Influence of Autochthonous and Commercial Yeast Strains on Fermentation and Quality of Wines Produced from Vranec and Cabernet Sauvignon Grape Varieties from Tikveš Wine-Growing Region, Republic of North Macedonia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grapes from Vranec and Cabernet Sauvignon Variety for Winemaking and Chemicals
2.2. Isolation, Selection, and Identification of Autochthonous Yeast Strains F-8 and F-78 from the Tikveš Wine-Growing Region
2.3. Inoculation of Autochthonous and Commercial Yeast Strains and Monitoring of the Alcoholic Fermentation toward the Production of Vranec and Cabernet Sauvignon Wines
2.4. Determination of Oenological Parameters in Trial Wines
2.5. Statistical Analysis
3. Results
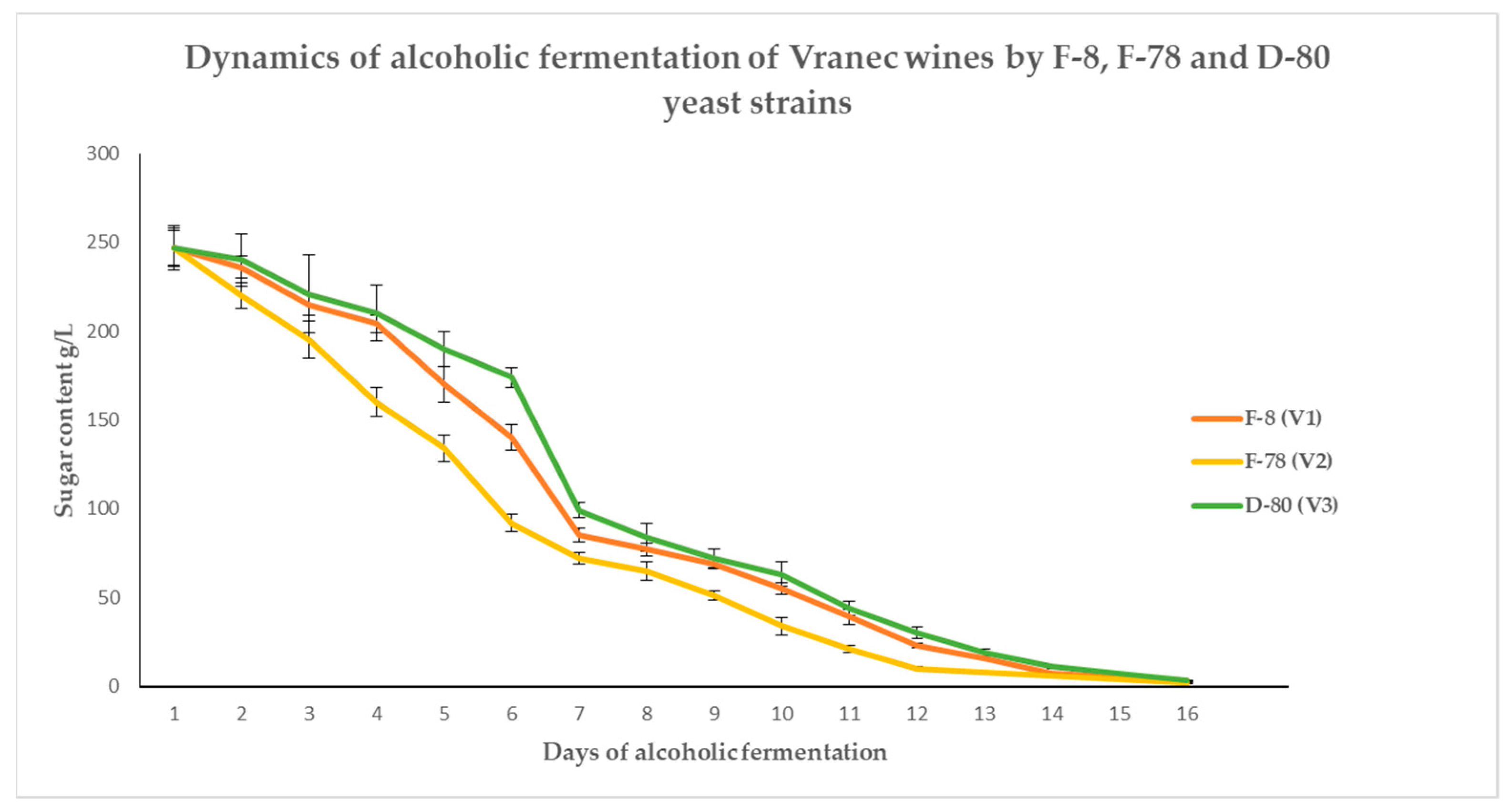
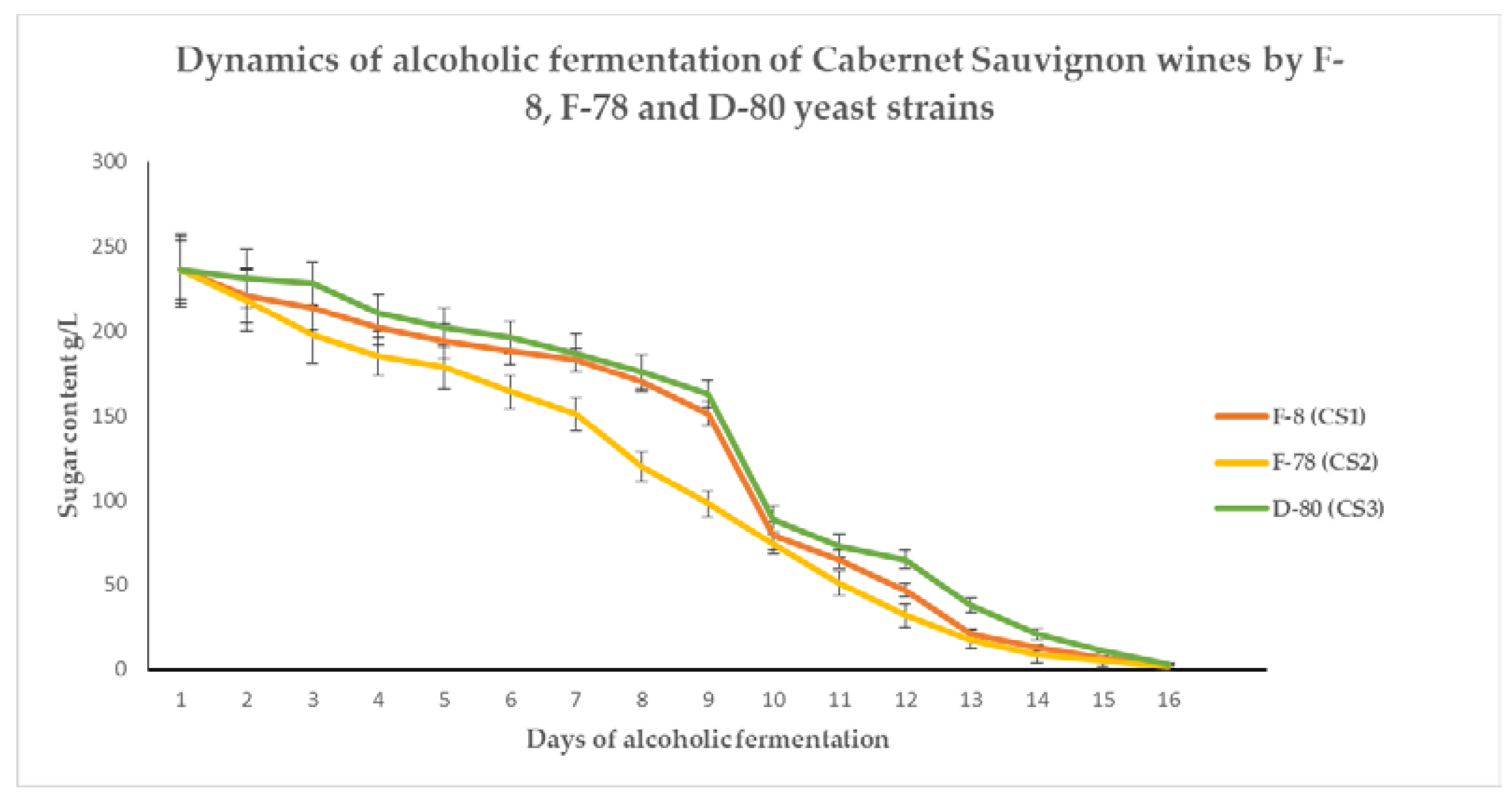
3.1. Dynamics of the Alcoholic Fermentation throughout Time to Produce Wines from Vranec and Cabernet Sauvignon Grape Varieties
3.2. Oenochemical Parameters of Vranec and Cabernet Sauvignon Wines Produced by Autochthonous and Commercial Yeast Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cadière, A.; Aguera, E.; Caillé, S.; Ortiz-Julien, A.; Dequin, S. Pilot-scale evaluation the enological traits of a novel, aromatic wine yeast strain obtained by adaptive evolution. Food Microbiol. 2012, 32, 332–337. [Google Scholar] [CrossRef]
