Modification of Vegetable Proteins to Release Bioactive Peptides Able to Treat Metabolic Syndrome—In Silico Assessment
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioactive Peptides Selection
2.2. Scaffold Selection
2.3. Protein Design
2.4. In Silico Evaluation
3. Results and Discussion
3.1. Peptides to Treat Metabolic Syndrome
3.2. Scaffold Selection
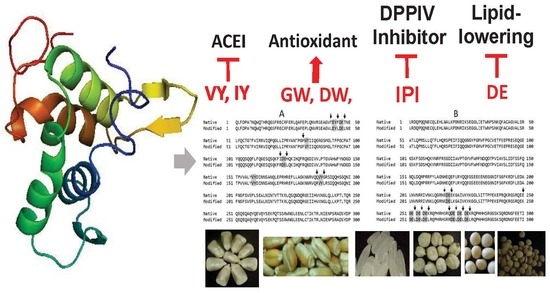
3.3. In Silico Design of a Protein against Metabolc Syndrome
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Mameli, C.; Zuccotti, G.V.; Carnovale, C.; Galli, E.; Nannini, P.; Cervia, D.; Perrotta, C. An update on the assessment and management of metabolic syndrome, a growing medical emergency in pediatric populations. Pharmacol. Res. 2017, 119, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Barbagallo, M. The biology of the metabolic syndrome and aging. Curr. Opin. Clin. Nutr. Metab. Care 2015, 19, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new worldwide definition. Diabetic Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Wagh, A.; Stone, N.J. Treatment of metabolic syndrome. Expert Rev. Cardiovasc. Ther. 2014, 2, 213–228. [Google Scholar] [CrossRef]
- Ilanne-Parika, P.; Toumilehto, J. Lifestyle Intervention of Complications to the Metabolic Syndrome. In The Metabolic Syndrome; Beck-Nielsen, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; p. 64. [Google Scholar]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Daliri, E.D.; Oh, D.H.; Lee, B.H. Bioactive Peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef]
- Iwaniak, A.; Darewicz, M.; Minkiewicz, P. Peptides derived from foods as supportive diet components in the prevention of metabolic syndrome. Compr. Rev. Food Scif. 2017, 17, 63–81. [Google Scholar] [CrossRef]
- Dullius, A.; Fassina, P.; Giroldi, M.; Goettert, M.I.; Volken de Souza, C.F. A biotechnological approach for the production of branched chain amino acid containing bioactive peptides to improve human health: A review. Food Res. Int. 2020, 131, 109002. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, L.; Han, X.; Xin, L.; Meng, Z.; Gong, P.; Cheng, D. Yak milk casein as potential precursor of angiotensin I-converting enzyme inhibitory peptides based on in silico proteolysis. Food Chem. 2018, 254, 340–347. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, W.; Zhu, M.; Xiao, Z. In silico assessment of the potential of patatin as a precursor of bioactive peptides. J. Food Biochem. 2016, 40, 366–370. [Google Scholar] [CrossRef]
- Kang, N.J.; Jin, H.S.; Lee, S.E.; Kim, H.J.; Koh, H.; Lee, D.W. New approaches toward the discovery and evaluation of bioactive peptides from natural resources. Crit. Rev. Environ. Sci. Tec. 2020, 1, 72–103. [Google Scholar] [CrossRef]
- Shewry, P.R.; Napier, J.A.; Tatham, A.S. Seed storage proteins: Structures and biosynthesis. Plant Cell 1995, 7, 945–956. [Google Scholar] [PubMed]
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef]
- Song, M.; Fung, T.T.; Hu, F.; Willett, W.; Longo, V.; Chan, A.; Giovannucci, E. Association of animal and plant protein intake with all-cause cause-specific mortality. JAMA Intern. Med. 2016, 176, 1453–1463. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Dziuba, J.; Iwaniak, A.; Dziuba, M.; Darewicz, M. BIOPEP database and other programs for processing bioactive peptide sequences. J. AOAC Int. 2008, 91, 965–980. [Google Scholar] [CrossRef]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Raghava, G. In silico approach for predicting toxicity of peptides and proteins. PLoS ONE 2013, 8, e73957. [Google Scholar] [CrossRef]
