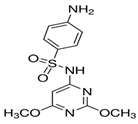
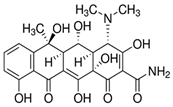
3.1. Phytotoxic Assessment
Instead of the number of germinated seeds, root length was taken as an endpoint for analysis, an approach consistent with the previous study for organic contaminants [
12,
23].
Table 2 lists the EC
50 values of the VAs tested on lettuce, carrot, and pepper seeds after the seed germination test. Carrot seeds were found to be the most susceptible to VAs and had the lowest EC
50 value of 7.49 mg kg
−1 for SDZ antibiotics. On the other hand, pepper seeds presented the least susceptibility to the VAs. STR antibiotics were the least toxic compound, especially towards pepper seeds with EC
50 value > 300 mg kg
−1 (306.84 mg kg
−1). Comparatively, SDZ, and OTC antibiotics were more toxic with EC
50 values < 100 mg kg
−1.
From the seed germination test, it was evident that the effect of VAs on plant seed germination varied depending on both the type of VAs and the plant species used. Among the three-plant species studied, carrot was the most susceptible to the VAs depending upon the antibiotic type. SDZ antibiotics were most toxic to plant seed germination whereas; STR antibiotic was the least toxic.
Based on the plant growth tests in soil, only SDZ antibiotics affected the growth of plants significantly, EC
50 value less than 100 mg kg
−1 (
Table 3). SDZ was the most toxic and visible plant growth observed at concentrations of 300 mg kg