- Berbegal de Gracia, C.; Garofalo, C.; Russo, P.; Pati, S.; Capozzi, V.; Spano, G. Use of Autochthonous yeasts and bacteria in order to control Brettanomyces bruxellensis in wine. Fermentation 2017, 3, 1–11. [Google Scholar]
- Zabukovec, P.; Čadež, N.; Čuš, F. Isolation and Identification of indigenous wine yeasts and their use in alcoholic fermentation. Food Technol. Biotechnol. 2020, 58, 337–347. [Google Scholar] [CrossRef]
- Raymond Eder, M.L.; Conti, F.; Rosa, A.L. Differences between indigenous yeast populations in spontaneously fermenting musts from V. vinifera L. and V. labrusca L. grapes harvested in the same geographic location. Front. Microbiol. 2018, 9, 1320. [Google Scholar] [CrossRef]
- Brossaud, F.; Cheynier, V.; Noble, A. Bitterness and astringency of grape and wine polyphenols. Aust. J. Grape Wine Res. 2001, 7, 33–39. [Google Scholar] [CrossRef]
- Nel, A.P.; Van Rensburg, P.; Lambrechts, M.G. The influence of different winemaking techniques on the extraction of grape tannins and anthocyanins. S. Afr. J. Enol. Vitic. 2014, 35, 304–320. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Norton, E.L. Chemistry and reactivity of tannins in Vitis spp.: A review. Molecules 2020, 25, 2110. [Google Scholar] [CrossRef]
- Yacco, R.S.; Watrelot, A.A.; Kennedy, J.A. Red wine tannin structure–Activity relationships during fermentation and maceration. J. Agric. Food Chem. 2016, 64, 860–869. [Google Scholar] [CrossRef]
