wines produced in different oak barrels. Int. Sch. Sci. Res. Innov. 2017, 11, 413–418. [Google Scholar]
- Genovese, A.; Gambuti, A.; Piombino, P.; Moio, L. Sensory properties and aroma compounds of sweet Fiano wine. Food Chem. 2007, 103, 1228–1236. [Google Scholar] [CrossRef]
- Roldán, A.M.; Sánchez-García, F.; Pérez-Rodríguez, L.; Palacios, V.M. Influence of different vinification techniques on volatile compounds and the aromatic profile of Palomino fino wines. Foods 2021, 10, 453. [Google Scholar] [CrossRef]
- Moio, L.; Ugliano, M.; Genovese, A.; Gambuti, A.; Pessina, R.; Piombino, P. Effect of antioxidant protection of must on volatile compounds and aroma shelf life of Falanghina (Vitis vinifera L.) wine. J. Agric. Food Chem. 2004, 52, 891–897. [Google Scholar] [CrossRef]
- Ullrich, F.; Grosch, W. Identification of the most intense volatile flavour compounds formed during autoxidation of linoleic acid. Z. Für Lebensm.-Unters. Und Forsch. 1987, 184, 277–282. [Google Scholar] [CrossRef]
- Ferreira, V.; Fernández, P.; Peña, C.; Escudero, A.; Cacho, J.F. Investigation on the role played by fermentation esters in the aroma of young Spanish wines by multivariate analysis. J. Sci. Food Agric. 1995, 67, 381–392. [Google Scholar] [CrossRef]
- Ugliano, M.; Bartowsky, E.J.; McCarthy, J.; Moio, L.; Henschke, P.A. Hydrolysis and transformation of grape glycosidically bound volatile compounds during fermentation with three Saccharomyces yeast strains. J. Agric. Food Chem. 2006, 54, 6322–6331. [Google Scholar] [CrossRef]
- Ugliano, M.; Moio, L. Free and hydrolytically released volatile compounds of Vitis vinifera L. cv. Fiano grapes as odour-active constituents of Fiano wine. Anal. Chim. Acta 2008, 621, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Mauricio, J.C.; Moreno, J. Aromatic series in sherry wines with gluconic acid subjected to different biological aging conditions by Saccharomyces cerevisiae var. capensis. Food Chem. 2006, 94, 232–239. [Google Scholar] [CrossRef]
- Francis, I.; Newton, J. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Franco, M.; Peinado, R.A.; Medina, M.; Moreno, J. Off-vine grape drying effect on volatile compounds and aromatic series in must from Pedro Ximénez grape variety. J. Agric. Food Chem. 2004, 52, 3905–3910. [Google Scholar] [CrossRef]
- Genovese, A.; Lamorte, S.A.; Gambuti, A.; Moio, L. Aroma of Aglianico and Uva di Troia grapes by aromatic series. Food Res. Int. 2013, 53, 15–23. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Santiago, J.; Martínez, M.; Simal-Gándara, J. Aroma potential of Brancellao grapes from different cluster positions. Food Chem. 2012, 132, 112–124. [Google Scholar] [CrossRef] [Green Version]
| Compound | Control | s.d. | QA23 | s.d. | D47 | s.d. | CY3079 | s.d. | VL1 | s.d. | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols | |||||||||||