−1 and above. Similar observations have been seen by Migliore et al. 1998, where sulphamethoxine at 300 mg kg
−1 depressed the growth of
Hordeum disthicum both in vitro and in soil [
24]. In contrast to the seed germination test, which showed significant inhibitory effects, the EC
50 values of OTC were near or more than 200 mg kg
−1 for plant growth in soil. STR antibiotics showed no evident growth inhibition to the test plants.
Through this study, we established species variability to VAs toxicity. As found through the seed germination test, pepper was the least sensitive to the VAs, which was translated in the plant growth tests in the soil as well (
Table 3). On the other hand, carrot plants were the most sensitive to VAs administration. The negative effect of VAs in lettuce plants, sampled after 30 days of growth in soil, was evident. The increasing concentration of SDZ in the soil-manure mixture hampered both root length and foliar development. On the other hand, in comparison with control, root length, instead of foliar development, was more compromised with OTC and STR treatments. Previous studies have reported species variability to VAs treatment [
5,
12]. Chlortetracycline and oxytetracycline enhanced the growth of radish and wheat, but corn plants remained unaffected [
5]. A study reported a higher sensitivity of sweet oat and rice in comparison with cucumber [
12]. Based on the EC
50 values (in this study), only SDZ antibiotics may affect the growth of lettuce, carrot, and pepper plants in soil.
3.2. VAs Accumulation and Bio-Concentration
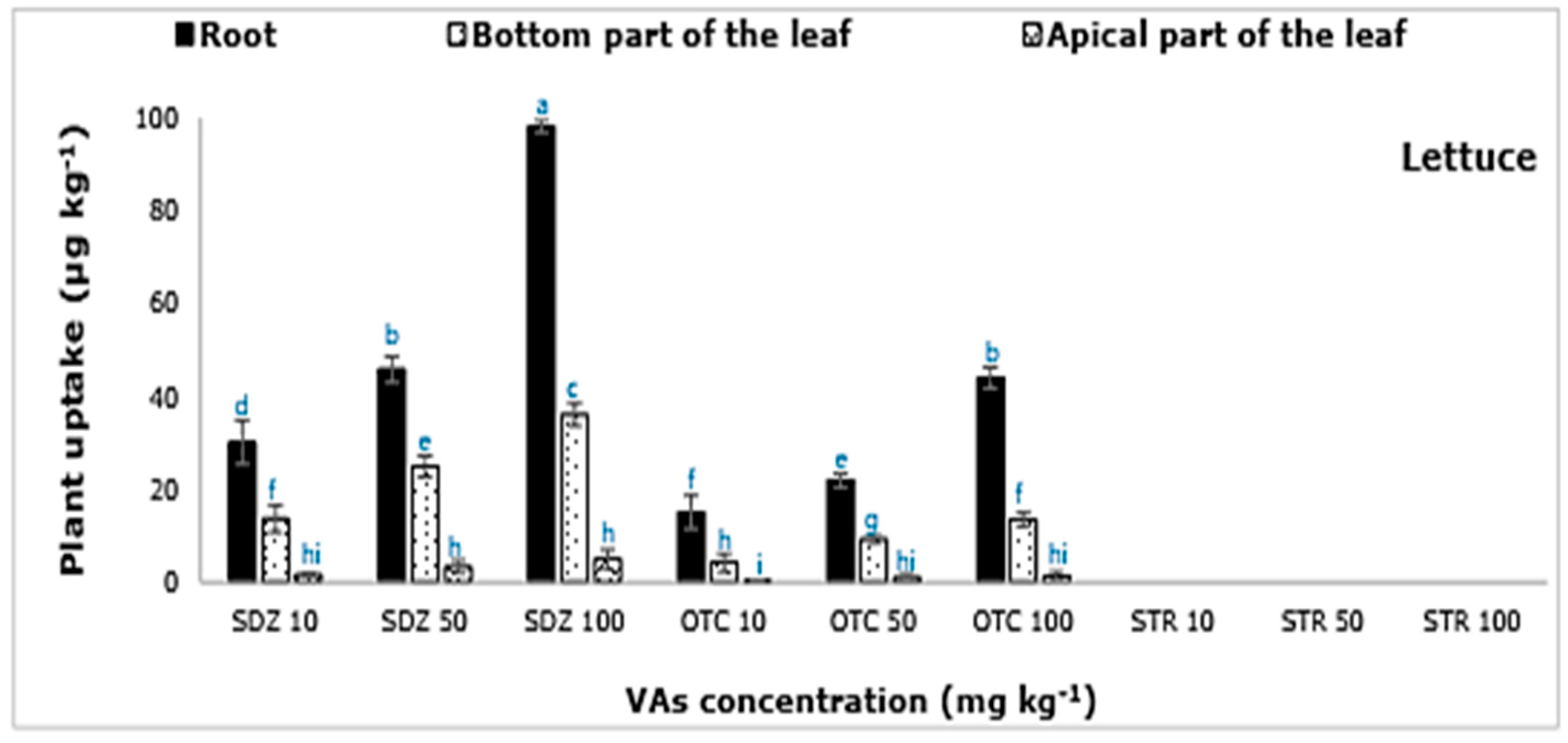
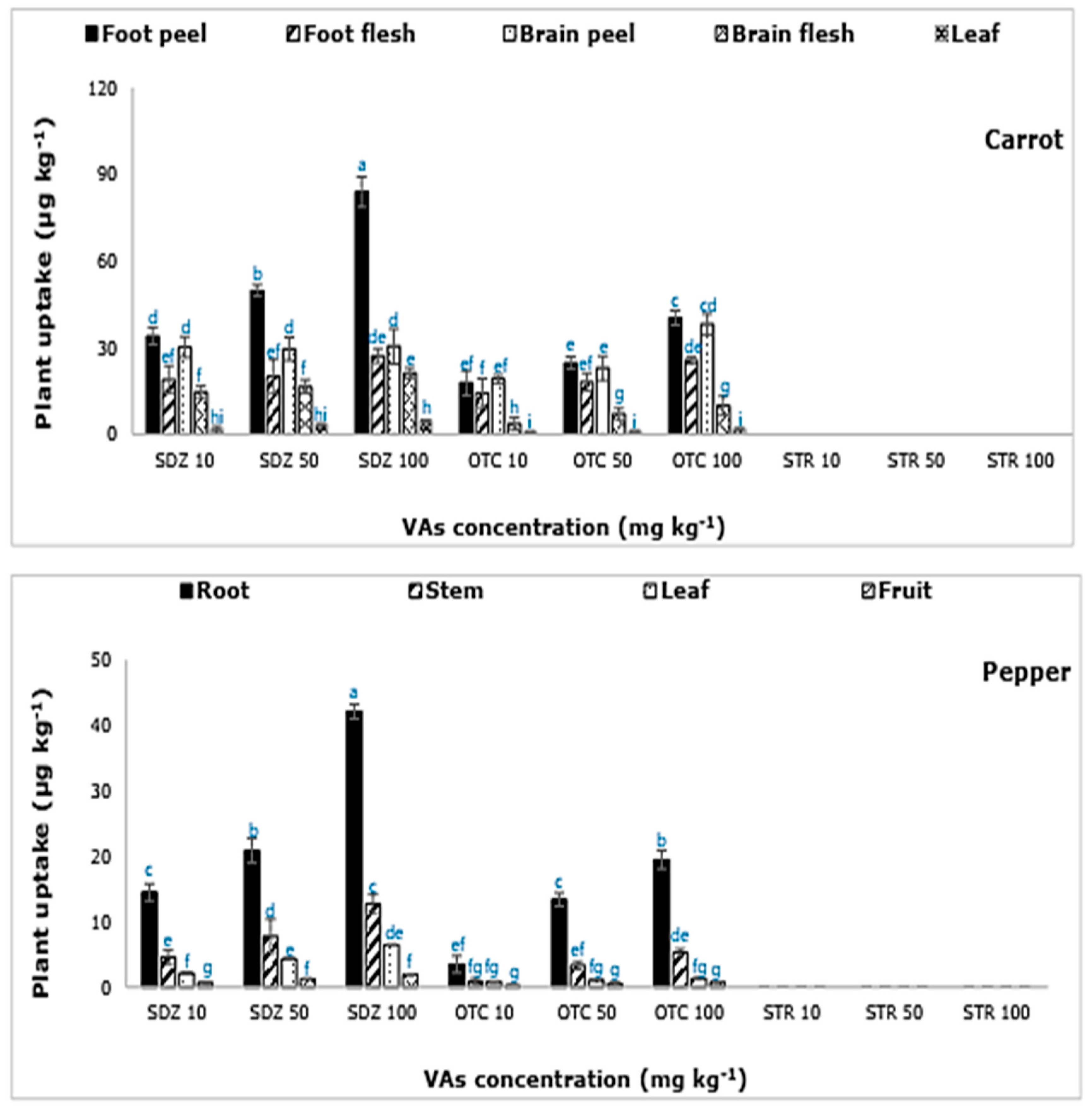
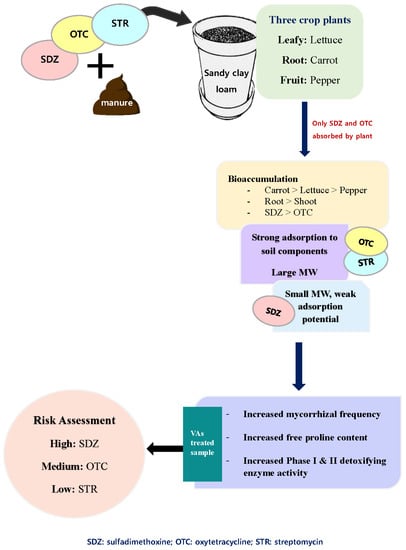
In this study, only the active compounds of SDZ and OTC were extracted from the plant samples (
Figure 1). However, STR antibiotics could be successfully extracted from soil (
Table 4), indicating the failure of test plants to absorb and translocate the antibiotic. Interestingly all three test plants absorbed SDZ and OTC but not STR antibiotics, which can be explained based on the differences in adsorption coefficients (K
d) of these antibiotics in soil. STR is a strongly basic antibiotic known to form strong complexes with the soil components, especially clay, making it highly unavailable [
25]. Since the soil used in this study was sandy clay loam with 30.73% clay content, STR can quickly form strong complexes with clay. Another explanation is the higher molecular weight of STR antibiotics (728.69 g mol
−1), almost double the mass of SDZ and OTC molecule. Such a large molecule can’t be taken up by plants easily, both in mass flow (transpiration) or as active uptake. Similar conclusions have been drawn from a previous study where tylosin antibiotic with a large molar mass of 916.1 g mol
−1 was not taken up by green onion, cabbage, and corn plants [
6].