- García-Estévez, I.; Cristina Alcalde-Eon, C.; Víctor Puente, V.; Escribano-Bailón, T.M. Enological tannin effect on red wine color and pigment composition and relevance of the yeast fermentation products. Molecules 2017, 22, 2046. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.A.; McRae, J.M.; Bindon, K.A. Impact of winemaking practices on the concentration and composition of tannins in red wine. Aust. J. Grape Wine Res. 2015, 21, 601–614. [Google Scholar] [CrossRef]
- Pereira de Freitas, V.A.; Fernandes, A.; Oliveira, J.; Teixeira, N.; Mateus, N. A review of the current knowledge of red wine colour. OENO One 2017, 51. [Google Scholar] [CrossRef]
- De Freitas, V.; Mateus, N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2010, 401, 1463–1473. [Google Scholar] [CrossRef]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and their variation in red wines I. monomeric anthocyanins and their color expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef] [Green Version]
- Kostadinović Veličkovska, S.; Mirhosseini, H.; Bogeva, E. Isolation of anthocyanins by high-speed countercurrent chromatography and application of the color activity concept to different varieties of red grape pomace from Macedonia. J. Nutr. Food. Sci. 2013, 3, 243–250. [Google Scholar]
- OIV-AS2-02-SUCREF Compendium of International Methods of Analysis of Wines and Musts; International Organization of Vine and Wine: Paris, France, 2007; Volume 2.
