Featured Application
Bioactive peptides derived from meat by-products have generated increasing interest in the development of functional foods. This application promotes the principles of a sustainable circular economy, since it allows an efficient use of meat by-products. Therefore, the use of by-products generated from slaughterhouses, which are not appropriate for human consumption, would maximize the use of existing nutritional sources and avoid the contamination caused by these waste products. In addition, the antioxidant and antimicrobial activities associated with these bioactive compounds would allow them to replace the synthetic products used today, which are associated with adverse effects on human health.
Abstract
In order to make the by-products generated from the porcine industry more valuable, pig livers were used in this trial to obtain protein hydrolysates. Three proteases (alcalase, bromelain, and papain) were utilized for enzymatic hydrolysis with two different durations, 4 and 8 hours. Ultrafiltration process was used for the recovery of the extracts, employing three different membrane pore sizes (30, 10, and 5 kDa). The porcine livers contained considerable amounts of protein (19.0%), considering they are almost composed of water (74.1%). The antioxidant activity of the obtained hydrolysates was investigated using four antioxidant methods (2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, 2-2′-Azino-di-[3-ethylbenzthiazoline sulfonate] (ABTS) radical scavenging activity, ferric reducing antioxidant power assay (FRAP), and oxygen radical absorbance capacity assay (ORAC)). Antibacterial properties were also measured against Gram-negative and Gram-positive bacteria. Results indicated that the three studied factors (type of enzyme, membrane pore size, and time) significantly affected the parameters evaluated. Hydrolysates obtained at 8 hours with alcalase had the best antioxidant properties. The 30 kDa alcalase extracts exhibited the highest DPPH (562 µg Trolox/g), FRAP (82.9 µmol Fe2+/100 g), and ORAC (53.2 mg Trolox/g) activities, while for ABTS the 10 kDa alcalase showed the higher values (1068 mg ascorbic acid/100 g). Concerning the antibacterial activity, 30 kDa hydrolysates obtained with bromelain for 4 hours exhibited the highest antimicrobial capacity, providing an inhibition of 91.7%.
1. Introduction
In recent years, the consumption of protein-rich food has increased in the world. Meat and meat products are good sources of protein [1]. These proteins contain essential amino acids with high bioavailability that humans cannot synthesize [2,3]. Regrettably, huge quantities of low-value by-products are generated every day by the meat industry and slaughterhouses, representing economic and environmental issues for meat processors [4]. Therefore, their utilization in the production chain could mean the substantial reduction of meat wastes [5]. These by-products consist of parts of animals or entire bodies, animal origin products, or derivatives obtained from animals that cannot be directly consumed by humans [6]. Porcine liver is an example of an edible meat processing waste. This organ is cheap and is readily available from butchers and grocery stores. However, its peculiar organoleptic characteristics (color and odor) mean it is mainly used for the manufacture of animal feed, pâtés, and sausages. This contrasts with the fact that the nutritional profile of liver is exceptional, so it is considered one of the most valuable proteins and nutrient sources [7]. It is characterized by its high levels of carbohydrates and polyunsaturated fatty acids (PUFA) and by its low levels of monounsaturated fatty acids (MUFA). It provides numerous vitamins, such as retinol (A), riboflavin (B2), niacin (B3), pyridoxine (B6), folacin (B9), cobalamin (B12), and ascorbic acid (C). It also stands out for its high mineral content, especially iron and manganese.
The Spanish porcine sector accounts for around 14% of the overall agricultural production [8]. It is the most economically important sector in terms of overall livestock production, accounting for roughly 39% of the total economic output. Globally, China is the largest pig meat producer, whereas the European Union is the second. Spain is the world’s fourth biggest producer, behind China, USA, and Germany. Additionally, Spain ranks second in production in the European Union, at 19% of the total [9]. Accordingly, there is a large amount of available pork liver, offering an excellent scope and opportunities for its utilization [10]. There is growing consumer concern about maintaining a healthy diet. Diseases associated with bad food habits are increasing worldwide. Therefore, the new trend of consumption of foodstuffs with bioactive properties is gaining importance. Bioactive peptides are characterized by short sequences of approximately 2–20 amino acid residues encrypted in their parent protein. These biopeptides are considered as the main compounds used for the production of functional foods and nutraceuticals, which have advantageous properties for human health [11]. Additionally, they can improve the storage stability of perishable foods by preventing the growth of food-spoilage organisms [12].
Although biopeptides have functional effects, they must be released from the entire sequence of the protein through enzymatic hydrolysis, microbial fermentation, or solvent extraction methods [4]. The former procedure is the most common for obtaining biopeptides in a reliable and effective manner [13]. It is widely used to enhance the functional and nutritional features of proteins [14]. It is mainly affected by several factors, such as the protein substrate and concentration, the specificity of the enzyme (protease), the enzyme/substrate ratio, temperature, pH, and time [15]. Furthermore, it should be emphasized that the type of enzyme plays an important role in the effectiveness of the breakdown of proteins into functional peptides [16]. Due to the small size and specificity of bioactive peptides, they are capable of inhibiting protein–protein interactions [17].
Biopeptides have attracted rising attention due to their antioxidant capacity. Moreover, they have many other biological activities that are still under study, such as anticancer, antihypertensive (angiotensin-converting enzyme—ACE inhibitory), antiviral, antithrombotic, opioid, and immunomodulatory activities [18]. Furthermore, many researchers are focused on the development of antimicrobial peptides as human therapeutics, as a result of the fast growth of resistance of conventional antibiotics. This study intends to highlight the antioxidant and antimicrobial potential of porcine liver protein hydrolysates for developing new healthy foods. As mentioned, the enzymes used for liver hydrolysis influence the resulting activity. Alcalase, papain, pepsin, and trypsin are commonly used in the extraction of hydrolysates from porcine liver [7,19]. However, scarce information is available on the antioxidant and antimicrobial activities of hydrolysates and their fractions obtained with bromelain. These enzymes are less known and are usually used in the enzymatic hydrolysis of legume seed extracts [20].
For the purpose of finding attractive applications for the huge amount of wasted porcine liver, samples were subjected to enzymatic hydrolysis, employing several commercial enzymes in order to release the biopeptides through protein cleavage. Subsequently, a recovery step was performed using low molecular mass cut-off membranes to separate small peptides from high molecular residues and remaining enzymes. Finally, the obtained extracts were assayed for antioxidant (2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2-2′-Azino-di-[3-ethylbenzthiazoline sulfonate] (ABTS), Ferric Reducing Antioxidant Power (FRAP) and Oxygen Radical Absorbance Capacity (ORAC) assays) and antimicrobial properties against Gram-positive (Brochothrix thermosphacta, Listeria monocytogenes, Staphylococcus aureus) and Gram-negative (Pseudomonas aeruginosa, Escherichia coli, Salmonella enterica) bacteria.