- Ganapathy, V.; Gupta, N.; Martindale, R.G. Protein Digestion and Absorption. In Physiology of the Gastrointestinal Tract; Johnson, L.R., Ed.; Academic Press: Cambridge, MA, USA, 2006; pp. 1667–1692. [Google Scholar]
- Kaiser, S.; Martin, M.; Lunoe, D.; Rudolph, S.; Mertten, S.; Möckel, U.; Deuβen, A.; Henle, T. Tryptophan-containing dipeptides are bioavailable and inhibit plasma human angiotensin-converting enzyme in vivo. Int. Dairy J. 2016, 52, 107–114. [Google Scholar] [CrossRef]
- Tokunaga, K.; Yoshida, C.; Suzuki, K.; Maruyama, H.; Futamura, Y.; Araki, Y.; Mishima, S. Antihypertensive effect of peptides from royal jelly in spontaneously hypertensive rats. Biol. Pharm. Bull. 2004, 27, 189–192. [Google Scholar] [CrossRef]
- Sato, M.; Hosokawa, T.; Yamaguchi, T.; Nakano, T.; Maramoto, K.; Kahara, T.; Funayama, K.; Kobayashi, A.; Nakano, T. Angiotensin I-converting enzyme inhibitory peptides derived from Wakame (Undaria pinnatifida) and their antihypertensive effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2002, 50, 6245–6252. [Google Scholar] [CrossRef]
- Wu, J.; Ding, X. Hypotensive and physiological effect of angiotensin converting enzyme inhibitory peptides derived from soy protein on spontaneously hypertensive rats. J. Agric. Food Chem. 2001, 49, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Wang, B.; Jiang, L.; Liu, C.; Li, B. Hydrophobicity exert different effects on bioavailability and stability of antioxidant peptide fractions from casein during simulated gastrointestinal digestion and Caco-2 cell absorption. Food Res. Int. 2015, 76, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, H.; Aoyagi, T.; Ogawa, K.; Naganawa, H.; Hamada, M.; Takeuchi, T. Diprotins A and B inhibitors of dipeptidyl aminopeptidase IV produced by bacteria. J. Antibiot. 1984, 37, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Nogonierma, A.B.; FitzGerald, R.J. Features of dipeptidyl peptidase IV (DPPIV) inhibitory peptides from dietary proteins. J. Food Biochem. 2017, 43, e12451. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Dong, H.; Su, G.; Zhao, Q.; Zhao, M. Radical scavenging activities of Tyr-, Trp, Cys, and Met-Gly and their protective effects against AAPH-induced oxidative damage in human erythrocytes. Food Chem. 2016, 197, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Fatma, Z.N.; Wahyu, B. The effect of peptide (Asp-Glu) synthetic base on sterilized fermented soymilk on lipid profile of Sprague Dawley Rats. Int. Food Res. J. 2013, 20, 3047–3052. [Google Scholar]
- Pooj, K.; Rani, S.; Prakash, B. In silico approaches towards the exploration of rice bran proteins-derived angiotensin-I-converting enzyme inhiitory peptides. Int. J. Food Prop. 2017, 20, 2178–2191. [Google Scholar]
- Matsui, T.; Hayshi, A.; Tamaya, K.; Matsumoto, K.; Kawasaki, T.; Mursksmi, K.; Kimoto, K. Depressor effect induced by dipeptide, Val-Tyr, in hypertensive transgenic mice is due, in part, to the suppresion of human circulating renin-angiotensin system. Clin. Exp. Pharmacol. Physiol. 2003, 30, 262–265. [Google Scholar] [CrossRef]
- Saito, Y.; Wanezaki, K.; Kawato, A.; Imayasu, S. Structure and activity of angiotensin I converting enzyme inhibitory peptides from sake and sake less. Biosci. Biotechnol. Biochem. 1994, 58, 1767–1771. [Google Scholar] [CrossRef]
- Kahijara, R.; Shibata, K.; Nakatsu, S.; Sakamoto, K. Production of angiotensin I-converting enzyme-inibitory peptides in a freeze-thaw infusión-treated soybean. Food Sci. Technol. Res. 2011, 17, 561–565. [Google Scholar]
- Wu, H.; He, H.; Chen, X.; Sun, C.; Zhang, Y.; Zhou, B. Purification and identification of novel angiotensin-I-converting enzyme inhibitory peptide from shark meat hydrolysate. Process Biochem. 2008, 43, 457–461. [Google Scholar] [CrossRef]