- OIV-AS-313-01-ACITOT Compendium of International Methods of Analysis of Wines and Musts; International Organization of Vine and Wine: Paris, France, 2009; Volume 2.
- Ilieva, F.; Kostadinović Veličkovska, S.; Dimovska, V.; Spasov, H. The impact of some wine-making practices on the quality of Vranec red wines from Macedonia produced by the newly-selected local strain “F-78”. Food Chem. 2016, 194, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, F.; Kostadinović Veličkovska, S.; Dimovska, V.; Mirhosseini, H.; Spasov, H. Selection of 80 newly isolated autochthonous yeast strains from the Tikveš region of Macedonia and their impact on the quality of red wines produced from Vranec and Cabernet Sauvignon grape varieties. Food Chem. 2017, 216, 309–315. [Google Scholar] [CrossRef]
- Ilieva, F.; Kostadinović Veličkovska, S.; Dimovska, V.; Mirhosseini, H.; Spasov, H. Isolation of Saccharomyces cerevisiae yeast strains from Macedonian “Tikveš” wine-growing region and their impact on the organoleptic characteristics of Vranec and Cabernet Sauvignon wines. Res. J. Biotechnol. 2019, 14, 100–110. [Google Scholar]
- Bambalov, G.; Spasov, C.; Bambalov, K. Isuliorung und selection von Weinhefen aus dem Westtilchen teil der Thrazischeen tiefeben. Wein Wiss. 2000, 55, 55–57. [Google Scholar]
- Zoecklein, B.W.; Gugelsang, K.C.; Gump, B.H.; Nury, F.S. Wine Analysis and Production; Chapman and Hall: New York, NY, USA, 2000. [Google Scholar]
- ISI 28-1e Determination of Reducing Sugar, DE by Luff-Schoorl’s Method. Available online: http://www.starch.dk/isi/methods/28luff.htm (accessed on 1 January 2018).
- AOAC. Official Method 2005.02 Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Winesand. J. AOAC Int. 2005, 88, 1269. [Google Scholar]
- AOAC SMPR 2015.009: Estimation of Total Phenolic Content Using the Folin-C Assay; AOAC International: Rockville, MD, USA, 2015.
- OIV-MA-AS313-01 Compendium of International Methods of Analysis of Wines and Musts. R; International Organization of Vine and Wine: Paris, France, 2015; Volume 2.
- Barrajón, N.; Arévalo-Villena, M.; Úbeda, J.; Briones, A. Enological properties in wild and commercial Saccharomyces cerevisiae yeasts: Relationship with competition during alcoholic fermentation. World J. Microbiol. Biotechnol. 2011, 27, 2703–2710. [Google Scholar] [CrossRef]
- García-Ríos, E.; Gutiérrez, A.; Salvadó, Z.; Arroyo-López, F.N.; Guillamon, M. The fitness advantage of commercial wine yeasts in relation to the nitrogen concentration, temperature, and ethanol content under microvinification conditions. Appl. Environ. Microbiol. 2014, 80, 704–713. [Google Scholar] [CrossRef] [Green Version]
- Vigentini, I.; Fabrizio, V.; Faccincani, M.; Picozzi, C.; Comasio, A.; Foschino, R. Dynamics of Saccharomyces cerevisiae populations in controlled and spontaneous fermentations for Franciacorta D.O.C.G. base wine production. Ann. Microbiol. 2014, 64, 639–651. [Google Scholar] [CrossRef]