On the contrary, all three plants (lettuce, carrot, and pepper) absorbed SDZ and OTC antibiotics (
Figure 1). The active molecules of SDZ and OTC were recovered from all the plant parts examined. The amount of SDZ and OTC extracted, from the plant parts, increased with increasing concentration of the VAs in the soil-manure mixture, indicating a higher absorption of VAs by plants as the amount of VAs increases in the soil-manure mix. The maximum amount of SDZ and OTC was extracted from carrot plants, followed by lettuce and pepper. The highest plant-tissue concentration was found in the carrot and lettuce plant, followed by pepper (
Figure 1). The concentration of SDZ extracted from lettuce, carrot, and pepper tissues were ≈ 0.2%, 0.4%, and 0.1% of the amount applied to the soil through manure. On the other hand, OTC concentration in lettuce, carrot, and pepper tissues was ≈ 0.1%, 0.2%, and 0.05%, respectively. A higher accumulation of VAs in the roots than in aerial parts was evident. In carrot roots, the concentration of VAs was higher in the peel/skin in comparison to the flesh. A similar observation where
Solanum tuberosum tubers accumulated a higher percentage of sulfamethazine in their peels [
26].
The calculated BCF values for root and shoots of lettuce, carrot, and pepper plants grown in VAs amended soil presented in
Table 5. BCF values increased with increasing concentration of VAs in the soil-manure mixture. Among the three test plants, the highest BCF was reported in carrot plants, followed by lettuce and pepper. The BCF values for lettuce plants ranged from 0.065 ± 0.001 to 2.307 ± 0.008 and 0.227 ± 0.004 to 6.103 ± 0.005 μg kg
−1 for OTC and SDZ, respectively. In carrot, BCF for OTC and SDZ ranged from 0.010 ± 0.054 to 2.132 ± 0.003 and 0.018 ± 0.023 to 8.859 ± 0.002 μg kg
−1, respectively. On the other hand, BCF in pepper ranged from 0.033 ± 0.010 to 0.474 ± 0.118, and 0.066 ± 0.005 to 3.361 ± 0.099 μg kg
−1 for OTC and SDZ, respectively.
The level of VAs sensitivity and eventual bio-accumulation was greatly affected by plant type. For instance, carrot is a root vegetable and significantly affected by VAs. Since roots are the first plant organ to come in contact with soil and its contaminants, carrot a root vegetable was more sensitive to VAs. As a result, there was a higher accumulation of VAs by carrot roots that functions as its primary storage organ. A study funded by the United States Department of Agriculture showed that root crops such as carrots and radishes, that directly come in direct contact with soil, may be particularly vulnerable to VAs contamination [
