Chromium(III) Removal from Wastewater by Chitosan Flakes
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
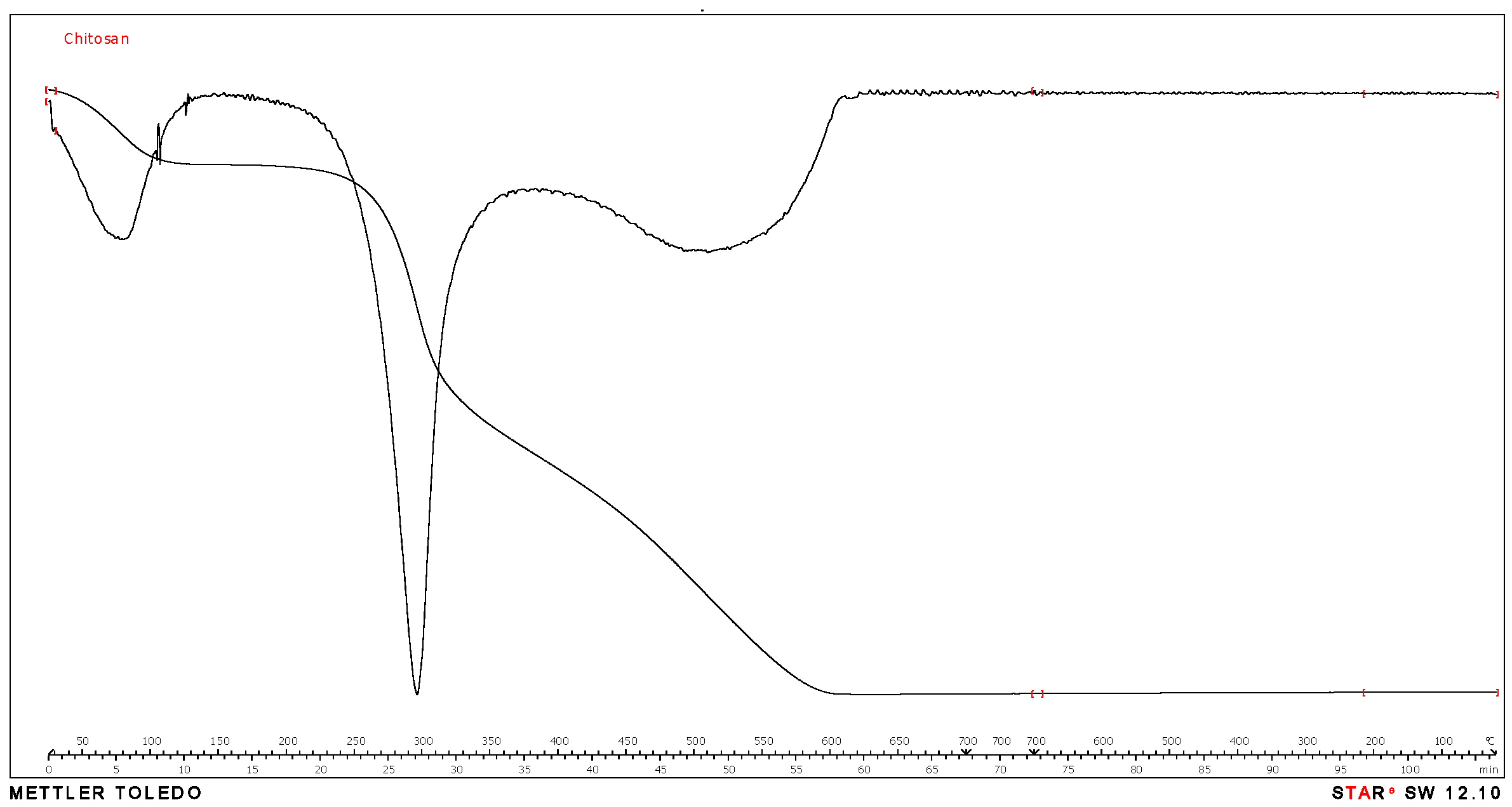
2.1. Material and Reagents
2.2. Adsorption Tests
3. Results
3.1. Adsorption Dynamics
- i.
- the pseudo-first-order equation described by Lagergren [25], which can be rearranged to obtain a linear form as shown by Equation (1):
- ii.