- Gao, C.; Fleet, G.H. The effects of temperature and pH on the ethanol tolerance of the wine yeasts, Saccharomyces cerevisiae, Candida stellata and Kloeckera apiculata. J. Appl. Bacteriol. 1988, 65, 405–410. [Google Scholar] [CrossRef]
- Torija, M.J.; Rozès, N.; Poblet, M.; Guillamón, J.M.; Mas, A. Effects of fermentation temperature on the strain population of Saccharomyces cerevisiae. Int. J. Food Microbiol. 2003, 80, 47–53. [Google Scholar] [CrossRef]
- Ciani, M.; Capece, A.; Comitini, F.; Canonico, L.; Siesto, G.; Romano, P. Yeast interactions in inoculated wine fermentation. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Caridi, A.; Cufari, A.; Lovino, R.; Palumbo, R.; Tedesco, I. Influence of yeast on polyphenol composition of wine. Food Technol. Biotechnol. 2004, 42, 37–40. [Google Scholar]
- Tofalo, R.; Patrignani, F.; Lanciotti, R.; Perpetuini, G.; Schirone, M.; Di Gianvito, P.; Pizzoni, D.; Arfelli, G.; Suzzi, G. Aroma profile of Montepulciano d’abruzzo wine fermented by single and co-culture starters of autochthonous Saccharomyces and non-Saccharomyces yeasts. Front. Microbiol. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Petruzzi, L.; Capozzi, V.; Berbegal, C.; Corbo, M.R.; Bevilacqua, A.; Spano, G.; Sinigaglia, M. Microbial resources and enological significance: Opportunities and benefits. Front. Microbiol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Perpetuini, G.; Tittarelli, F.; Battistelli, N.; Suzzi, G.; Tofalo, R. Contribution of Pichiamanshurica strains to aroma profile of organic wines. Eur. Food Res. Technol. 2020, 246, 1405–1417. [Google Scholar] [CrossRef]
- Lallemand Oenology Inc., Vienna. Available online: https://www.lallemandwine.com/en/china/ (accessed on 25 April 2019).
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Cell wall anthocyanin adsorption by different Saccharomyces strains during the fermentation of Vitis vinifera L. cv Graciano grapes. Eur. Food Res. Technol. 2005, 220, 341–346. [Google Scholar] [CrossRef]
- Stockley, C.S.; Høj, P.B. Better wine for better health: Factor fiction? Aust. J. Grape Wine Res. 2005, 11, 127–138. [Google Scholar] [CrossRef]
- Caridi, A.; Sidari, R.; Giuffrè, A.M.; Pellicanò, T.M.; Sicari, V.; Zappia, C.; Poiana, M. Test of four generations of Saccharomyces cerevisiae concerning their effect on antioxidant phenolic compounds in wine. Eur. Food Res. Technol. 2017, 243, 1287–1294. [Google Scholar] [CrossRef]
- Mazauric, J.P.; Salmon, J.M. Interactions between yeast lees and wine polyphenols during simulation of wine aging: I. Analysis of remnant polyphenolic compounds in the resulting wines. J. Agric. Food Chem. 2005, 53, 5647–5653. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Dellacassa, E.; Carrau, F. Yeast interactions with anthocyanins during red wine fermentation. Am. J. Enol. Vitic. 2005, 56, 104–109. [Google Scholar]
- Kostadinović, S.; Wilkens, A.; Stefova, M.; Ivanova, V.; Vojnoski, B.; Mirhosseini, H.; Winterhalter, P. Stilbene levels and antioxidant activity of Vranec and Merlot wines from Macedonia: Effect of variety and enological practices. Food Chem. 2012, 135, 3003–3009. [Google Scholar] [CrossRef] [PubMed]