- Jahandideh, F.; Chakrabart, S.; Davige, S.T.; Wu, J. Antioxidant peptides identified from ovotransferrin by the ORAC method did not show anti-inflammatory and antioxidant activities in endotelial cells. J. Agric. Food Chem. 2015, 645, 113–119. [Google Scholar]
- Chai, H.J.; Wu, C.J.; Yang, S.H.; Li, T.L.; Pan, B.S. Peptides from hydrolysate of lantern fish (Benthosema pterotum) proved neuroprotective in vitro and in vivo. J. Funct. Foods. 2016, 24, 438–449. [Google Scholar] [CrossRef]

| Peptide | Activity | IC50 (µM) | In Vivo | Reference |
|---|---|---|---|---|
| VY | ACEI | 7.1 | ✓ | [30] |
| VW | ACEI | 1.4 | x | [31] |
| IW | ACEI | 4.7 | ✓ | [20] |
| IY | ACEI | 2.69 | ✓ | [32] |
| EY | ACEI | 2.68 | x | [33] |
| DG | ACEI | 12.3 | ✓ | [22] |
| IPI | DPPIV Inhibitor | 1.1 µg/ml | x | [26] |
| GW | Antioxidant | - | x | [34] |
| DW | Antioxidant | - | x | [35] |
| CG | Antioxidant | - | x | [24] |
| DE | Lipid-lowering | - | ✓ | [28] |
| Uniprot Entry | Sequence Lenght | IW | VW | VY | IY | EY | DG | IPI | GW | CG | DW | DE | Total | A | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soy | |||||||||||||||
| β-conglicinin chain alfa | P11827 | 617 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 7 | 0.011 |
| β-conglicinin chain beta | P25974 | 426 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 0.007 |
| Albumin 2S | P19594 | 137 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0.015 |
| Basic 7S Globulin | P13917 | 403 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 5 | 0.012 |
| Amaranth | |||||||||||||||
| 11S Globulin | Q38712 | 501 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 8 | 0.016 |
| Oat | |||||||||||||||
| Avenin 3 | P80356 | 201 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.005 |
| 11S Globulin | Q38780 | 503 | 0 | 0 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 8 | 0.016 |
| Beans | |||||||||||||||
| Phaseolin | P80463 | 404 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0.007 |
| Globulin-1 | A6YNT0 | 224 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 3 | 0.013 |
| Alpha-zein 16 | P04700 | 242 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.004 |
| Gamma-zein | C0P381 | 267 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 3 | 0.011 |
| Rice | |||||||||||||||
| Prolamin PPROLINE 4E | Q0DJ45 | 131 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.015 |
| Cupincin | B8AL97 | 436 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 5 | 7 | 0.016 |
| Globulin | P29835 | 164 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0.012 |
| Glutelin | Q6T725 | 471 | 0 | 0 | 3 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 3 | 11 | 0.023 |
| Glutelin Type A-2 | P07730 | 475 | 0 | 0 | 3 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 8 | 0.017 |
| Glutelin Type B-2 | Q02897 | 481 | 0 | 0 | 4 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 2 | 10 | 0.021 |
| Maize | |||||||||||||||
| Globulin-1 S Allele | P15590 | 487 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 3 | 0.006 |
| 22 kDa alpha zein 4 | O48966 | 245 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.000 |
| 50 kDa gamma zein | C0P381 | 267 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 3 | 0.011 |
| Globulin-2 | Q7M1Z8 | 431 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 4 | 0.009 |
| Globulin-1 | A6YNT0 | 224 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 3 | 0.013 |
| 18 kD delta zein | Q946V9 | 190 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.000 |