- a pseudo-second-order equation based on the equilibrium adsorption capacity, which can be expressed as in Equation (2):
3.2. Grain Size Effect
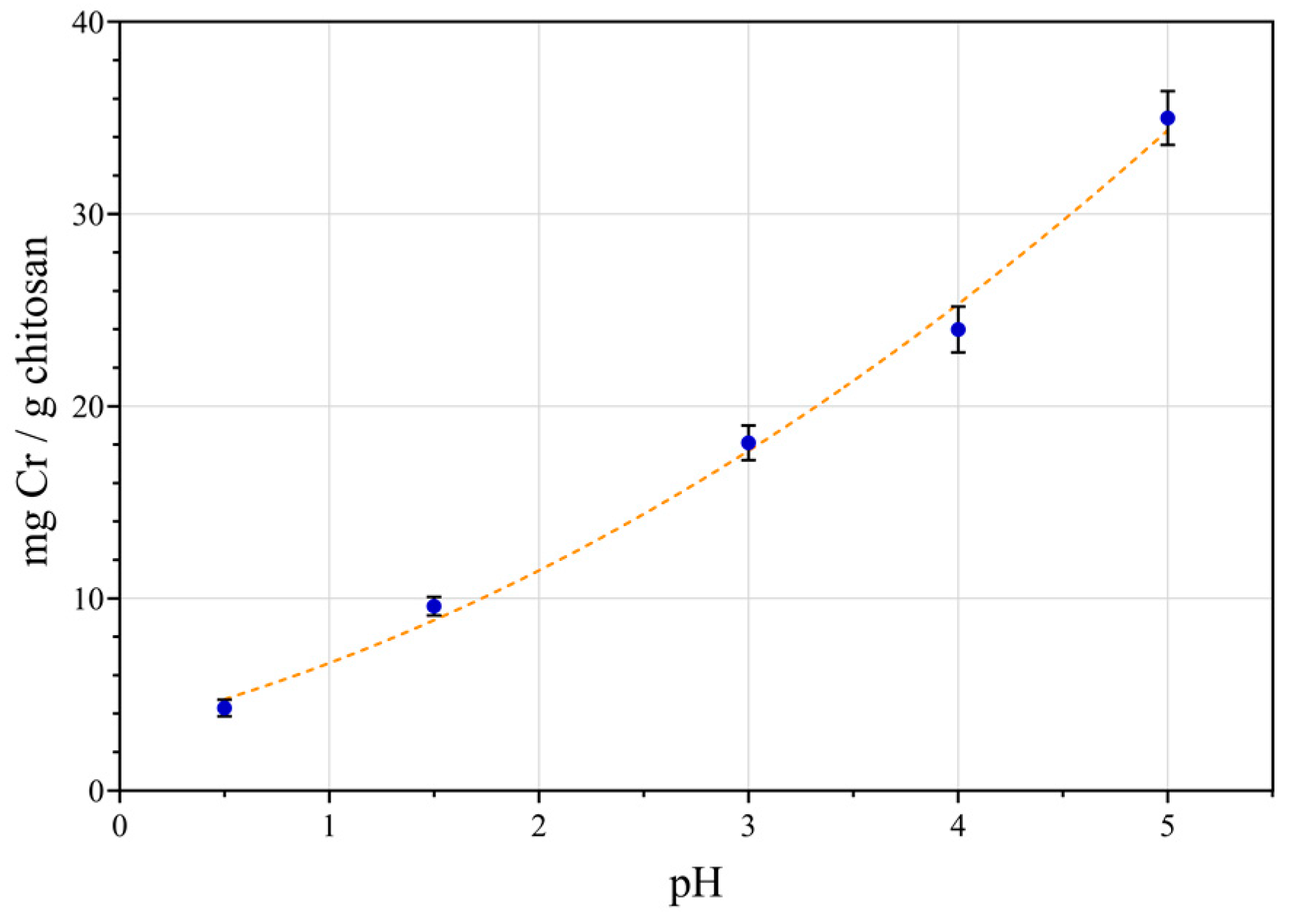
3.3. Effect of pH
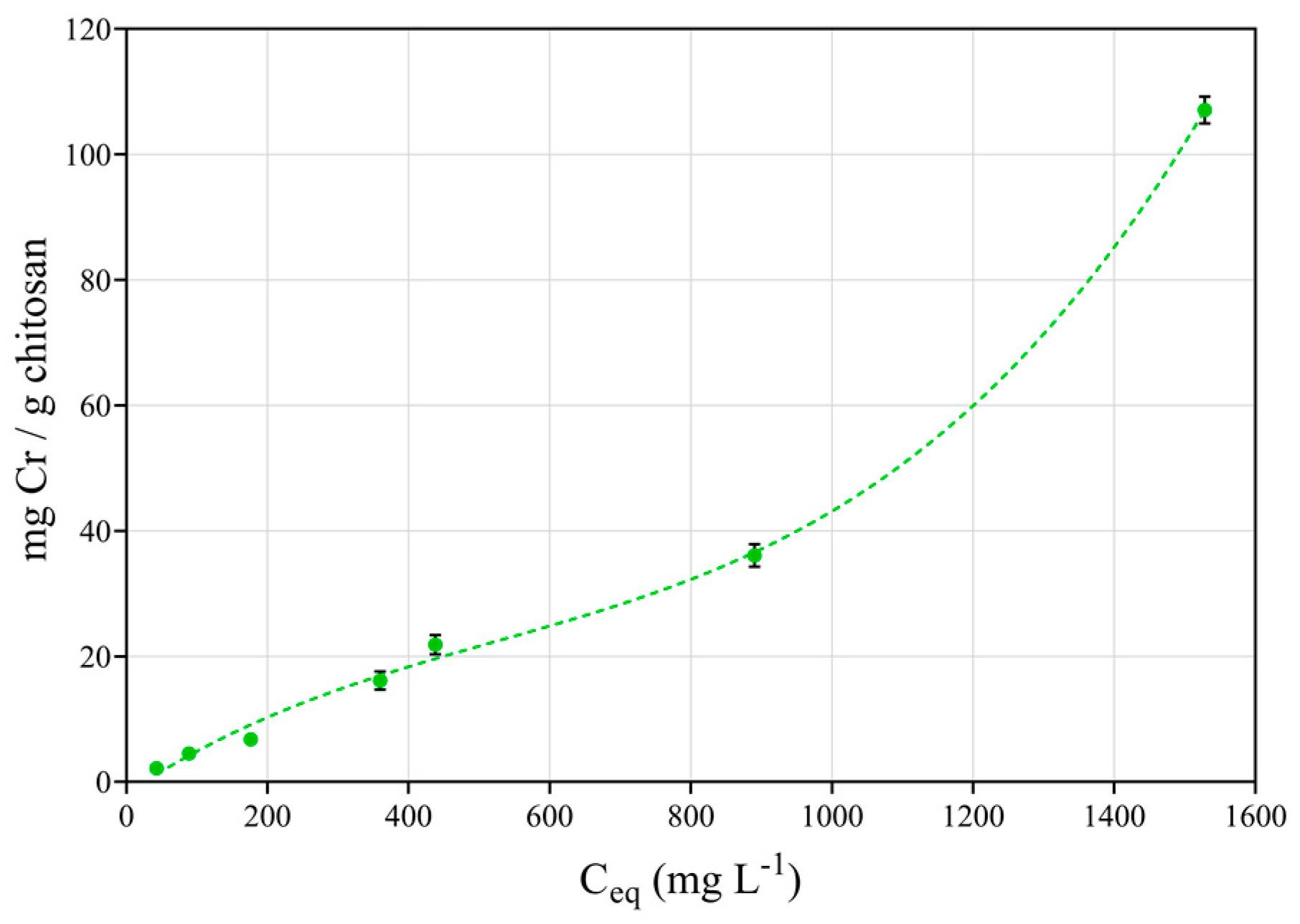
3.4. Adsorption Isotherms
3.5. Desorption
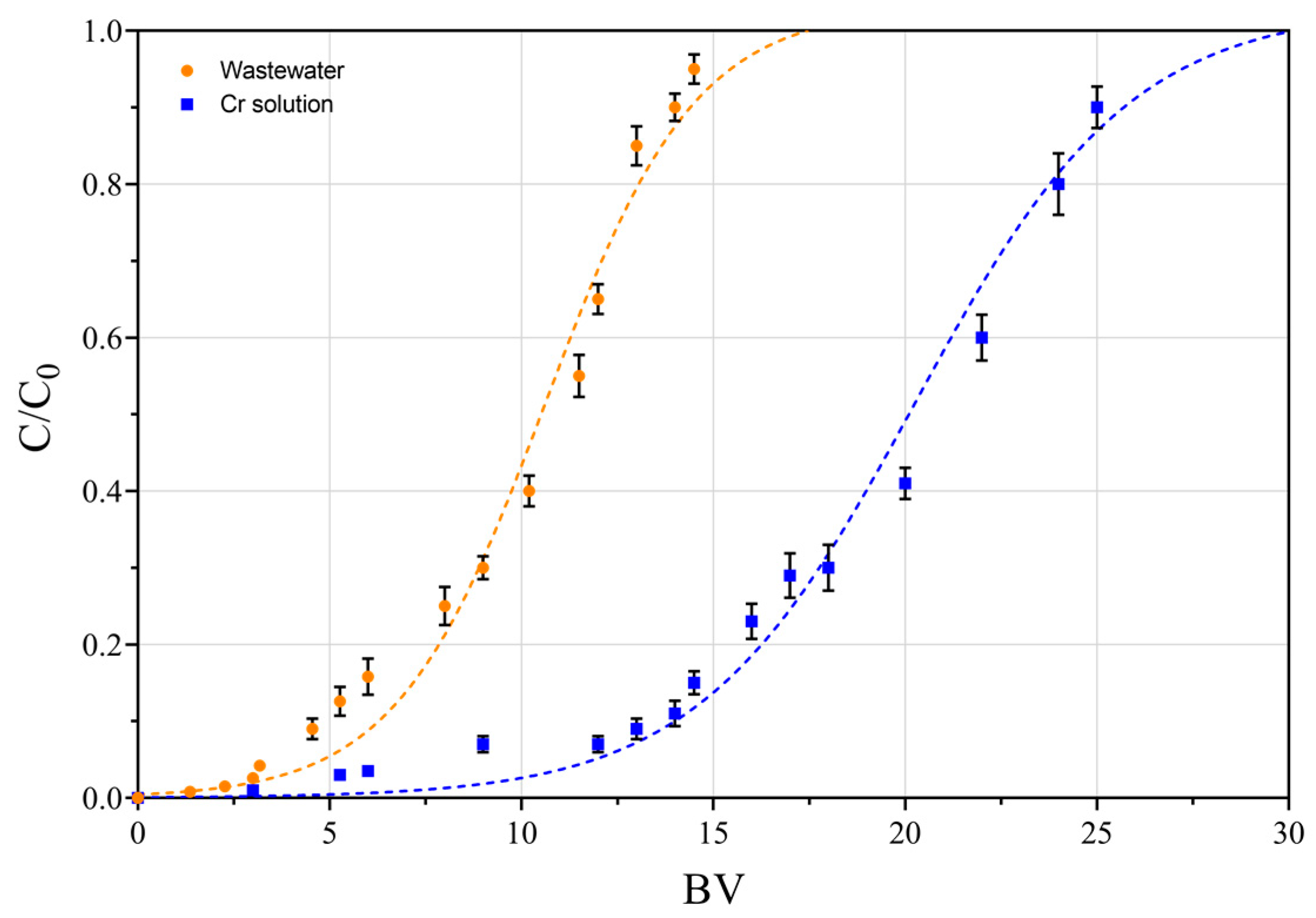
3.6. Dynamic Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pietrelli, L.; Ippolito, N.M.; Reverberi, A.P.; Vocciante, M. Heavy Metals Removal and Recovery from Hazardous Leather Sludge. Chem. Eng. Trans. 2019, 76, 1327–1332. [Google Scholar]
- Rengaraj, S.; Yeon, K.H.; Moon, S.H. Removal of chromium from water and wastewater by ion exchange resins. J. Hazard. Mater. 2001, 87, 273–287. [Google Scholar] [CrossRef]
- Vocciante, M.; Reverberi, A.P.; Pietrelli, L.; Dovì, V.G. Improved remediation processes through cost-effective estimation of soil properties from surface measurements. J. Clean. Prod. 2017, 167, 680–686. [Google Scholar] [CrossRef]
- Vocciante, M.; Finocchi, A.; De Folly, D.; Auris, A.; Conte, A.; Tonziello, J.; Pola, A.; Reverberi, A.P. Enhanced Oil Spill Remediation by Adsorption with Interlinked Multilayered Graphene. Materials 2019, 12, 2231. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Vocciante, M.; De Folly, D.; Auris, A.; Finocchi, A.; Tagliabue, M.; Bellettato, M.; Ferrucci, A.; Reverberi, A.P.; Ferro, S. Adsorption of ammonium on clinoptilolite in presence of competing cations: Investigation on groundwater remediation. J. Clean. Prod. 2018, 198, 480–487. [Google Scholar] [CrossRef]
- El-Shafey, E.I.; Cox, M.; Pichugin, A.A.; Appleton, Q. Application of a carbon sorbent for the removal of cadmium and other heavy metal ions from aqueous solution. J. Chem. Technol. Biotechnol. Intern. Res. Process Environ. Clean Technol. 2002, 77, 429–436. [Google Scholar] [CrossRef]