27]. Some of the staples enjoyed worldwide are root vegetables, and the uncontrolled introduction of VAs in the agriculture ecosystem can jeopardize food security. Alternately, lettuce plants accumulated VAs mostly in the shoot (leaves), and the BCF values for VAs in the shoot were in the order: lettuce > pepper > carrot. Lettuce is a leafy vegetable; therefore, VAs were accumulated mostly in the leaf, its primary storage organ. Among the three test plants, the fruit plant (pepper) had the least accumulation of VAs. This study reported a species variation in VA absorption and accumulation, depending upon the plant type and the nature of the plant storage system.
3.3. Detoxification of VAs
In this study, we failed to detect the metabolites of the active molecules SDZ and OTC in the plant samples. Some possible reasons for this can be-
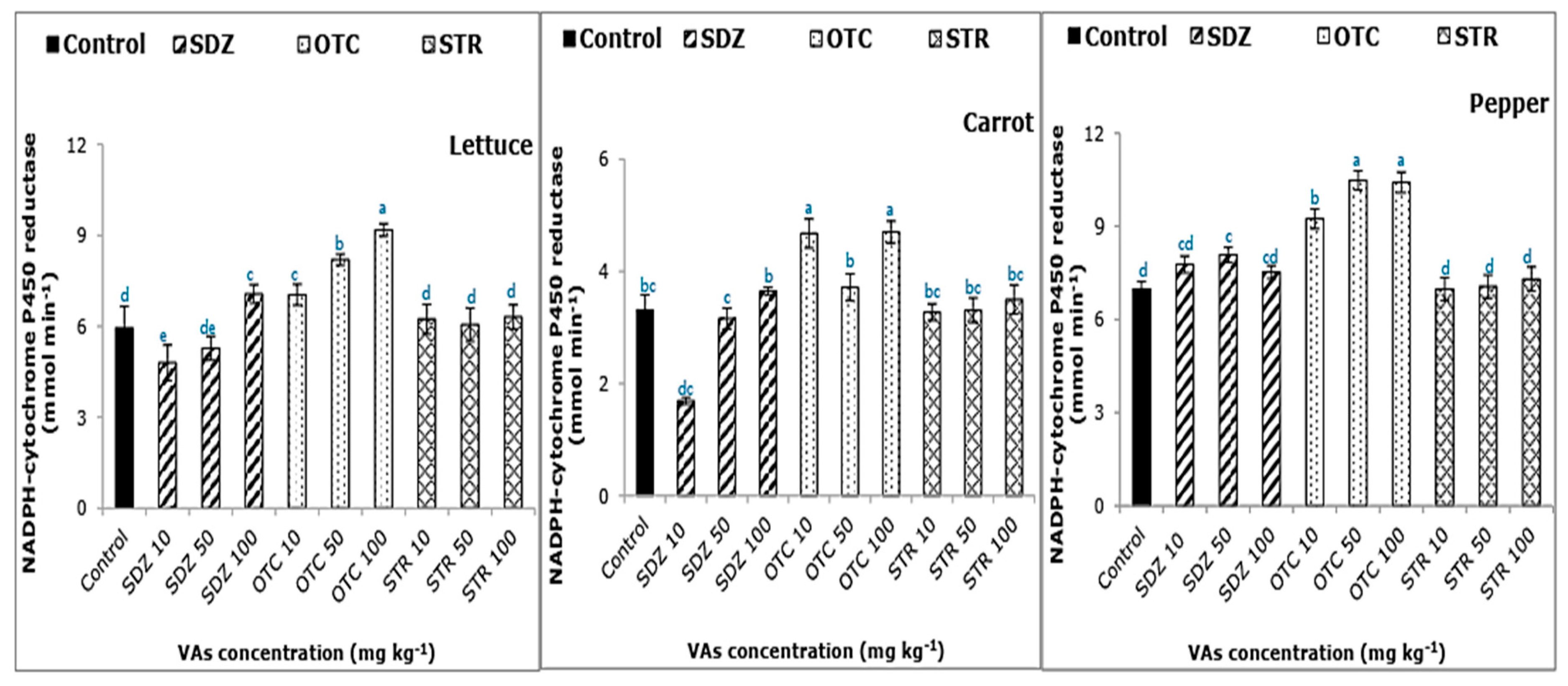
The phase I (CPR) and phase II (GST) detoxifying enzymes, of the plant detoxification system, were analyzed. The activity of the CPR enzyme increased with increasing concentration of SDZ and OTC antibiotics in the soil-manure mixture, irrespective of the plant type (
Figure 2). On the other hand, there was no significant difference between control and STR antibiotic. CPR enzyme activity ranged from 3.69 to 9.17, 1.69 to 4.70, and 6.98 to 10.47 mmol min
−1 for lettuce, carrot, and pepper, respectively. CPR enzymes function to metabolize potentially toxic compounds, including drugs. Therefore, increased CPR enzyme activity signifies the onset of detoxification mechanism or the increase in metabolic activity. A previous study has reported the induction of CPR activity in Oryza sativa exposed to the herbicide atrazine [
27]. The five-liganded heme-proteins of cytochromes P450 can exist as many isoforms, which enables them to accommodate a wide range of substrates and catalyze a variety of reactions [
28]. However, we were unable to find any published reports of plant CPR activity under VA stress. The highest CPR activity of 10.47 mmol min
−1 reported at OTC 50 mg kg
−1 in pepper and the lowest (1.69 mmol min
−1) at SDZ 10 mg kg
−1 in carrot. The response of the CPR varied depending on the plant species, the plant tissue analyzed, and the intensity of stress (VAs concentration).