| Prolamin PPROL 17 | B6UH22 | 164 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 4 | 0.024 |
| Chickpea | |||||||||||||||
| Legumin | Q9SMJ4 | 475 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 7 | 10 | 0.021 |
| Globulin-1 S Allele | A0A1S2YZ56 | 621 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 1 | 1 | 0 | 3 | 9 | 0.014 |
| 11S Globulin seed storage | A0A1S2YGT3 | 348 | 1 | 1 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 7 | 0.020 |
| Glutelin Type-A 2-Like | A0A1S2YJV5 | 200 | 0 | 1 | 1 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 6 | 0.030 |
| Lentil | |||||||||||||||
| Albumin S | P86782 | 37 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.000 |
| Broad Bean | |||||||||||||||
| Legumin type B | P05190 | 462 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 6 | 0.013 |
| Vicilin | P08438 | 436 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 5 | 0.011 |
| Convicilin | B0BCL8 | 469 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 5 | 0.011 |
| Wheat | |||||||||||||||
| Glutenin subunit DX5 | P10388 | 827 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.000 |
| Avenin-like B1 | Q2A783 | 267 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.007 |
| Alpha/Beta Gliadin | P02863 | 266 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.004 |
| Alpha/Beta Gliadin A-I | P04721 | 242 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.000 |
| Alpha/Beta Gliadin A-II | P04722 | 271 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.004 |
| Alpha/Beta Gliadin A-III | P04723 | 262 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.004 |
| Alpha/Beta Gliadin A-IV | P04724 | 277 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.004 |
| Alpha/Beta Gliadin A-V | P04725 | 299 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.003 |
| Glutelin Type A-1 | M7ZVJ6 | 299 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 1 | 0 | 6 | 0.020 |
| Protein | Number of Cleavage Sites | Fragments | Selected Peptides Released | |||
|---|---|---|---|---|---|---|
| Native | Modified | Native | Modified | Native | Modified | |
| Glutelin Q6T725 | 160 | 182 | 144 | 160 | 1 | 8 |
| Legumin Q9SMJ4 | 192 | 206 | 167 | 180 | 0 | 10 |
| Glutelin type-A 2 A0A1S2YJV5 | 143 | 154 | 123 | 134 | 1 | 6 |
| Prolamin PPROL 17 B6UH22 | 42 | 49 | 39 | 47 | 0 | 4 |
| Glutelin type-B 2 Q02897 | 165 | 179 | 144 | 159 | 2 | 9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado-Torres, D.A.; Fernández-Velasco, D.A.; Morales-Olán, G.; Rosas-Cárdenas, F.d.F.; Luna-Suárez, S. Modification of Vegetable Proteins to Release Bioactive Peptides Able to Treat Metabolic Syndrome—In Silico Assessment. Appl. Sci. 2020, 10, 2604. https://doi.org/10.3390/app10072604
Maldonado-Torres DA, Fernández-Velasco DA, Morales-Olán G, Rosas-Cárdenas FdF, Luna-Suárez S. Modification of Vegetable Proteins to Release Bioactive Peptides Able to Treat Metabolic Syndrome—In Silico Assessment. Applied Sciences. 2020; 10(7):2604. https://doi.org/10.3390/app10072604
Chicago/Turabian StyleMaldonado-Torres, Diego Armando, D. Alejandro Fernández-Velasco, Gema Morales-Olán, Flor de Fátima Rosas-Cárdenas, and Silvia Luna-Suárez. 2020. "Modification of Vegetable Proteins to Release Bioactive Peptides Able to Treat Metabolic Syndrome—In Silico Assessment" Applied Sciences 10, no. 7: 2604. https://doi.org/10.3390/app10072604
APA StyleMaldonado-Torres, D. A., Fernández-Velasco, D. A., Morales-Olán, G., Rosas-Cárdenas, F. d. F., & Luna-Suárez, S. (2020). Modification of Vegetable Proteins to Release Bioactive Peptides Able to Treat Metabolic Syndrome—In Silico Assessment. Applied Sciences, 10(7), 2604. https://doi.org/10.3390/app10072604