2. Materials and Methods
2.1. Materials and Reagents
Fresh porcine livers were provided by a local meat market (Cárnicas M. Boo, San Cibrao das Viñas, Ourense). The 2,2′-Azobis (2-methylpropionamidine) dihydrochloride (AAPH) 98% and (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid 97% (Trolox) were acquired from Acros organics (New Jersey, USA). L-(+)-ascorbic acid 98+%, 2,4,6-Tri(2-pyridyl)-1,3,5-triazine (TPTZ) 98%, and 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS) 98% were purchased from Alfa Aesar (Kandel, Germany). Gallic acid monohydrate extra pure, di-potassium hydrogen phosphate anhydrous extra pure, potassium dihydrogen phosphate reagent grade, sodium hydroxide 32%, and hydrochloric acid 37% extra pure were supplied by Scharlau S.L. (Barcelona, Spain). Potassium peroxodisulphate and sodium acetate were dispensed by VWR Chemicals (Leuven, Belgium), iron (III) chloride hexahydrate and iron (II) sulphate heptahydrate were from VWR Chemicals (Germany), and Folin–Ciocalteu reagent was from VWR chemicals (France). The 2,2-diphenyl-1-picrylhydrazyl (DPPH) and nisin from Lactococcus lactis were procured from Sigma Aldrich (Germany), methanol was from Chem-Lab NV (Zedelgem, Belgium), gentamicin sulfate was from Biowest (France), and fluorescein sodium was from Fisher (UK). Papain 6000 USP, Bromelain 2000 U/g, and bioprotease LA 660 (alcalase) were supplied by Biocon (Spain). Brochothrix thermosphacta (CECT 847) was distributed by CECT (Spain). Listeria monocytogenes (MKTD 11994/WDCM 00019), Staphylococcus aureus (MKTD 799/WDCM 00032), Salmonella enterica (MKTD 17058), Pseudomonas aeruginosa (MKTD 1128/WDCM 00026), and Escherichia coli (DSMZ 1103/WDCM 00013) were from Microkit (Spain). Amicon stirred cells (400 mL, membrane diameter 76 mm) were purchased from Millipore (Germany), and 5, 10, and 30 kDa molecular weight cut-off (MWCO) (76 mm) membranes were from Millipore (Jaffrey, NH, USA). Cellulose acetate filters with pore size of 0.22 µm were supplied by Filter Lab (Spain).
2.2. Chemical Composition of Porcine Liver
Ash [21], moisture [22], and protein [23] contents of the porcine liver were quantified according to the ISO recommended standards, while fat was measurement by the American Oil Chemists’ Society (AOCS) official procedure [24]. The carbohydrate content was calculated numerically by the difference of the aforementioned contents (carbohydrate = 100 − (ash + fat + moisture + protein)).
2.3. Peptic Hydrolysis of Porcine Liver
The process of obtaining protein hydrolysates from porcine liver is shown in Figure 1.
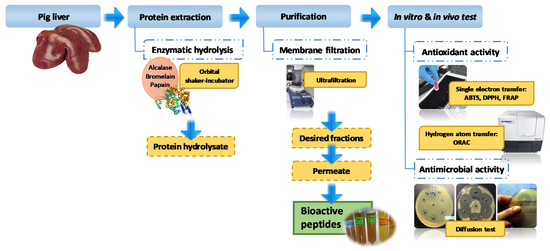
Figure 1.
Process of obtaining protein hydrolysates from porcine liver. DPPH: 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Activity; ABTS: 2-2′-Azino-di-[3-ethylbenzthiazoline sulfonate] Radical Scavenging Activity; FRAP: Ferric Reducing Antioxidant Power; ORAC: Oxygen Radical Absorbance Capacity.
Firstly, fatty and connective tissues were removed from the livers. Then, they were chopped into small cubes and frozen at −20 °C to reduce their viscosity. This facilitated their homogenization with ice at a ratio of 1:1 (w/w) in a cutter Talsa K3 (Talsa, Valencia, Spain). Hydrolysis of samples was carried out using three different enzymes. Each was preincubated for 30 minutes at its optimal temperature. Samples were further preincubated at the optimal pH and temperature conditions for each protease. These were: 37 °C, pH = 6.0 for papain; 40 °C, pH = 6 for bromelain; 50 °C, pH = 8 for alcalase. Protases were added in an enzyme/substrate ratio of 1:100 (w/w). Enzymatic hydrolysis reactions were performed for 10 hours in an orbital shaker-incubator (125 rpm), maintaining the above pH conditions through the addition of NaOH or HCl 1N. Reactions were deactivated by heating the samples for 3 minutes at 95 °C. The mixtures were then rapidly cooled to room temperature in an ice bath. Subsequently, they were centrifuged at 10,000× g for 10 min using an Allegra X-22R centrifuge (Beckman Coulter, Nyon, Switzerland). Supernatants were purified by vacuum ultrafiltration technique using regenerated cellulose membrane filters with MWCO values of 30, 10, and 5 kDa.
2.4. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
DPPH radical scavenging activity is a method routinely used for the assessment of free radical scavenging potentials, and is regarded as a standard for the in vitro evaluation of antioxidants [25]. The DPPH assay was performed according to the method reported by Brand-Williams et al. [26], with minor modifications. Aliquots measuring 100 μL of samples were added to 3900 μL of DPPH solution (60 μM in methanol) and incubated for 10 min at 37 °C. Absorbance was read at 515 nm in a spectrophotometer UV-1800 (Shimatzu Corporation, Kyoto, Japan). Trolox was the standard used in this method to determine the DPPH radical scavenging activity of hydrolysates. Results were expressed as μg Trolox equivalents (TE)/g sample.
2.5. 2-2′-Azino-di-[3-ethylbenzthiazoline sulfonate] (ABTS) Radical Scavenging Activity
ABTS radical cation decolorization assays of samples were carried out following the method previously described by Re et al. [27], with some modifications. This assay is based on the ability of antioxidants to quench the long-lived ABTS radical cation, a bluish-green chromophore with a specific absorption line at 734 nm. The radical scavenging capacity was measured by employing the standard curve of ascorbic acid. ABTS was prepared by mixing 7 mM ABTS stock solution with 2.45 mM potassium persulfate, a strong antioxidant agent, leaving the mixture to stand in the dark at room temperature for 12–16 h before use. Prior to use, the ABTS stock solution was diluted with distilled water to achieve an absorbance of 0.70 at 734 nm, then equilibrated at 30 °C. Then, 980 μL of working solutions was added to an aliquot of 20 μL each of hydrolysate and standard. Absorbance was measured at the specific wavelength after 10 min in darkness. Results were expressed as mg AA (ascorbic acid)/100 g sample.