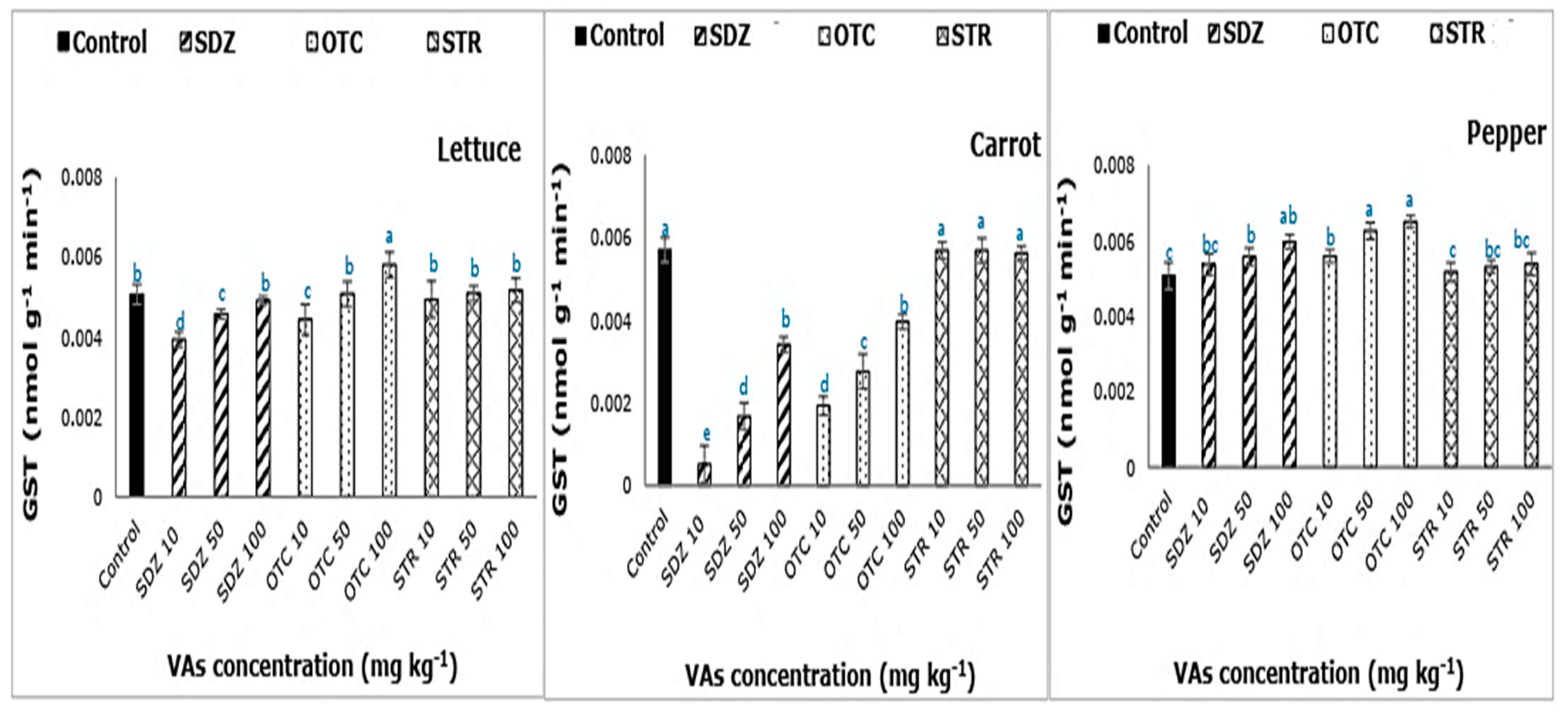
With increasing concentrations of SDZ and OTC in the soil-manure mixture, a gradual increase in GST activity was observed (
Figure 3). A previous study showed a significant increase in GST activity of pinto beans grown un chlortetracycline treated soil [
11]. GSTs are known to safeguard the cells against chemical-induced toxicity and provide tolerance [
29]. In plants, GSTs reportedly provide tolerance to herbicides [
30]. In carrot, the overall GST activity for SDZ and OTC was lower, in comparison with control, enforcing the inhibitory effect of SDZ and OTC on GST activity. Alternately in lettuce, low doses (10 mg kg
−1) resulted in significant inhibition of GST activity. On the contrary, in comparison with control, no significant difference (
p < 0.05) in GST activity was observed with STR treatment.
Therefore, under VA stress, there was an onset of the plant detoxification system, which varied depending upon the type of VAs and plant species.
