Retained Austenite Destabilization during Tempering of Low-Temperature Bainite
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Initial Microstructure
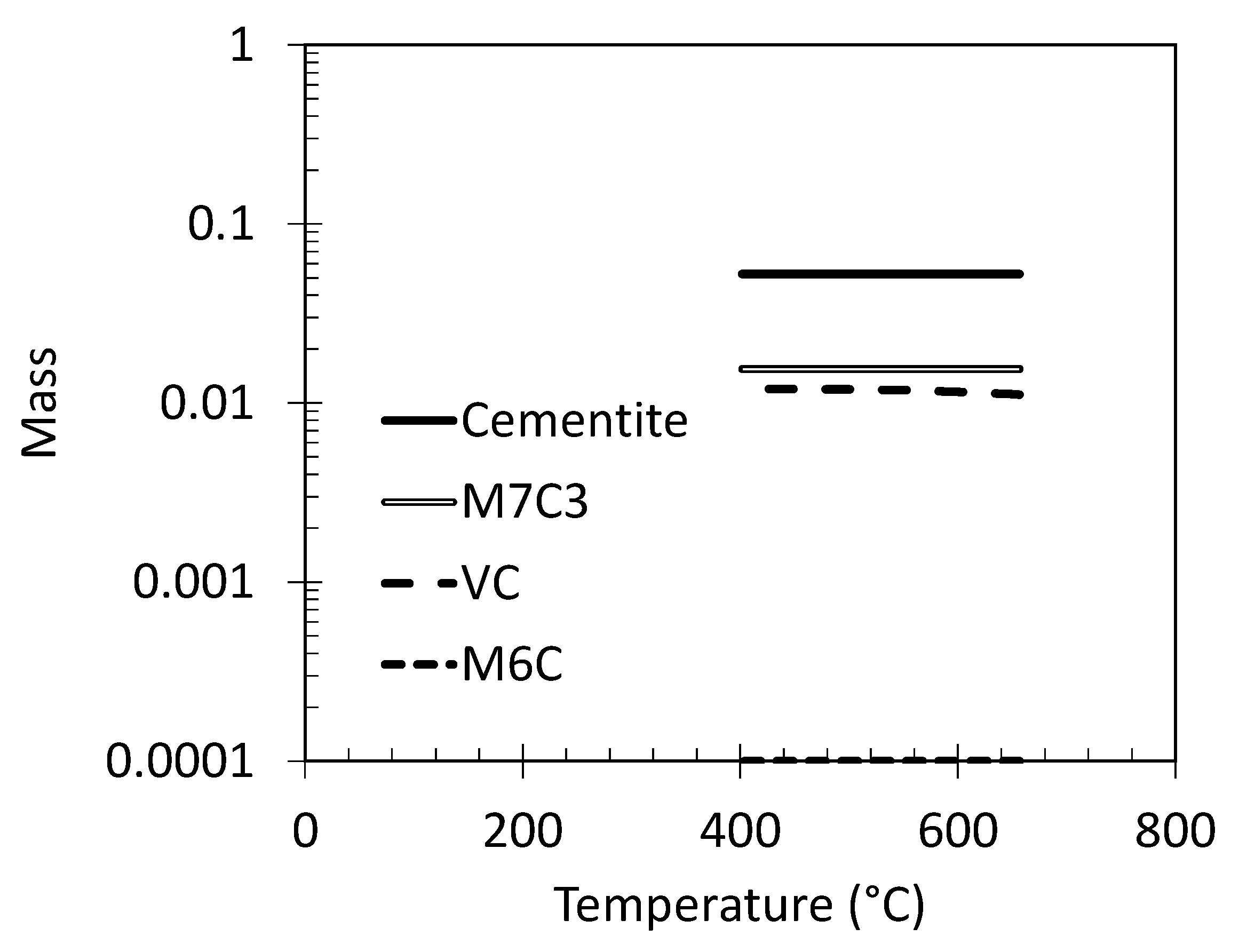