2.6. Ferric Reducing Antioxidant Power Assay (FRAP)
Measurement of the reducing power of the samples was monitored according to the work previously described by Benzie and Strain [28], with slight changes. This test is based on the capability of the antioxidants species to reduce iron (III) to the ferrous form (II) in an acid medium. The assay measures the change in absorbance at 593 nm, which involves the formation of an intense navy-blue colored compound of Fe2+ with 2,4,6-Tri(2-pyridyl)-1,3,5-triazine (TPTZ) from the colorless oxidized Fe3+. FRAP reagent was freshly prepared from 0.3 M acetate buffer (pH 3.6), 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mM HCl, and 20 mM FeCl3:6H2O at the ratio of 10:1:1 (v:v:v). Then, 900 μL of this fresh FRAP solution was mixed with 30 μL of properly diluted samples and 90 μL of distilled water. After incubation for 20 minutes at 37 °C in darkness, the absorbance was measured at 593 nm. The FRAP value was expressed as μmol Fe+2/100g sample based on a calibration curve that was prepared using FeSO4 as the reference standard.
2.7. Oxygen Radical Absorbance Capacity Assay (ORAC)
The ORAC assay was assayed according to the protocol of Huan et al. [29], with the following modifications. The reaction was carried out in 75 mM phosphate buffer (pH 7.4), with a final reaction mixture volume of 200 µL. Then, 25 µL of dilute sample and 150 µL of 0.8 µM fluorescein (oxidizable substrate) were added into the internal wells of a black 96-well microplate (FluoroNunc™ F96-MicroWell™ plate) and immediately incubated at 37 °C for 30 minutes in the fluorescence instrument. Then, 25 µL of AAPH 184 mM (2,2-azobis(2-methylpropionamidine)dihydrochloride) solution was added rapidly to each well, using the fluorescence device injectors to initiate the reaction in the microplate reader. The fluorescence was recorded with excitation and emission filters of 485 and 528 nm, respectively. Samples were stirred prior to each reading. Trolox reagent was used as a standard reference compound and phosphate buffer was used as blank. Results were calculated on the basis of the differences of areas under the curves of fluorescence decay of the fluorescein between the blank and the sample (net area under the curve). They were expressed as mg Trolox equivalent (TE)/g sample.
2.8. Antimicrobial Activity
Antimicrobial capacity was assessed as per the Kirby–Bauer test, following an agar well diffusion method described by Aristizabal and Marín [30]. This method is based on the relation between the concentration of the substance (in our case, the antimicrobial peptides) necessary to inhibit the bacterial strain and the growth inhibition halo on the surface of an agar plate with a suitable culture medium, which is inoculated homogeneously with the specific bacteria to be tested. A total of 6 strains were selected in this study. The nutrient broths employed were Pseudomonas agar base for P. aeruginosa, Baird–Parker agar medium for S. aureus, streptomycin thallous acetate (STAA) for B. thermosphacta, and trypticase soy agar (TSA) for L. monocytogenes, S. enterica and E. coli. Strains were prepared in saline solution following the commercial instructions. Then, 0.2 mL (B. thermosphacta, P. aeruginosa, and S. aureus) or 1 mL (L. monocytogenes, S. enterica, and E. coli) of broth was spread with a sterile Drigralsky spatula or poured onto plates, respectively. The bacterial concentration of the inoculum should be approximately 108 colony-forming units (CFU)/mL to ensure the highest growth of the microorganism inoculated in the growth medium. Seven wells measuring 6 mm in diameter were made using a sterile cork-borer. About 25 µL of each hydrolysate was added into 5 wells onto the solid media in the nutrient agar. The rest of the wells contained 25 µL of controls, one for the positive control and the other for the negative. All components were sterilized before their addition onto the plates through a sterile 0.22 µm pore cellulose acetate filter. Positive controls were 50 ppm of gentamicin and 105IU nisin/mL of Nisin for Gram-negative bacteria and Gram-positive bacteria, respectively. Sterile water was the negative control employed for both assays. The incubation conditions were 24 hours at 37 °C. The diameter of the inhibitory zone surrounding the wells (mm) was measured using a manual Vernier caliper. The percentage of inhibition was calculated following the formula shown below:
2.9. Statistical Analysis
A total of 72 samples (6 porcine livers × 2 two different processing batches × 3 types of enzymes × 2 hydrolysis times) were used to analyze the statistical significance differences of antioxidant and antimicrobial properties of the obtained hydrolysates. The statistical analyses were performed using IBM SPSS Statistics version 23 (SPSS Inc., NY, USA). The normal distribution and homogeneity of variance were previously tested (Shapiro–Wilk test). Data were submitted for analysis of variance (ANOVA) and Duncan tests for comparison when ANOVA had a significant effect (p < 0.05).
3. Results
3.1. Chemical Composition
The mean values of the chemical composition of porcine liver are presented in Table 1. The results showed that liver had a high nutritional content. Although almost all of its composition was water (mean moisture values of 74.1%), liver offered a good source of protein, with mean values of 19%. Fat and carbohydrate contents reached mean values of 3.56% and 1.97%, respectively. Finally, liver displayed an ash content of 1.4%.

Table 1.
Chemical composition of porcine liver. Results expressed as mean ± standard error.
3.2. DPPH Radical Scavenging Activity
The enzyme and the hydrolysis time used had significant effects (P < 0.05) on the DPPH radical scavenging activity of the porcine liver protein hydrolysates (Table 2).

Table 2.
Antioxidant activity of porcine liver protein hydrolysates obtained using alcalase, bromelain, and papain.
For the same hydrolysis time, alcalase was the enzyme that displayed the highest activity, reaching values of 562 µg Trolox/g. In contrast, samples hydrolyzed with papain showed the lowest outcomes, with an activity that in some cases was half of the values shown by the other two enzymes used (145 vs. 344 and 477 µg Trolox/g for papain vs. bromelain and alcalase, respectively). Except for the results found in the samples extracted with bromelain, the activity of porcine liver protein hydrolysates increased with the hydrolysis time.
The filtration process also had a significant effect (P < 0.05) on DPPH. However, the behavior depended on the enzymatic hydrolysis carried out. In the case of alcalase, the results showed that DPPH activity increased when the pore size of the membrane was larger. In this way, when the hydrolysis time was 8 h, values of 562, 542, and 443 µg Trolox/g for 30, 10, and 5 kDa were found, respectively; while the capacity decreased at low hydrolysis time, with values of 477, 408, and 372 µg Trolox/g for 30, 10, and 5 kDa, respectively. For bromelain, the intermediate pore size showed the best results for both hydrolysis times (427 and 379 µg Trolox/g for hydrolysis times of 4 h and 8 h, respectively, ultrafiltered at 10 kDa). Finally, the trend for papain 4 h was inverse to alcalase, since activity values increased as the pore size decreased (249, 228, and 145 µg Trolox/g for 5, 10, and 30 kDa, respectively), while protein hydrolysates filtered with a pore of 10 kDa were more effective for longer hydrolysis.