3.4. Secondary Experiments
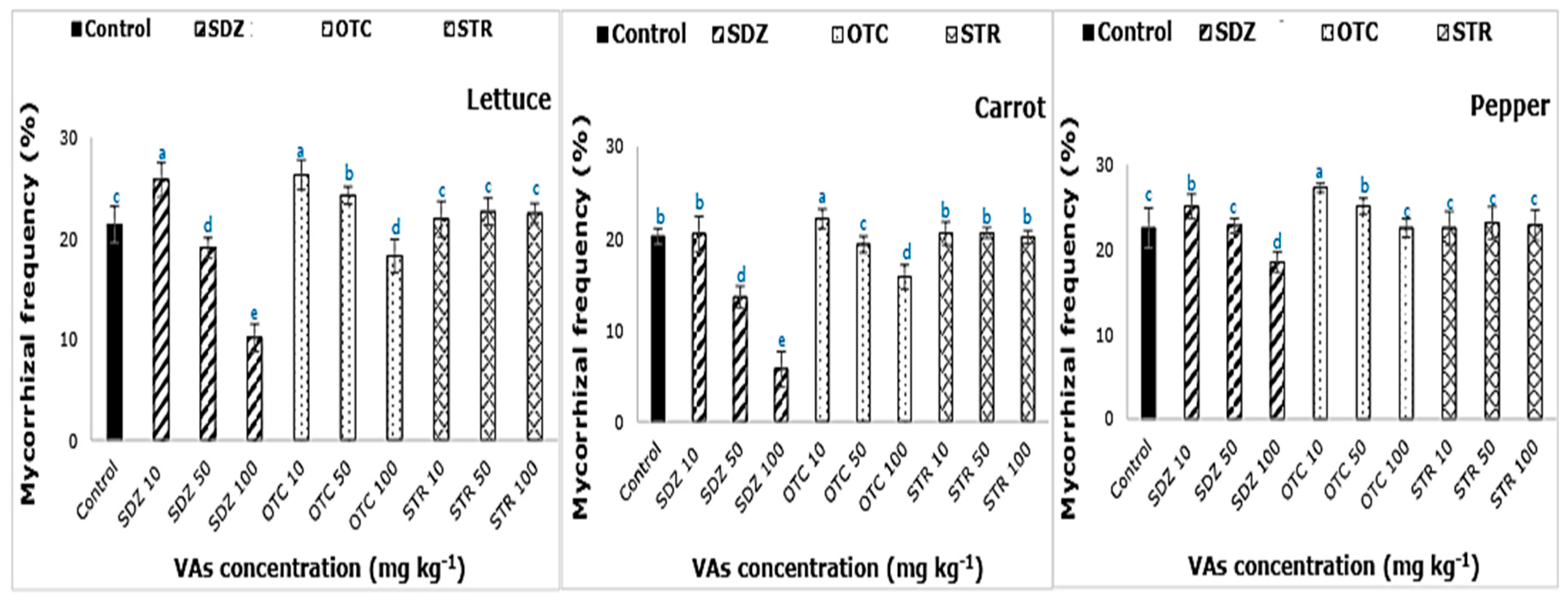
In
Figure 4, the mycorrhizal frequency (F%) of lettuce, carrot, and pepper plants grown in VAs amended soil is given. Upon comparison with control, F% was affected the most by SDZ, followed by OTC antibiotics. The effect of STR antibiotics on F% of lettuce, carrot, and pepper plants was found not to be significantly different (
p < 0.05) from control. At minimum VAs dosage of 10 mg kg
−1, the frequency of mycorrhizal colonization was higher than that of control. As described in previous studies, under abiotic stress conditions, increased colonization of plant roots by AM fungi is observed [
31,
32,
33]. An increase in F% at low concentrations followed a steady decline with the increasing dosage of VAs in the soil-manure mix. The formation of mycorrhizal structures in root is associated with the effective colonization of root by various AMF species [
31,
33]. Roots being the first plant organ to come in contact with the VAs, the physiology of the root affected by VAs treatment, thereby affected the intensity of mycorrhizal colonization. The observed F% varied depending on plant type indicating species variability. The F% of carrot, a root vegetable, reporting the highest accumulation of VAs was affected the most, followed by lettuce and then pepper.