- Giudici, P.; Solieri, L.; Pulvirenti, A.M.; Cassanelli, S. Strategies and perspectives for genetic improvement of wine yeasts. Appl. Microbiol. Biotechnol. 2005, 66, 622–628. [Google Scholar] [CrossRef]
- De Vero, L.; Bonciani, T.; Verspohl, A.; Mezzetti, F.; Giudici, P. High-glutathione producing yeasts obtained by genetic improvement strategies: A focus on adaptive evolution approaches for novel wine strains. AIMS Microbiol. 2017, 3, 155–170. [Google Scholar] [CrossRef] [Green Version]
- Mezzetti, F.; Fay, J.C.; Giudici, P.; De Vero, L. Genetic variation and expression changes associated with molybdate resistance from a glutathione producing wine strain of Saccharomyces cerevisiae. PLoS ONE 2017, 12, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulvirenti, A.; De Vero, L.; Blaiotta, G.; Sidari, R.; Iosca, G.; Gullo, M.; Caridi, A. Selection of wine Saccharomyces cerevisiae strains and their screening for the adsorption activity of pigments, phenolics and ochratoxin A. Fermentation 2020, 6, 80. [Google Scholar] [CrossRef]
- Bisson, L.F. Geographic origin and diversity of wine strains of Saccharomyces. Am. J. Enol. Vitic. 2012, 63, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Combina, M.; Elía, A.; Mercado, L.; Catania, C.; Ganga, A.; Martinez, C. Dynamics of indigenous yeast populations during spontaneous fermentation of wines from Mendoza, Argentina. Int. J. Food Microbiol. 2005, 99, 237–243. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Siesto, G.; Romano, P. Diversity of Saccharomyces cerevisiae yeasts associated to spontaneously fermenting grapes from an Italian “heroic vine-growing area”. Food Microbiol. 2012, 31, 159–166. [Google Scholar] [CrossRef]
- Versavaud, A.; Courcoux, P.; Roulland, C.; Dulau, L.; Hallet, J.N. Genetic diversity and geographical distribution of wild Saccharomyces cerevisiae strains from the wine-producing area of Charentes, France. Appl. Environ. Microbiol. 1995, 61, 3521–3529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganucci, D.; Guerrini, S.; Mangani, S.; Vincenzini, M.; Granchi, L. Quantifying the effects of ethanol and temperature on the fitness advantage of predominant Saccharomyces cerevisiae strains occurring in spontaneous wine fermentations. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Vezinhet, F.; Hallet, J.N.; Valade, M.; Poulard, A. Ecological survey of wine strains by molecular methods of identification. Am. J. Enol. Vitic. 1992, 43, 83–86. [Google Scholar]
- Sabate, J.; Cano, J.; Querol, A.; Guillamon, J.M. Diversity of Saccharomyces strains in wine fermentations: Analysis for two consecutive years. Lett. Appl. Microbiol. 1998, 26, 452–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granchi, L.; Ganucci, D.; Viti, C.; Giovannetti, L.; Vincenzini, M. Saccharomyces cerevisiae biodiversity in spontaneous commercial fermentations of grape musts with adequate and inadequate assimilable-nitrogen content. Lett. Appl. Microbiol. 