3.3. ABTS Radical Scavenging Activity
The effects of the enzyme used on ABTS radical scavenging activity of the porcine liver protein hydrolysates are shown in Table 2. All samples were strongly influenced by the type of membrane and the enzyme used (P < 0.001). The porcine hydrolysates obtained in the hydrolysis with alcalase provided higher activity than those obtained with the other two enzymes at both hydrolysis times studied. These values even tripled the maximum value obtained for the other enzymes (936 and 1068 mg AA/100 g vs. 335 and 416 mg AA/100 g, and 392 and 335 mg AA/100 g for alcalase, papain and bromelain extracts obtained from a hydrolysis time of 4 h and 8 h, respectively).
The ultrafiltration step significantly (P < 0.05) affected the activity of the samples analyzed. The behavior was different depending on the enzyme and the pore size used. For papain the best choice of membrane was 30 kDa (416, 368, and 352 mg AA/100 g resulted from hydrolysis time of 8 h and with membranes measuring 30 kDa, 10 kDa, and 5 kDa, respectively) and for bromelain the best choice of membrane was 10 kDa (392, 346, and 281 mg AA/100 g resulted from hydrolysis time of 4 h and with membranes measuring 10 kDa, 5 kDa, and 30 kDa, respectively), since the antioxidant properties obtained were greater. For alcalase, at 4 h the 30 kDa membrane was the best option, while at 8 h the best option was 10 kDa (936 and 1068 mg AA/100 g, respectively).
As can be seen in the results obtained, time also had an important impact on the ABTS activity. Papain and alcalase showed higher activities when the hydrolysis time increased, while 4 h hydrolyzing period was enough to hydrolyze porcine livers with bromelain (392 vs. 335 mg AA/100 g for 4 h and 8 h processes, respectively).
3.4. Ferric Reducing Antioxidant Power Assay (FRAP)
In general, the results obtained showed that FRAP activity varied significantly (P < 0.05) with the three variables evaluated (Table 2). Regarding the enzyme used, bromelain hydrolysates had more antioxidant power than the ones obtained for alcalase and papain. In contrast to the results of DPPH and ABTS assays, alcalase only showed a greater activity at a longer time of hydrolysis and in the extracts filtered with larger pore membranes (82.9 vs. 69.8 µmol Fe+2/100 g for alcalase and bromelain, respectively).
It can be highlighted that for longer reaction times the results were better, excluding papain 30 kDa samples, where the effect was inverse and not significant (49.0 vs. 36.9 µmol Fe+2/100 g for hydrolysis times of 4 h and 8 h, respectively). Moreover, no significant differences (P > 0.05) were found between enzymes employing 4 h and 30 kDa, and 8 h and 10 kDa.
The antioxidant character increased significantly (P < 0.05) as the membrane pore size increased, in some cases doubling the values obtained. In this way, extracts filtered with 30 kDa pore size displayed values of 24.3 vs. 49.0 µmol Fe+2/100 g in the extracts hydrolyzed with papain for 4 h; and 43.3 vs. 82.9 µmol Fe+2/100 g for extracts hydrolyzed with alcalase for 8 h. Samples obtained with papain after 8 h were an exception to this trend, since the maximum activity was reached with 10 kDa (57.0 vs. 46.2 and 36.9 µmol Fe+2/100 g for 10 kDa vs. 5 kDa and 10 kDa, respectively).
3.5. Oxygen Radical Absorbance Capacity Assay (ORAC)
The effects of enzyme, membrane, and hydrolysis time on the ORAC capacity are shown in Table 2. In most of the samples, significant differences were observed (P < 0.05) due to the aforementioned parameters. In general, longer reaction times seemed to result in better antioxidant ability. This fact could be observed in alcalase and bromelain. In fact, the enzyme which provided the best results was alcalase hydrolyzed for 8 h, while in papain the effect was not clear.
On the other hand, the larger the pore diameter of the membrane used, the greater the antioxidant capacity. This trend was observed in the hydrolysates obtained with papain and alcalase for both hydrolysis times evaluated. Nevertheless, for bromelain this behavior was inverse, since the lowest value was achieved with 30 kDa (31.5 and 28.7 mg trolox/g for extracts hydrolyzed for 4 h and 8 h, respectively).
3.6. Antimicrobial Activity
The antibacterial activities of all hydrolysates were assessed against Gram-positive (B. thermosphacta, L. monocytogenes, and S. aureus) and Gram-negative (P. aeruginosa, E. coli, and S. enterica) bacteria (Figure 2). The antibacterial activity of samples was evaluated by the zone of inhibition (mm), expressing the results obtained as the percentage of inhibition.
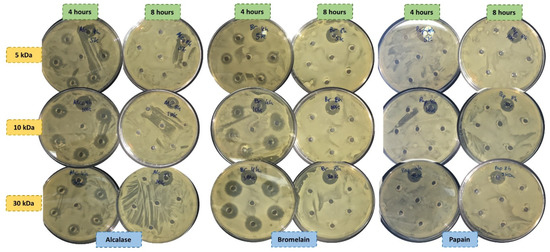
Figure 2.
Antimicrobial activity of porcine liver protein hydrolysates obtained with alcalase, bromelain, and papain against Brochothrix thermosphacta.
None of the samples were able to block the Gram-negative bacteria proliferation, while bromelain and alcalase extracts were capable to paralyze the B. thermosphacta and L. monocytogenes expansion. In contrast, peptidic fractions of papain did not show antimicrobial activity for any of the strains evaluated. Hydrolysates obtained with bromelain after 4 hours and at 30 kDa exhibited the strongest activity against Brochothrix, with a percentage of inhibition of 91.7%, activity that was reduced to 75.0% and 50.0% with pore sizes of 10 and 5 kDa, respectively. In the extractions carried out for 8 hours with this enzyme, only the 5 kDa extracts showed activity (8.30%).
Lower activities than those obtained with bromelain were observed in the hydrolysates obtained with alcalase. The extracts that were hydrolyzed for 4 hours and filtered with 5 and 30 kDa exhibited the same inhibition ability (50.0%), while the fraction of 10 kDa exerted a greater activity (83.3%). Concerning L. monocytogenes, only bromelain hydrolyzed for 4 hours at 30 kDa and alcalase hydrolyzed for 4 hours at 10 kDa showed antimicrobial activity against this microorganism, displaying inhibition percentages of 67.0% and 100.0%, respectively.