| 1-Propanol | 0.352 | 0.027 | 0.396 | 0.002 | 0.388 | 0.041 | 0.519 | 0.053 | 0.374 | 0.010 | *** |
| 2-Methyl-1-propanol | 2.645 | 0.100 | 0.920 | 0.091 | 1.598 | 0.247 | 2.654 | 0.787 | 3.082 | 0.419 | *** |
| 1-Butanol | 0.048 | 0.019 | 0.046 | 0.012 | 0.061 | 0.018 | 0.065 | 0.001 | 0.044 | 0.007 | NS |
| 3 + 2-Methyl-1-butanol | 97.870 | 5.237 | 96.216 | 5.426 | 89.024 | 4.292 | 84.125 | 5.701 | 108.606 | 6.566 | * |
| 1-Hexanol | 2.918 | 0.287 | 3.050 | 0.068 | 2.718 | 0.602 | 2.769 | 0.171 | 2.993 | 0.212 | NS |
| cis-3-Hexen-1-ol | 0.030 | 0.001 | 0.033 | 0.002 | 0.023 | 0.003 | 0.026 | 0.003 | 0.035 | 0.008 | * |
| Linalool | 0.030 | 0.001 | 0.032 | 0.002 | 0.031 | 0.001 | 0.031 | 0.003 | 0.023 | 0.006 | * |
| α-Terpineol | 0.081 | 0.005 | 0.083 | 0.004 | 0.078 | 0.015 | 0.084 | 0.005 | 0.078 | 0.008 | NS |
| Benzyl alcohol | 0.191 | 0.005 | 0.198 | 0.011 | 0.412 | 0.117 | 0.365 | 0.098 | 0.190 | 0.003 | ** |
| 2-Phenylethanol | 50.854 | 1.034 | 69.411 | 4.210 | 47.705 | 7.100 | 50.375 | 4.137 | 57.107 | 4.286 | ** |
| Esters | |||||||||||
| Ethyl butanoate | 0.352 | 0.027 | 0.396 | 0.002 | 0.388 | 0.041 | 0.519 | 0.053 | 0.374 | 0.010 | *** |
| 3-Methylbutyl acetate | 0.390 | 0.017 | 0.345 | 0.008 | 0.348 | 0.074 | 0.330 | 0.022 | 0.246 | 0.012 | ** |
| Ethyl hexanoate | 0.979 | 0.025 | 1.340 | 0.059 | 0.784 | 0.130 | 0.847 | 0.014 | 0.904 | 0.038 | *** |
| Hexyl acetate | 0.132 | 0.015 | 0.022 | 0.002 | 0.084 | 0.010 | 0.076 | 0.002 | *** | ||
| Ethyl lactate | 37.655 | 0.635 | 15.459 | 6.822 | 32.481 | 3.086 | 46.027 | 2.075 | 43.613 | 2.232 | *** |
| Ethyl octanoate | 2.054 | 0.090 | 2.405 | 0.094 | 1.408 | 0.299 | 1.730 | 0.084 | 1.764 | 0.113 | *** |
| 3-Hydroxyethyl butanoate | 0.043 | 0.012 | 0.060 | 0.013 | 0.095 | 0.014 | 0.075 | 0.001 | 0.085 | 0.006 | *** |
| Ethyl decanoate | 0.679 | 0.036 | 0.906 | 0.012 | 0.550 | 0.122 | 0.544 | 0.052 | 0.518 | 0.031 | *** |
| Diethyl ester of butanedioic acid | 5.292 | 0.111 | 6.386 | 0.470 | 7.589 | 1.302 | 7.162 | 0.088 | 7.131 | 0.570 | * |
| 2-Phenylethyl acetate | 0.103 | 0.004 | 0.088 | 0.001 | 0.092 | 0.017 | 0.088 | 0.007 | 0.069 | 0.012 | *** |
| Aldehydes and ketones | |||||||||||
| Acetoin | 0.791 | 0.070 | 0.386 | 0.054 | 0.616 | 0.053 | 0.661 | 0.099 | 0.066 | 0.013 | *** |
| Furfural | 0.176 | 0.005 | 2.866 | 0.118 | 2.648 | 0.418 | 0.299 | 0.010 | 0.225 | 0.012 | *** |
| Ethylphenyl acetaldehyde | 0.021 | 0.007 | 0.066 | 0.005 | 0.028 | 0.007 | 0.033 | 0.005 | 0.030 | 0.009 | *** |
| β-Damascenone | 0.012 | 0.001 | 0.006 | 0.002 | 0.011 | 0.000 | 0.012 | 0.000 | NS | ||
| 5-Hydroxymethyl furfural | 0.358 | 0.043 | 0.129 | 0.015 | 0.293 | 0.083 | 0.351 | 0.083 | 0.077 | 0.028 | *** |
| Lactones | |||||||||||
| Butyrolactone | 5.085 | 0.131 | 4.220 | 0.659 | 4.262 | 1.068 | 8.200 | 0.428 | 5.428 | 0.127 | *** |
| trans-3-Methyl-γ-octalactone | 0.053 | 0.002 | 0.011 | 0.001 | 0.067 | 0.020 | 0.068 | 0.004 | 0.044 | 0.004 | *** |