2003, 36, 54–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granchi, L.; Ganucci, D.; Buscioni, G.; Mangani, S.; Guerrini, S. The Biodiversity of Saccharomyces cerevisiae in spontaneous wine fermentation: The occurrence and persistence of winery-strains. Fermentation 2019, 5, 86. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Caballero, V.; Ayala, F.; Echávarri, J.R.; Negueruela, A.I. Proposal for a new standard OIV method for determination of chromatic characteristics of wine. Am. J. Enol. Vitic. 2003, 54, 59–62. [Google Scholar]

| Wine Designation | Grape Variety | Yeast Strain | Oenological Parameters |
|---|---|---|---|
| V1 | Vranec | F-8 | Alcohol (ethanol) Total reducing sugars * Titratable acidity (TA) * Volatile acidity (VA) Color intensity (IC) Total monomeric anthocyanins Total phenolic compounds |
| V2 | Vranec | F-78 | |
| V3 | Vranec | D-80 | |
| CS1 | Cabernet Sauvignon | F-8 | |
| CS2 | Cabernet Sauvignon | F-78 | |
| CS3 | Cabernet Sauvignon | D-80 |
| Grape Variety | Vintage Year | Sugar Content (g/L) | Titratable Acidity (TA) (g/L) | pH |
|---|---|---|---|---|
| Vranec | 2016 | 245 | 234 | 3.51 |
| Vranec | 2016 | 249 | 238 | 3.54 |
| Cabernet Sauvignon | 2017 | 246 | 235 | 3.41 |
| Cabernet Sauvignon | 2017 | 248 | 237 | 3.40 |
| Days of Fermentation | Type of Yeast | Content of Sugar in Must from Vranec Grapes (g/L) | Days of Fermentation | Type of Yeast | Content of Sugar in Must from Cabernet Sauvignon Grapes (g/L) |
|---|---|---|---|---|---|
| 3 | F-8 | 215.0 ± 9.2 b | 3 | F-8 | 214.1 ± 12.9 b |
| F-78 | 195.5 ± 8.9 a | F-78 | 198.6 ± 10.8 a | ||
| D-80 | 221.3 ± 11.0 b | D-80 | 228.4 ± 13.6 b | ||
| 4 | F-8 | 204.2 ± 10.8 b | 4 | F-8 | 202.5 ± 11.8 b |
| F-78 | 160.4 ± 12.2 a | F-78 | 185.8 ± 14.1 a | ||
| D-80 | 210.9 ± 9.7 b | D-80 | 211.9 ± 12.8 b | ||
| 5 | F-8 | 170.5 ± 8.3 b | 5 | F-8 | 194.4 ± 10.0 b |
| F-78 | 134.8 ± 10.1 a | F-78 | 179.0 ± 12.8 a | ||
| D-80 | 190.1 ± 9.9 c | D-80 | 202.1 ± 13.1 b | ||
| 6 | F-8 | 140.7 ± 10.8 b | 6 | F-8 | 188.5 ± 12.6 b |
| F-78 | 92.8 ± 9.2 a | F-78 | 164.7 ± 13.1 a | ||
| D-80 | 174.3 ± 12.4 c | D-80 | 196.5 ± 13.0 b,c | ||
| 7 | F-8 | 85.5 ± 9.5 b | 7 | F-8 | 183.3 ± 12.5 b |
| F-78 | 72.6 ± 10.4 a | F-78 | 151.8 ± 12.4 a | ||
| D-80 | 99.9 ± 7.1 c | D-80 | 187.6 ± 9.8 b | ||
| 8 | F-8 | 170.3 ± 13.0 b | |||
| F-78 | 120.1 ± 9.6 a | ||||
| D-80 | 176.9 ± 12.1 b | ||||
| 9 | F-8 | 151.4 ± 13.4 b | |||
| F-78 | 98.5 ± 7.5 a | ||||
| D-80 | 163.8 ± 11.5 b,c | ||||
| ANOVA factors and interactions | |||||
| p-values | |||||
| Days of fermentation | 0.00 | 0.001 | |||
| Type of yeast | 0.01 | 0.02 | |||
| Grape Variety | 0.12 | 0.49 | |||
| Days of fermentation x type of yeast | 0.01 | 0.03 | |||
| Days of fermentation x grape variety | 0.14 | 0.09 | |||
| Type of yeast x grape variety | 0.32 | 0.58 | |||
| Wine Designation | Alcohol % (vol.) | Total Reducing Sugars (gsugars/Lwine) | Titratable Acids (TA) (gTA/Lwine) | Volatile Acids (VA) (gVA/Lwine) | pH |
|---|---|---|---|---|---|
| V1 | 14.96 ± 1.51 a | 2.2 ± 0.0 a | 6.89 ± 1.01 b | 0.68 ± 0.09 a | 3.32 ± 0.11 b |