4. Discussion
4.1. Chemical Composition
The demand for new sources of protein has continued to grow tremendously in the past decade [31]. The results obtained in this work highlight the importance of the liver as a source of peptides, giving added value to this product. Regarding fat content, the values observed were lower than 5% in organs such as the heart, kidney, lung, spleen, and thymus [6]. In contrast, carbohydrate contents were higher than the values found in other meat by-products, with mean values of 1.97% [32].
Results acquired in this study are similar to those reported by Kakimov et al. [33], who determined the proximate composition of beef liver, but with higher ash and lower carbohydrate contents (1.8% and 0.60%, respectively). Lower values of fat, moisture, and carbohydrate contents were also found by these authors in horse liver, while the protein content was higher (2.62%, 70.9%, 25.1%, and 0.02%, respectively). Chicken liver had higher moisture (76.7%) and lower fat (2.89%) and protein (17.7%) contents according to the data described by Seong et al. [34]. These parameters were also assessed by the same authors [35] in bovine liver, however these values were lower than those obtained in the present study (ash: 1.18%, fat: 3.15%, moisture: 69.2%, protein: 18.6%).
Regarding porcine liver, higher contents of protein, fat, and carbohydrates (20.6%, 4.97%, and 2.58%, respectively), lower moisture content (70.3%), and an equal ash percentage were observed by Verma et al. [10]. In contrast, Seong et al. [36] obtained less protein and carbohydrate contents (17.6% and 1.15%) in edible pork by-products.
4.2. DPPH Radical Scavenging Activity
Liver is an important source of peptides with antioxidant activities [4,37]. This was reflected in the DPPH radical scavenging activity observed in the hydrolysates obtained in the present study from the enzymatic hydrolysis of porcine liver.
As mentioned before, the enzymes used for their extraction conditioned their activity [4]. The values found were in accordance with those found by other authors in porcine liver [10], where alcalase hydrolysates exhibited better DPPH values than papain (42.3% vs. 40.3%, respectively). Similar trends and DPPH activity were observed by Yu et al. [7] in porcine liver proteins hydrolyzed with the aforementioned enzymes (42.0% and 37.0%, respectively). Alcalase also showed good DPPH values when it was compared with those found by other enzymes, such as pepsin and pancreatin [38]. On the contrary, Kim et al. [39] observed that venison hydrolysates obtained with papain displayed higher antioxidant activity than the hydrolysates produced with alcalase. Moreover, the DPPH ability depends on the material used to carry out the hydrolysis, since higher DPPH activity could be obtained using the same enzyme in different matrices. This trend was observed when pepsin was used to hydrolyze chicken and porcine liver, with higher antioxidant activities observed in hydrolysates obtained from chicken liver (95.0% vs. 55.0%, respectively).
Regarding hydrolysis time, except for bromelain, DPPH values increased as the process time increased, with increases of up to 53.0% and 25.0% observed for papain and alcalase, respectively. These findings were previously found by other authors [10,40]. In this way, the antioxidant activity of protein hydrolysate extracted from porcine liver was affected by the different hydrolyzing periods, increasing from 23.2% to 42.3% for alcalase and from 21.1% to 40.3% for papain during the six hour of hydrolysis [10]. This could be due to relevant roles of the peptide structure, molecular weight, and amino acid sequence in the radical scavenging activity [10].
Ultrafiltration with MWCO membranes is the primary perm-selective barrier commonly used to separate proteins from meat processing by-products, with pore sizes ranging from 4 to 30 kDa being the most effective for the separation of macro- and microsolutes, such as protein hydrolysates [41]. Previous studies also confirmed that low molecular weight peptides were stronger as antioxidants than high molecular weight peptides [38,39]. This behavior was observed in the present study for papain and bromelain hydrolysates, suggesting that lower molecular weight peptides (10 kDa) are more effective as potent DPPH radical scavengers than high molecular weight peptides (30 kDa). Moreover, in the case of papain at 4 h, the activity values increased as the pore size decreased (249, 228, and 145 µg Trolox/g for 5, 10, and 30 kDa, respectively). This outcome agrees with data observed by Liu et al. [42], who noticed that fractions with a small molecular weight (<3 kDa) possessed better antioxidant potential, with evidence of higher DPPH. On the other hand, the alcalase values were in contrast to the current observations, since an increase in the DPPH scavenging activity was obtained when the peptide size of the protein hydrolysate increased. The same trend was observed in the results obtained from bovine liver sarcoplasm, poultry viscera, and yellowstripe trevally (Selaroides leptolepis) protein hydrolysates [37,43,44].
4.3. ABTS Radical Scavenging Activity
ABTS radical scavenging activity is among the most used methods employed to determine antioxidant capacity [16]. Enzyme content, degree of hydrolysis, solubility of hydrolysates, types of peptides, and free amino acid content are some of the factors that could have effect on the ability of hydrolysates to scavenge ABTS radicals [45].
As for DPPH, the ABTS activities of extracts are closely related to the hydrolysis time and type of enzyme. The variation with the enzyme treatment could be due to the changes in the structures of hydrolysate proteins due to the different cleavage sites of the enzymes [19]. In this way, alcalase hydrolysates displayed higher levels of ABTS than papain, and the values were also better as time increased [10]. In the present study, these increases resulting in an increment of 20.0% and 26.0% for papain and alcalase, respectively. Alcalase also gave rise to hydrolysates with antioxidant activity in other porcine tissues (appendix, colon, lung, pancreas, and rectum). In this regard, Damgaard et al. [46] confirmed that hydrolysates obtained from these porcine by-products can also generate antioxidant activity, showing ABTS values even higher than those obtained for liver.
An opposite effect was observed in bromelain, since the activity decreased as the hydrolysis time increased, meaning that hydrolysis for 4 h would be sufficient to obtain the higher activity of the extracts obtained with this enzyme. Better activities were also found by other authors in protein hydrolysate extracted from porcine liver when hydrolysis time was increased, with increments of inhibition percentages until 47.0% and 49.0% for papain and alcalase hydrolysates, respectively, for 0–6 h of reaction time [10].
The ultrafiltration step also significantly affected the activities of the samples analyzed. ABTS radical scavenging activity increase as the size of the peptide increased. This was in contrast with the results found in previous studies, where the fractions smaller than 3 kDa reached higher values (795 µM TEAC/mg) [44]. This behavior could be related to the type of enzyme used, which in addition to conditioning the bioactivity of the hydrolysates obtained, could influence the composition of the extracts obtained, and therefore the filtration process. This may affect the fouling mechanisms that occur in polymeric membranes, such as the one used in the present study, decreasing permeate fluxes through the membrane, and consequently the separation performance and the ABTS activity [41].