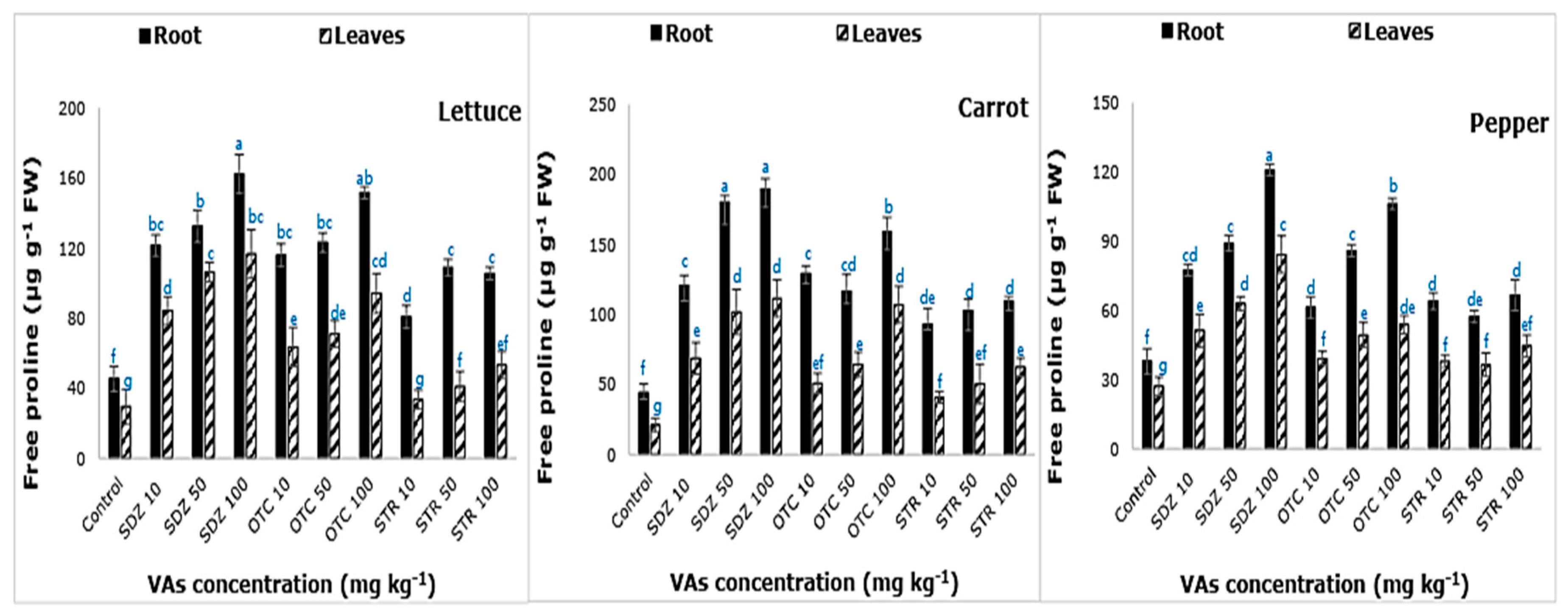
Figure 5 depicts the free proline content in the root and leaf tissues examined. In all three plants, we observed a variation in the accumulation of free proline in response to VAs stress. Free proline concentration increased with increasing abiotic stress (VAs concentration) and was the highest in carrot plants followed by lettuce and then pepper plants. The highest proline accumulation occurred at the highest VAs concentration of 100 mg kg
−1. As reported in a previous study, the accumulation of proline in
Medicago polymorpha and
Medicago ciliaris under NaCl stress, was the greatest at the highest NaCl concentration of 100 mM [
34]. Proline contributes to osmotic adjustment, stabilization, and protection of membranes integrity and macromolecules from the damage as a ROS scavenger [
35,
36]. It is significant in maintaining growth when plants are under stress [
37]. Under unfavorable conditions, plants accumulate amino acid metabolites, a constituent of proteins, that play a significant role in plant development and metabolism. Among the amino acids, ‘proline’ has been reported to play a beneficial role in plants exposed to various stress conditions [
38]. Proline plays three significant roles during stress, i.e., as a metal chelator, antioxidant molecule, and a signaling molecule. The high proline concentration in roots, in comparison to leaves, is likely due to the higher concentration of VAs in the roots. Previous studies have also reported an increase in proline accumulation in plants under stress [
39].
Free proline content varied between the plant roots and leaves. Free proline content was higher in the root than in leaves, likely due to the higher accumulation of VAs in plant root. Another possibility is the direct exposure of the roots to the soil contaminated with VAs. The highest accumulation of free proline occurred in carrot roots at 100 mg kg−1 SDZ (189.84 ± 7.23 μg g−1).