- Garcia-Reyes, R.B.; Rangel-Mendez, J.R. Adsorption kinetics of chromium (III) ions on agro-waste materials. Bioresour. Technol. 2010, 101, 8099–8108. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Liang, H.W.; Lu, Y.; Cui, C.H.; Yu, S.H. Synthesis of an attapulgite clay@ carbon nanocomposite adsorbent by a hydrothermal carbonization process and their application in the removal of toxic metal ions from water. Langmuir 2011, 27, 8998–9004. [Google Scholar] [CrossRef]
- Pietrelli, L.; Ippolito, N.M.; Ferro, S.; Dovì, V.G.; Vocciante, M. Removal of Mn and As from drinking water by red mud and pyrolusite. J. Environ. Manag. 2019, 237, 526–533. [Google Scholar] [CrossRef]
- Pietrelli, L.; Ferro, S.; Vocciante, M. Raw materials recovery from spent hydrochloric acid-based galvanizing wastewater. Chem. Eng. J. 2018, 341, 539–546. [Google Scholar] [CrossRef]
- Chiou, M.S.; Li, H.Y. Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads. Chemosphere 2003, 50, 1095–1105. [Google Scholar] [CrossRef]
- Pietrelli, L.; Xingrong, L. Chitosan membrane: Tool for chromium (III) recovery from aqueous solutions. Ann. Chim. J. Anal. Environ. Cult. Herit. Chem. 2004, 94, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.A.; Kim, B.K.; Park, H.J. Preparation of acetylated chitosan sponges (chitin sponges). J. Appl. Polym. Sci. 2010, 117, 1618–1623. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Bakshia, P.S.; Selvakumara, D.; Kadirvelub, K.; Kumara, N.S. Chitosan as an Environment Friendly Biomaterial—A Review on Recent Modifications and Applications. Int. J. Biol. Macromol. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of chitosan aerogels as promising carriers for drug delivery: A review. Carbohydr. Polym. 2019, 231, 115744. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology: A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