4.4. Ferric Reducing Antioxidant Power Assay (FRAP)
The results found for FRAP in all of the porcine liver enzymatic hydrolysates were lower than the activities observed in ABTS and DPPH assays. Moreover, the type of enzyme selected for hydrolysis had a significant effect (P < 0.05) on the outcomes obtained. In this case, alcalase was the second most important enzyme after bromelain. The lower values of FRAP obtained in alcalase and papain could be due to the lower ability of these liver hydrolysate extracts to reduce ferric ion to its ferrous form. In contrast, other authors found that papain presented better results than those obtained for alcalase and bromelain in bovine blood and lung hydrolysates [47,48]. In addition, the values obtained in FRAP assay showed lower values than those found in other liver hydrolysates [10].
Large hydrolysis periods intensified the FRAP activity of the peptides released by enzyme hydrolysis. This increase of the FRAP activity with longer hydrolysis time was also reported with other protein hydrolysates obtained from meat by-products [49,50]. This behavior was reported in the enzymes studied in the present work and in others, such as flavorzyme [47,48]. Verma et al. [10] observed that hydrolysis performed with alcalase and papain increased FRAP activity by 40.0% after 6 h of hydrolysis, while increases of about 76.0% were observed by Chang et al. [50] in porcine hemoglobin hydrolysates after 10 h of hydrolysis.
According to the results previously reported by other authors for porcine protein hydrolysates, ultrafiltration process is one of the factors that has a marked effect on the FRAP activity. In contrast with the results found by other authors [49,51], larger size peptides obtained from the enzyme hydrolysis exhibited higher FRAP values than those obtained in extracts that contained lower molecular weight peptides. A similar trend was observed in whole porcine liver and blood hydrolysates [19,52]. This fact confirmed that the structure and size of the peptides of the resulting hydrolysates could be responsible for these differences in antioxidant activities among hydrolysates [43].
4.5. Oxygen Radical Absorbance Capacity Assay (ORAC)
In addition to the previous methods, which are the most widely used to assess antioxidant capacity, the hydrolysates of porcine liver proteins were also evaluated using ORAC. This method is widely applied to evaluate the antioxidant activity of phenolic compounds, however few studies have used it to evaluate the antioxidant activity of peptides [42]. The enzyme which provided the best results for ORAC was alcalase. This is in agreement with the results found by other authors in bovine lung hydrolysates, where alcalase achieved higher ORAC activity than papain and pepsin [47]. Better results were also found for alcalase than those found by the mixture of pepsin and pancreatin in chicken skin enzymatic protein hydrolysates (35.0% vs. 33.0%, 95.0% vs. 80.0%, and 3800 vs. 3200 μM Trolox equivalent/g for DPPH, metal chelating effect, and ORAC, respectively).
Regarding hydrolysis time, its increase resulted in the increase of the ORAC antioxidant activity [53]. This trend was observed for all the enzymes evaluated, but was in contrast with the results found in other studies with porcine liver protein. These authors found that this behavior was only observed for alcalase, while the activity of papain extracts decreased [7].
On the other hand, Onuh et al. [38] reported higher ORAC values in small peptides. This could be due to the greater ability to interact and donate electrons compared to larger peptides, which could have a reduced ability to interact with the free radical. However, the results obtained in the present study showed that hydrolysates filtered through intermediate and higher pore size membranes were those that showed the best capabilities (36.4 and 45.2 mg Trolox/g for bromelain 10 kDa, 40.1 and 53.2 mg Trolox/g for alcalase 30 kDa, and 37.4 and 44.3 mg Trolox/g for papain 30 kDa).
4.6. Antimicrobial Activity
The use of different enzymes and hydrolysis times are crucial in the production of antibacterial peptides [20]. The activity of these antimicrobial peptides allows the selective disruption of cell membranes, which leads to membrane permeabilization, depolarization, dissipation of electrochemical gradients, and eventual cell death [54]. Microbial cell walls are covered by polyanionic molecules such as lipoteichoic acids in Gram-positive bacteria, while in Gram-negative bacteria the peptidoglycan layer is thinner and is surrounded by two phospholipids membranes, with the outer membrane being topped by lipopolysaccharides [55]. Therefore, the effectiveness of the porcine liver hydrolysates could be due to the cationic and hydrophobic properties of the peptides, which allow the reaction with the internal cytoplasmic anionic membranes of the microbes, causing their breakdown [10].
The hydrolysates obtained in this study showed low activity. For Gram-negative bacteria this could be due to the high resistance of these strains, which is greater than the Gram-positive bacteria [56]. Bromelain was the enzyme that resulted in hydrolysates with the highest antimicrobial activity, followed by alcalase. A similar behavior was observed by Ee et al. [20], who noticed higher activities of bromelain and no activity against Gram-negative bacteria.
On the contrary, papain did not show activity against the bacterial strains evaluated. These outcomes disagree with the findings reported by Verma et al. [10], who utilized 3 enzymes to evaluate the antimicrobial capacity of protein hydrolysates obtained from the same matrix. They studied the type of enzyme and the effect of hydrolysis time on the inhibition of L. monocytogenes, E. coli, and S. aureus. They achieved the greatest inhibition using trypsin and papain, while with alcalase the effect obtained was lower.
5. Conclusions
Porcine liver is, undoubtedly, a valuable by-product for obtaining bioactive peptides. Based on the obtained results, it can be confirmed that the type of enzyme and duration influenced the hydrolysis of liver proteins, and therefore the related activities. Moreover, the ultrafiltration process through membranes with several pore sizes had different effects with respect to the antioxidant and antimicrobial power. The protein hydrolysates obtained from the enzymatic hydrolysis of porcine liver displayed antioxidant properties. The use of various antioxidant methods helps in understanding the types of mechanisms involved in their activity, although the lack of standardization complicates the comparison between assays. Alcalase hydrolysates showed the highest antioxidant activity. Even though the hydrolysates obtained with this enzyme also displayed antibacterial activities, bromelain was the enzyme that showed the best antimicrobial properties against Gram-positive bacteria (B. thermosphacta and L. monocytogenes). Therefore, it can be highlighted that porcine liver protein hydrolysates have good potential for developing new health foods, in addition to acting as natural antioxidants. Moreover, bromelain allowed hydrolysates with antimicrobial properties against Gram-positive bacteria to be obtained, which are commonly found in meat and meat products. Future studies are needed to evaluate the specific compounds responsible for the antioxidant and antimicrobial activity of porcine liver protein hydrolysates.
Author Contributions
Conceptualization, P.B., M.P., and J.M.L.; writing—original draft preparation, P.B., and M.P.; writing—review and editing, P.B., M.P., M.G., D.F., W.Z., and J.M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received external funding by grant RTA 2017-00024-CO4-04 from INIA (Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria, Spain).