| cis-3-Methyl-γ-octalactone | 0.151 | 0.007 | 0.114 | 0.012 | 0.091 | 0.022 | 0.136 | 0.009 | 0.143 | 0.015 | ** |
| 3-Hydroxy-2-pyranone | 0.089 | 0.009 | 0.618 | 0.017 | 0.225 | 0.077 | 0.156 | 0.041 | 0.107 | 0.016 | *** |
| Pantolactone | 0.012 | 0.001 | 0.021 | 0.008 | 0.013 | 0.001 | 0.011 | 0.001 | ** | ||
| Volatile phenols | |||||||||||
| 4-Vinylguaiacol | 0.248 | 0.038 | 0.466 | 0.200 | 0.278 | 0.074 | 0.190 | 0.018 | 0.336 | 0.038 | NS |
| Syringol | 0.013 | 0.002 | 0.015 | 0.002 | 0.037 | 0.004 | 0.009 | 0.001 | *** | ||
| Vanillin | 0.018 | 0.001 | 0.047 | 0.001 | 0.016 | 0.004 | NS | ||||
| Acids | |||||||||||
| Acetic acid | 0.062 | 0.006 | 0.260 | 0.060 | 0.220 | 0.007 | 0.069 | 0.004 | 0.081 | 0.027 | * |
| Propanoic acid | 0.017 | 0.004 | 0.015 | 0.001 | *** | ||||||
| Hexanoic acid | 11.369 | 0.354 | 7.591 | 2.685 | 8.261 | 0.427 | 8.405 | 0.523 | 10.172 | 0.743 | * |
| 2-Hexenoic acid | 0.150 | 0.012 | 0.111 | 0.012 | 0.198 | 0.071 | 0.133 | 0.012 | 0.148 | 0.024 | NS |
| Octanoic acid | 38.185 | 1.990 | 32.460 | 3.183 | 20.110 | 7.852 | 29.693 | 1.417 | 37.226 | 2.702 | ** |
| Decanoic acid | 9.464 | 0.570 | 6.535 | 0.705 | 4.811 | 0.723 | 6.805 | 0.505 | 9.300 | 0.732 | *** |
| Furans | |||||||||||
| 2-Propyl furan | 1.247 | 0.228 | 0.425 | 0.005 | 1.562 | 0.364 | 2.222 | 0.572 | 0.656 | 0.055 | *** |
| 2-Furanmethanol | 0.392 | 0.032 | 0.422 | 0.033 | 0.604 | 0.094 | 0.695 | 0.084 | *** | ||
| Others | |||||||||||
| 3-Methylthiopropanol | 0.180 | 0.003 | 0.192 | 0.032 | 0.140 | 0.010 | 0.227 | 0.025 | 0.229 | 0.032 | ** |
| N-2-Phenyl-acetamide | 0.128 | 0.012 | 0.075 | 0.012 | 0.061 | 0.014 | 0.236 | 0.010 | 0.221 | 0.031 | *** |
| N-butyl-acetamide | 0.191 | 0.005 | 0.198 | 0.011 | 0.412 | 0.117 | 0.365 | 0.098 | 0.190 | 0.003 | ** |
| Nr. | Compounds | Odor Descriptor | AEDA Value | Aromatic Series | ||||
|---|---|---|---|---|---|---|---|---|
| Control | QA23 | D47 | CY3079 | VL1 | ||||
| 1 | N.I. | Fruity, sweety | 1 | 1 | 0 | 1 | 25 | Fruity |
| 2 | Ethyl acetate | Fruity, apple | 5 | 25 | 125 | 625 | 625 | Fruity |
| 3 | Diacetyl | Butter | 125 | 5 | 5 | 5 | 5 | Butter/cheese |
| 4 | 1-Propanol | Fruity, sweety | 5 | 125 | 25 | 25 | 25 | Fruity |
| 5 | Ethyl butanoate | Fruity, apple | 25 | 5 | 125 | 0 | 125 | Fruity |
| 6 | 3-Methylbutyl acetate | Banana | 25 | 25 | 5 | 5 | 5 | Fruity |
| 7 | 1-Butanol | Winey, grass | 0 | 25 | 5 | 0 | 0 | Winey |
| 8 | 2+3-Methyl-1-butanol | Winey, grass | 125 | 625 | 125 | 125 | 625 | Winey |
| 9 | Ethyl hexanoate | Apple | 25 | 125 | 5 | 25 | 25 | Fruity |
| 10 | 2-Propyl furan | Winey, pungent | 125 | 25 | 25 | 625 | 625 | Winey |
| 11 | N.I. | Toasted nutty | 625 | 125 | 125 | 25 | 125 | Nutty/toasty |
| 12 | Ethyl octanoate | Ananas | 125 | 625 | 625 | 625 | 625 | Fruity |
| 13 | Acetic acid | Vinegar | 5 | 5 | 5 | 5 | 5 | Vinegar |