- Fendorf, S.E. Surface reactions of chromium in soils and waters. Geoderma 1995, 67, 55–71. [Google Scholar] [CrossRef]
- Maruca, R.; Suder, B.J.; Wightman, J.P. Interaction of heavy metals with chitin and chitosan. III. Chromium. J. Appl. Polym. Sci. 1982, 27, 4827–4837. [Google Scholar] [CrossRef]
- Chui, V.W.D.; Mok, K.W.; Ng, C.Y.; Luong, B.P.; Ma, K.K. Removal and recovery of copper (II), chromium (III), and nickel (II) from solutions using crude shrimp chitin packed in small columns. Environ. Int. 1996, 22, 463–468. [Google Scholar] [CrossRef]
- Ngah, W.W.; Kamari, A.; Fatinathan, S.; Ng, P.W. Adsorption of chromium from aqueous solution using chitosan beads. Adsorption 2006, 12, 249–257. [Google Scholar] [CrossRef]
- Lerivrey, J.; Dubois, B.; Decock, P.; Micera, G.; Urbanska, J.; Kozłowski, H. Formation of D-glucosamine complexes with Cu (II), Ni (II) and Co (II) ions. Inorg. Chim. Acta 1986, 125, 187–190. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. Kungliga Svenska Vetenskapsakademiens Handlingar 1898, 24, 1–39. [Google Scholar]
- Weber, W.J.; Morris, J.L. Kinetics of adsorption on carbon from solutions. J. Sanit. Eng. Div. Proc. Am. Soc. Civ. Eng. 1963, 89, 31–60. [Google Scholar]
- McKay, G. Adsorption of dyestuffs from aqueous solutions with activated carbon I: Equilibrium and batch contact-time studies. J. Chem. Technol. Biotechnol. 1982, 32, 759–772. [Google Scholar] [CrossRef]
- Annadurai, G.; Ling, L.Y.; Lee, J.F. Adsorption of reactive dye from an aqueous solution by chitosan: Isotherm, kinetic and thermodynamic analysis. J. Hazard. Mater. 2008, 152, 337–346. [Google Scholar] [CrossRef]