Acknowledgments
Authors would like to acknowledge to INIA for granting Paula Borrajo with a predoctoral scholarship (grant number CPD2016-0030). José M. Lorenzo is a member of the HealthyMeat network, funded by CYTED (Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo) (ref. 119RT0568). Thanks to GAIN (Axencia Galega de Innovación, Xunta de Galicia, Spain) for supporting this research (grant number IN607A2019/01).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lorenzo, J.M.; Sarriés, M.V.; Tateo, A.; Polidori, P.; Franco, D.; Lanza, M. Carcass characteristics, meat quality and nutritional value of horsemeat: A review. Meat Sci. 2014, 96, 1478–1488. [Google Scholar] [CrossRef]
- Siti, R.A.M.; Zainal, S.; Noriham, A. Determination of optimum condition of leucine content in beef protein hydrolysate using response surface methodology. Malays. J. Anal. Sci. 2016, 20, 829–837. [Google Scholar]
- Lorenzo, J.M.; Pateiro, M. Influence of type of muscles on nutritional value of foal meat. Meat Sci. 2013, 93, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Borrajo, P.; Pateiro, M.; Barba, F.J.; Mora, L.; Franco, D.; Toldrá, F.; Lorenzo, J.M. Antioxidant and Antimicrobial Activity of Peptides Extracted from Meat By-products: A Review. Food Anal. Methods 2019, 12, 2401–2415. [Google Scholar] [CrossRef]
- Mullen, A.M.; Álvarez, C.; Zeugolis, D.I.; Henchion, M.; O’Neill, E.; Drummond, L. Alternative uses for co-products: Harnessing the potential of valuable compounds from meat processing chains. Meat Sci. 2017, 132, 90–98. [Google Scholar] [CrossRef]
- Pateiro, M.; Borrajo, P.; Campagnol, P.C.B.; Domínguez, R.; Tomasevic, I.; Munekata, P.E.S.; Barba, F.J.; Lorenzo, J.M. Extraction of valuable compounds from meat by-products. In Green Extraction and Valorization of By-Products from Food Processing; Barba, F.J., Roselló-Soto, E., Brncic, M., Lorenzo, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 55–90. [Google Scholar]
- Yu, H.C.; Hsu, J.L.; Chang, C.I.; Tan, F.J. Antioxidant properties of porcine liver proteins hydrolyzed using Monascus purpureus. Food Sci. Biotechnol. 2017, 26, 1217–1225. [Google Scholar] [CrossRef]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef]
- MAPA Porcino. Sectores Ganaderos. Producción y Mercados Ganaderos. Ganadería. Ministerio de Agricultura, Pesca y Alimentación. Gobierno de España. Available online: https://www.mapa.gob.es/es/ganaderia/temas/produccion-y-mercados-ganaderos/sectores-ganaderos/porcino/ (accessed on 24 February 2020).
- Verma, A.K.; Chatli, M.K.; Kumar, P.; Mehta, N. Antioxidant and antimicrobial activity of protein hydrolysate extracted from porcine liver. Indian J. Anim. Sci. 2017, 87, 711–717. [Google Scholar]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef]
- Rai, M.; Pandit, R.; Gaikwad, S.; Kövics, G. Antimicrobial peptides as natural bio-preservative to enhance the shelf-life of food. J. Food Sci. Technol. 2016, 53, 3381–3394. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A.S. Production of bioactive peptides using enzymatic hydrolysis and identification antioxidative peptides from patin (Pangasius sutchi) sarcoplasmic protein hydolysate. J. Funct. Foods 2014, 9, 280–289. [Google Scholar] [CrossRef]
- Mokni Ghribi, A.; Maklouf Gafsi, I.; Sila, A.; Blecker, C.; Danthine, S.; Attia, H.; Bougatef, A.; Besbes, S. Effects of enzymatic hydrolysis on conformational and functional properties of chickpea protein isolate. Food Chem. 2015, 187, 322–330. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Zou, Y.; Sun, Z.; Xu, W. Optimization of flavourzyme hydrolysis condition for the preparation of antioxidant peptides from duck meat using response surface methodology. J. Poult. Sci. 2018, 55, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Banan-Mwine Daliri, E.; Oh, D.H.; Lee, B.H. Bioactive Peptides—Review. Foods 2017, 6, 1–21. [Google Scholar]
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Bioactive peptides of animal origin: A review. J. Food Sci. Technol. 2015, 52, 5377–5392. [Google Scholar] [CrossRef]
- Verma, A.K.; Chatli, M.K.; Kumar, P.; Mehta, N. In-vitro assessment of antioxidant and antimicrobial activity of whole porcine-liver hydrolysates and its fractions. Anim. Prod. Sci. 2019, 59, 641–646. [Google Scholar] [CrossRef]
- Ee, K.-Y.; Khoo, L.-Y.; Ng, W.-J.; Wong, F.-C.; Chai, T.-T. Effects of bromelain and trypsin hydrolysis on the phytochemical content, antioxidant activity, and antibacterial activity of roasted butterfly pea seeds. Processes 2019, 7, 534. [Google Scholar] [CrossRef]
- International Standards Meat and Meat Products—Determination of Ash Content; ISO 936; International Organization for Standarization: Geneva, Switzerland, 1998.
- International Standards Meat and Meat Products—Determination of Moisture Content; ISO 1442; International Organization for Standarization: Geneva, Switzerland, 1997.
- International Standards Meat and Meat Products—Determination of Nitrogen Content; ISO 937; International Organization for Standarization: Geneva, Switzerland, 1978.