| 14 | Linalool | Orange flowers | 5 | 5 | 25 | 5 | 5 | Floral |
| 15 | 2-Methylpropanoic acid | Cheese | 1 | 1 | 1 | 5 | 5 | Butter/cheese |
| 16 | Butanoic acid | Cheese | 25 | 25 | 25 | 5 | 25 | Butter/cheese |
| 17 | Acetophenone | Acacia honey | 125 | 0 | 0 | 125 | 125 | Honey |
| 18 | 3-Methylbutanoic acid | Cheese | 125 | 125 | 625 | 625 | 125 | Butter/cheese |
| 19 | 3-Methylthio-1-propanol | Potato, garlic | 25 | 1 | 5 | 5 | 5 | - |
| 20 | N.I. | Toasty | 1 | 0 | 0 | 1 | 5 | Nutty/toasty |
| 21 | 2-Phenylethyl acetate | Floral | 5 | 5 | 0 | 0 | 0 | Floral |
| 22 | β-Damascenone | Honey, tea | 625 | 625 | 625 | 625 | 625 | Honey |
| 23 | Hexanoic acid | Cheese | 25 | 5 | 25 | 5 | 25 | Butter/cheese |
| 24 | N.I. | Smoked | 625 | 625 | 125 | 125 | 125 | Smoked/phenolic |
| 25 | trans-3-Methyl-γ-octalactone | Coconut | 5 | 5 | 25 | 25 | 25 | Fruity |
| 26 | 2-Phenylethanol | Floral, rosa | 625 | 625 | 625 | 625 | 625 | Floral |
| 27 | N.I. | Floral, rosa | 0 | 5 | 1 | 5 | 5 | Floral |
| 28 | Pantolactone | Floral | 5 | 5 | 5 | 5 | 5 | Floral |
| 29 | Hydroxy diethyl butanoate | Caramel | 25 | 25 | 125 | 25 | 125 | Caramel |
| 30 | Octanoic acid | Cheese | 1 | 5 | 5 | 5 | 0 | Butter/cheese |
| 31 | N.I. | Medicinal/phenolic | 25 | 0 | 0 | 1 | 1 | Smoked/phenolic |
| 32 | N.I. | Smoked | 0 | 5 | 0 | 0 | 5 | Smoked/phenolic |
| 33 | N.I. | Caramel | 125 | 125 | 125 | 125 | 125 | Caramel |
| 34 | N.I. | Apricot | 125 | 125 | 125 | 5 | 125 | Fruity |
| 35 | N.I. | Medicinal/phenolic | 25 | 25 | 25 | 25 | 25 | Smoked/phenolic |
| 36 | N.I. | Smoked | 5 | 1 | 1 | 5 | 1 | Smoked/phenolic |
| 37 | 4-Vinylguaiacol | Smoked | 625 | 625 | 625 | 125 | 625 | Smoked/phenolic |
| 38 | Syringol | Smoked | 25 | 125 | 25 | 25 | 5 | Smoked/phenolic |
| 39 | N.I. | Smoked | 25 | 5 | 1 | 25 | 25 | Smoked/phenolic |
| 40 | N.I. | Medicinal/phenolic | 125 | 125 | 125 | 25 | 5 | Smoked/phenolic |
| 41 | N.I. | Floral | 625 | 125 | 125 | 125 | 25 | Floral |
| 42 | Phenylacetic acid | Honey | 125 | 625 | 125 | 25 | 25 | Honey |
| 43 | N.I. | Floral | 625 | 625 | 625 | 25 | 25 | Floral |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genovese, A.; Caporaso, N.; Moio, L. Influence of Yeast Strain on Odor-Active Compounds in Fiano Wine. Appl. Sci. 2021, 11, 7767. https://doi.org/10.3390/app11177767
Genovese A, Caporaso N, Moio L. Influence of Yeast Strain on Odor-Active Compounds in Fiano Wine. Applied Sciences. 2021; 11(17):7767. https://doi.org/10.3390/app11177767
Chicago/Turabian StyleGenovese, Alessandro, Nicola Caporaso, and Luigi Moio. 2021. "Influence of Yeast Strain on Odor-Active Compounds in Fiano Wine" Applied Sciences 11, no. 17: 7767. https://doi.org/10.3390/app11177767
APA StyleGenovese, A., Caporaso, N., & Moio, L. (2021). Influence of Yeast Strain on Odor-Active Compounds in Fiano Wine. Applied Sciences, 11(17), 7767. https://doi.org/10.3390/app11177767