| V2 | 14.11 ± 2.01 a,b | 2.0 ± 0.1 b | 7.13 ± 0.98 a | 0.43 ± 0.07 c | 3.26 ± 0.23 b |
| V3 | 14.53 ± 1.09 a | 2.2 ± 0.4 a | 7.34 ± 0.44 a | 0.61 ± 0.05 a | 3.24 ± 0.49 b |
| CS1 | 14.51 ± 0.98 a | 2.0 ± 0.0 b | 5.32 ± 0.67 c | 0.42 ± 0.01 c | 3.95 ± 0.57 a |
| CS2 | 14.68 ± 2.03 a | 2.0 ± 0.3 b | 6.29 ± 0.81 b | 0.65 ± 0.04 a | 3.84 ± 0.09 a |
| CS3 | 13.97 ± 1.77 b | 2.2 ± 0.7 a | 5.92 ± 0.39 c | 0.50 ± 0.07 b,c | 3.88 ± 0.41 a |
| ANOVA factors and interactions | |||||
| p-values | |||||
| Yeast strains | 0.03 | 0.04 | 0.01 | 0.01 | 0.04 |
| Grape varieties | 0.42 | 0.83 | 0.02 | 0.04 | 0.85 |
| Yeast strains x Grape varieties | 0.72 | 0.84 | 0.43 | 0.24 | 0.09 |
| Wine Designation | Total Phenolic Compounds (TPC) (mganalyt/Lwine) | Total Anthocyanins (mganalyt/Lwine) | Color Intensity (a.u.) |
|---|---|---|---|
| V1 | 1512.7 ± 23.55 b | 653.3 ± 11.08 a | 31.31 ± 4.21 a |
| V2 | 1528.9 ± 39.01 b | 583.7 ± 22.65 b | 21.02 ± 5.28 b |
| V3 | 1617.2 ± 24.72 a | 637.1 ± 19.87 a | 23.62 ± 3.97 b |
| CS1 | 1495.9 ± 23.55 b,c | 540.7 ± 12.04 c | 31.05 ± 2.99 a |
| CS2 | 1450.5 ± 34.21 c | 472.9 ± 20.11 c,d | 21.01 ± 4.01 b |
| CS3 | 1610.8 ± 13.90 a | 573.8 ± 17.91 b | 32.72 ± 3.87 a |
| ANOVA factors and interactions | |||
| p-values | |||
| Yeast strains | 0.04 | 0.01 | 0.02 |
| Grape varieties | 0.03 | 0.02 | 0.14 |
| Yeast strains x Grape varieties | 0.15 | 0.04 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilieva, F.; Petrov, K.; Veličkovska, S.K.; Gunova, N.; Dimovska, V.; Rocha, J.M.F.; Esatbeyoglu, T. Influence of Autochthonous and Commercial Yeast Strains on Fermentation and Quality of Wines Produced from Vranec and Cabernet Sauvignon Grape Varieties from Tikveš Wine-Growing Region, Republic of North Macedonia. Appl. Sci. 2021, 11, 6135. https://doi.org/10.3390/app11136135
Ilieva F, Petrov K, Veličkovska SK, Gunova N, Dimovska V, Rocha JMF, Esatbeyoglu T. Influence of Autochthonous and Commercial Yeast Strains on Fermentation and Quality of Wines Produced from Vranec and Cabernet Sauvignon Grape Varieties from Tikveš Wine-Growing Region, Republic of North Macedonia. Applied Sciences. 2021; 11(13):6135. https://doi.org/10.3390/app11136135
Chicago/Turabian StyleIlieva, Fidanka, Kire Petrov, Sanja Kostadinović Veličkovska, Natasa Gunova, Violeta Dimovska, João Miguel F. Rocha, and Tuba Esatbeyoglu. 2021. "Influence of Autochthonous and Commercial Yeast Strains on Fermentation and Quality of Wines Produced from Vranec and Cabernet Sauvignon Grape Varieties from Tikveš Wine-Growing Region, Republic of North Macedonia" Applied Sciences 11, no. 13: 6135. https://doi.org/10.3390/app11136135
APA StyleIlieva, F., Petrov, K., Veličkovska, S. K., Gunova, N., Dimovska, V., Rocha, J. M. F., & Esatbeyoglu, T. (2021). Influence of Autochthonous and Commercial Yeast Strains on Fermentation and Quality of Wines Produced from Vranec and Cabernet Sauvignon Grape Varieties from Tikveš Wine-Growing Region, Republic of North Macedonia. Applied Sciences, 11(13), 6135. https://doi.org/10.3390/app11136135