- AOCS. AOCS Official Procedure Am5-04. Rapid Determination of Oil/Fat Utilizing High Temperature Solvent Extraction; American Oil Chemists Society: Urbana, IL, USA, 2005. [Google Scholar]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH- assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Huan, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Aristizabal, L.S.; Marín, D. Evaluación de la actividad antibacteriana de aceites esenciales y extractos etanólicos utilizando métodos de difusión en agar y dilución en pozo. Sci. Tech. 2012, 17, 152–157. [Google Scholar]
- Alao, B.O.; Falowo, A.B.; Chulayo, A.; Muchenje, V. The potential of animal by-products in food systems: Production, prospects and challenges. Sustainability 2017, 9, 1089. [Google Scholar] [CrossRef]
- Honikel, K.O. Composition and calories. In Handbook of Analysis of Edible Animal By-Products; Nollet, L.M.L., Toldrá, F., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 105–123. [Google Scholar]
- Kakimov, A.; Suychinov, A.; Tsoy, A.; Mustambayev, N.; Ibragimov, N.; Kuderinova, N.; Mirasheva, G.; Yessimbekov, Z. Nutritive and biological value of liver and blood of various slaughtered animals. J. Pharm. Res. Int. 2018, 22, 1–5. [Google Scholar] [CrossRef]
- Seong, P.N.; Cho, S.H.; Park, K.M.; Kang, G.H.; Park, B.Y.; Moon, S.S.; Ba, H. Van Characterization of chicken by-products by mean of proximate and nutritional compositions. Korean J. Food Sci. Anim. Resour. 2015, 35, 179–188. [Google Scholar] [CrossRef]
- Seong, P.N.; Kang, G.H.; Park, K.M.; Cho, S.H.; Kang, S.M.; Park, B.Y.; Moon, S.S.; Ba, H. Van Characterization of Hanwoo bovine by-products by means of yield, physicochemical and nutritional compositions. Korean J. Food Sci. Anim. Resour. 2014, 34, 434–447. [Google Scholar] [CrossRef]
- Seong, P.N.; Park, K.M.; Cho, S.H.; Kang, S.M.; Kang, G.H.; Park, B.Y.; Moon, S.S.; Ba, H. Van Characterization of edible pork by-products by means of yield and nutritional composition. Korean J. Food Sci. Anim. Resour. 2014, 34, 297–306. [Google Scholar] [CrossRef]
- Di Bernardini, R.; Rai, D.K.; Bolton, D.; Kerry, J.; O’Neill, E.; Mullen, A.M.; Harnedy, P.; Hayes, M. Isolation, purification and characterization of antioxidant peptidic fractions from a bovine liver sarcoplasmic protein thermolysin hydrolyzate. Peptides 2011, 32, 388–400. [Google Scholar] [CrossRef]
- Onuh, J.O.; Girgih, A.T.; Aluko, R.E.; Aliani, M. In vitro antioxidant properties of chicken skin enzymatic protein hydrolysates and membrane fractions. Food Chem. 2014, 150, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-K.; Lee, S.-J.; Jeon, B.-T.; Moon, S.-H.; Kim, B.; Park, T.-K.; Han, J.-S.; Park, P.-J. Purification and characterisation of antioxidative peptides from enzymatic hydrolysates of venison protein. Food Chem. 2009, 114, 1365–1370. [Google Scholar] [CrossRef]
- Hidalgo, M.E.; Daroit, D.J.; Folmer Corrêa, A.P.; Pieniz, S.; Brandelli, A.; Risso, P.H. Physicochemical and antioxidant properties of bovine caseinate hydrolysates obtained through microbial protease treatment. Int. J. Dairy Technol. 2012, 65, 342–352. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Ruby-Figueroa, R. Membrane technology for the recovery of high-added value compounds from meat processing coproducts. In Sustainable Meat Production and Processing; Galanakis, C.M., Ed.; Academic Press: London, UK, 2019; pp. 127–143. [Google Scholar]
- Liu, R.; Xing, L.; Fu, Q.; Zhou, G.H.; Zhang, W.G. A review of antioxidant peptides derived from meat muscle and by-products. Antioxidants 2016, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Hayes, K.D.; Shahidi, F. Comparative study on antioxidative activity of yellow stripe trevally protein hydrolysate produced from Alcalase and Flavourzyme. Int. J. Food Sci. Technol. 2008, 43, 1019–1026. [Google Scholar] [CrossRef]
- Jamdar, S.N.; Rajalakshmi, V.; Sharma, A. Antioxidant and ace inhibitory properties of poultry viscera protein hydrolysate and its peptide fractions. J. Food Biochem. 2012, 36, 494–501. [Google Scholar] [CrossRef]
- Phanturat, P.; Benjakul, S.; Visessanguan, W.; Roytrakul, S. Use of pyloric caeca extract from bigeye snapper (Priacanthus macracanthus) for the production of gelatin hydrolysate with antioxidative activity. LWT Food Sci. Technol. 2010, 43, 86–97. [Google Scholar] [CrossRef]
- Damgaard, T.D.; Otte, J.A.; Meinert, L.; Jensen, K.; Lametsch, R. Antioxidant capacity of hydrolyzed porcine tissues. Food Sci. Nutr. 2014, 2, 282–288. [Google Scholar] [CrossRef]
- O’Sullivan, S.M.; Lafarga, T.; Hayes, M.; O’Brien, N.M. Bioactivity of bovine lung hydrolysates prepared using papain, pepsin, and Alcalase. J. Food Biochem. 2017, 41, e12406. [Google Scholar] [CrossRef]
- Bah, C.S.F.; Carne, A.; McConnell, M.A.; Mros, S.; Bekhit, A.E.D.A. Production of bioactive peptide hydrolysates from deer, sheep, pig and cattle red blood cell fractions using plant and fungal protease preparations. Food Chem. 2016, 202, 458–466. [Google Scholar] [CrossRef]
- Liu, Q.; Kong, B.; Xiong, Y.L.; Xia, X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010, 118, 403–410. [Google Scholar] [CrossRef]
- Chang, C.Y.; Wu, K.C.; Chiang, S.H. Antioxidant properties and protein compositions of porcine haemoglobin hydrolysates. Food Chem. 2007, 100, 1537–1543. [Google Scholar] [CrossRef]
- Ajibola, C.F.; Fashakin, J.B.; Fagbemi, T.N.; Aluko, R.E. Effect of Peptide Size on Antioxidant Properties of African Yam Bean Seed (Sphenostylis stenocarpa) Protein Hydrolysate Fractions. Int. J. Mol. Sci. 2011, 12, 6685–6702. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Chatli, M.K.; Mehta, N.; Kumar, P. Efficacy of antioxidant and antimicrobial activity of whole porcine blood hydrolysates and its fractions under in-vitro conditions. Anim. Prod. Sci. 2018, 58, 2084–2090. [Google Scholar] [CrossRef]
- Bah, C.S.F.; Bekhit, A.E.D.A.; Carne, A.; McConnell, M.A. Production of bioactive peptide hydrolysates from deer, sheep and pig plasma using plant and fungal protease preparations. Food Chem. 2015, 176, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.W.; Wong, G.C.L. Antimicrobial peptides and induced membrane curvature: Geometry, coordination chemistry, and molecular engineering. Curr. Opin. Solid State Mater. Sci. 2013, 17, 151–163. [Google Scholar] [CrossRef]
- Beaussart, A.; El-Kirat-Chatel, S. Microbial adhesion and ultrastructure from the single-molecule to the single-cell levels by atomic force microscopy. Cell Surf. 2019, 5, 100031. [Google Scholar] [CrossRef]
- Exner, M.; Bhattacharya, S.; Christiansen, B.; Gebel, J.; Goroncy-Bermes, P.; Hartemann, P.; Heeg, P.; Ilschner, C.; Kramer, A.; Larson, E.; et al. Antibiotic resistance: What is so special about multidrug-resistant Gram-negative bacteria? GMS Hyg. Infect. Control 2017, 12, 1–24. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).