- Annadurai, G.; Chellapandian, M.; Krishnan, M.R.V. Adsorption of reactive dye on chitin. Environ. Monit. Assess. 1999, 59, 111–119. [Google Scholar] [CrossRef]
- Udaybhaskar, P.; Iyengar, L.; Rao, A.P. Hexavalent chromium interaction with chitosan. J. Appl. Polym. Sci. 1990, 39, 739–747. [Google Scholar] [CrossRef]
- Singh, P.; Nagendran, R. A comparative study of sorption of chromium (III) onto chitin and chitosan. Appl. Water Sci. 2016, 6, 199–204. [Google Scholar] [CrossRef]
- Eiden, C.A.; Jewell, C.A.; Wightman, J.P. Interaction of lead and chromium with chitin and chitosan. J. Appl. Polymer Sci. 1980, 25, 1587–1599. [Google Scholar] [CrossRef]
- Boddu, V.M.; Abburi, K.; Talbott, J.L.; Smith, E.D. Removal of hexavalent chromium from wastewater using a new composite chitosan biosorbent. Environ. Sci. Technol. 2003, 37, 4449–4456. [Google Scholar] [CrossRef] [PubMed]
- Hsien, T.Y.; Rorrer, G.L. Effects of acylation and crosslinking on the material properties and cadmium ion adsorption capacity of porous chitosan beads. Sep. Sci. Technol. 1995, 30, 2455–2475. [Google Scholar] [CrossRef]
- Kubota, N.; Kikuchi, Y. Macromolecular complexes of chitosan. In Polysccharides; Dumitriu, S., Ed.; Dekker: New York, NY, USA, 1998; pp. 595–628. [Google Scholar]
- Bassi, R.; Prasher, O.; Simpson, B.K. Removal of selected metal ions from aqueous solutions using chitosan flakes. Sep. Sci. Technol. 2000, 35, 547–560. [Google Scholar] [CrossRef]
- Patel, H. Fixed-bed column adsorption study: A comprehensive review. Appl. Water Sci. 2019, 9, 45. [Google Scholar] [CrossRef]

| 1st Order Kinetic Model | 2nd Order Kinetic Model | Intraparticle Diffusion Model | |||||
|---|---|---|---|---|---|---|---|
| k1 | qe, cal | R2 | k2 | qe, cal | R2 | ki | R2 |
| (min−1) | (mg g−1) | (g mg−1 min−1) | (mg g−1) | (g mg−1 min−1) | |||
| 1.07 × 10−2 | 9.32 | 0.7838 | 5.07 × 10−3 | 23.89 | 0.9987 | 1.711 | 0.9199 |
| Cr(III) (ppm) | pH |
|---|---|
| 50 | 5.16 |
| 100 | 5.02 |
| 200 | 4.98 |
| 400 | 4.67 |
| 500 | 4.47 |
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| Q° (mg g−1) | kL | R2 | RL | kF (mg g−1) | 1/n | R2 |
| 138.04 | 3.7 × 10−4 | 0.9925 | 0.575 | 0.035 | 1.061 | 0.9694 |
| Reagent | 1st Cycle | 2nd Cycle | 3rd Cycle | 4th Cycle | 5th Cycle |
|---|---|---|---|---|---|
| EDTA 0.05 M | 53.3 | - | - | - | - |
| EDTA 0.1 M | 76.5 | 73.4 | 70.6 | 71.7 | 72.1 |
| H2SO4 0.05 M | 37.8 | - | - | - | - |
| H2SO4 0.1 M | 45.3 | 46.5 | 40.7 | 43.1 | 39.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrelli, L.; Francolini, I.; Piozzi, A.; Sighicelli, M.; Silvestro, I.; Vocciante, M. Chromium(III) Removal from Wastewater by Chitosan Flakes. Appl. Sci. 2020, 10, 1925. https://doi.org/10.3390/app10061925
Pietrelli L, Francolini I, Piozzi A, Sighicelli M, Silvestro I, Vocciante M. Chromium(III) Removal from Wastewater by Chitosan Flakes. Applied Sciences. 2020; 10(6):1925. https://doi.org/10.3390/app10061925
Chicago/Turabian StylePietrelli, Loris, Iolanda Francolini, Antonella Piozzi, Maria Sighicelli, Ilaria Silvestro, and Marco Vocciante. 2020. "Chromium(III) Removal from Wastewater by Chitosan Flakes" Applied Sciences 10, no. 6: 1925. https://doi.org/10.3390/app10061925
APA StylePietrelli, L., Francolini, I., Piozzi, A., Sighicelli, M., Silvestro, I., & Vocciante, M. (2020). Chromium(III) Removal from Wastewater by Chitosan Flakes. Applied Sciences, 10(6), 1925. https://doi.org/10.3390/app10061925