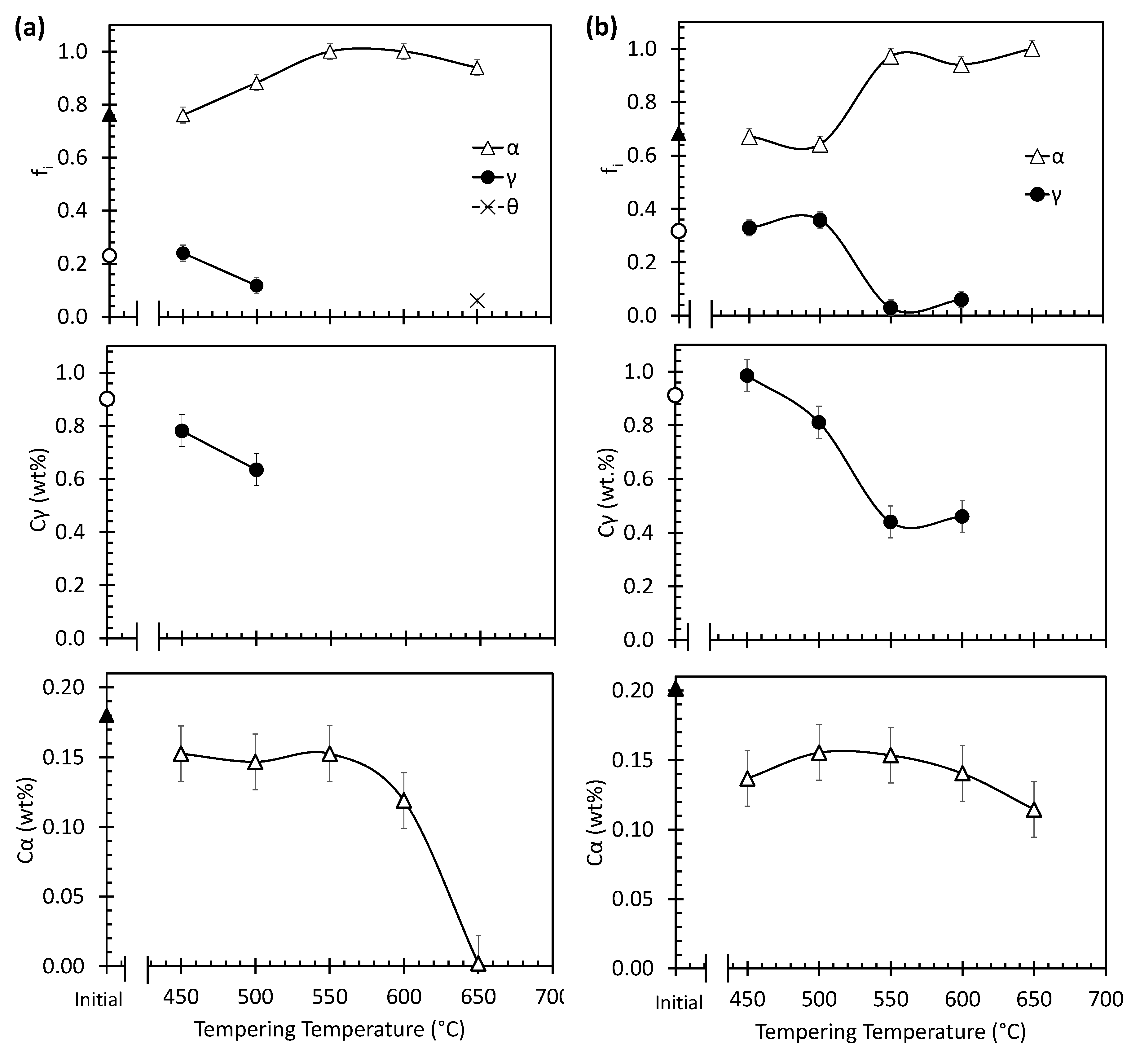
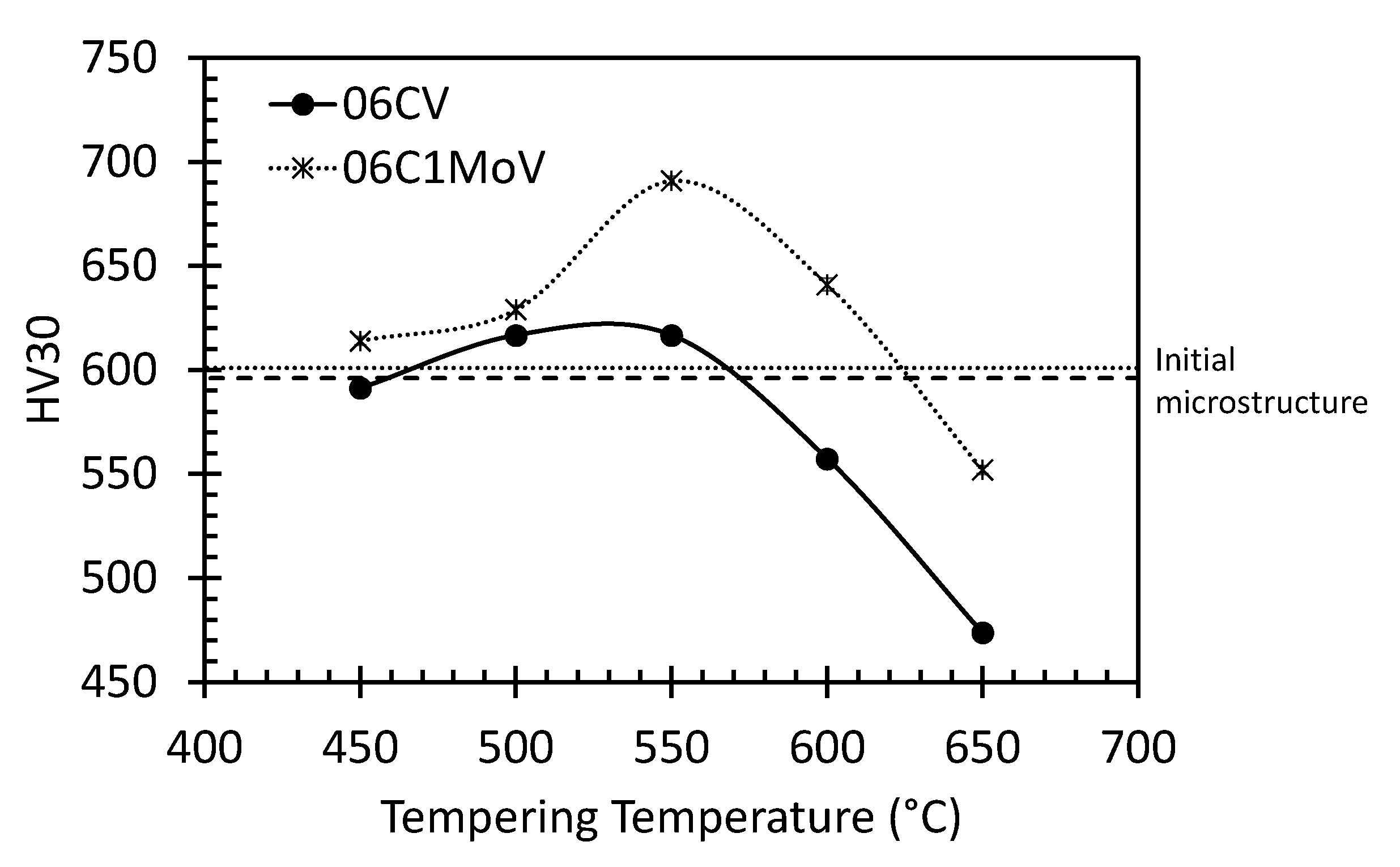
3.2. Selection of Tempering Conditions
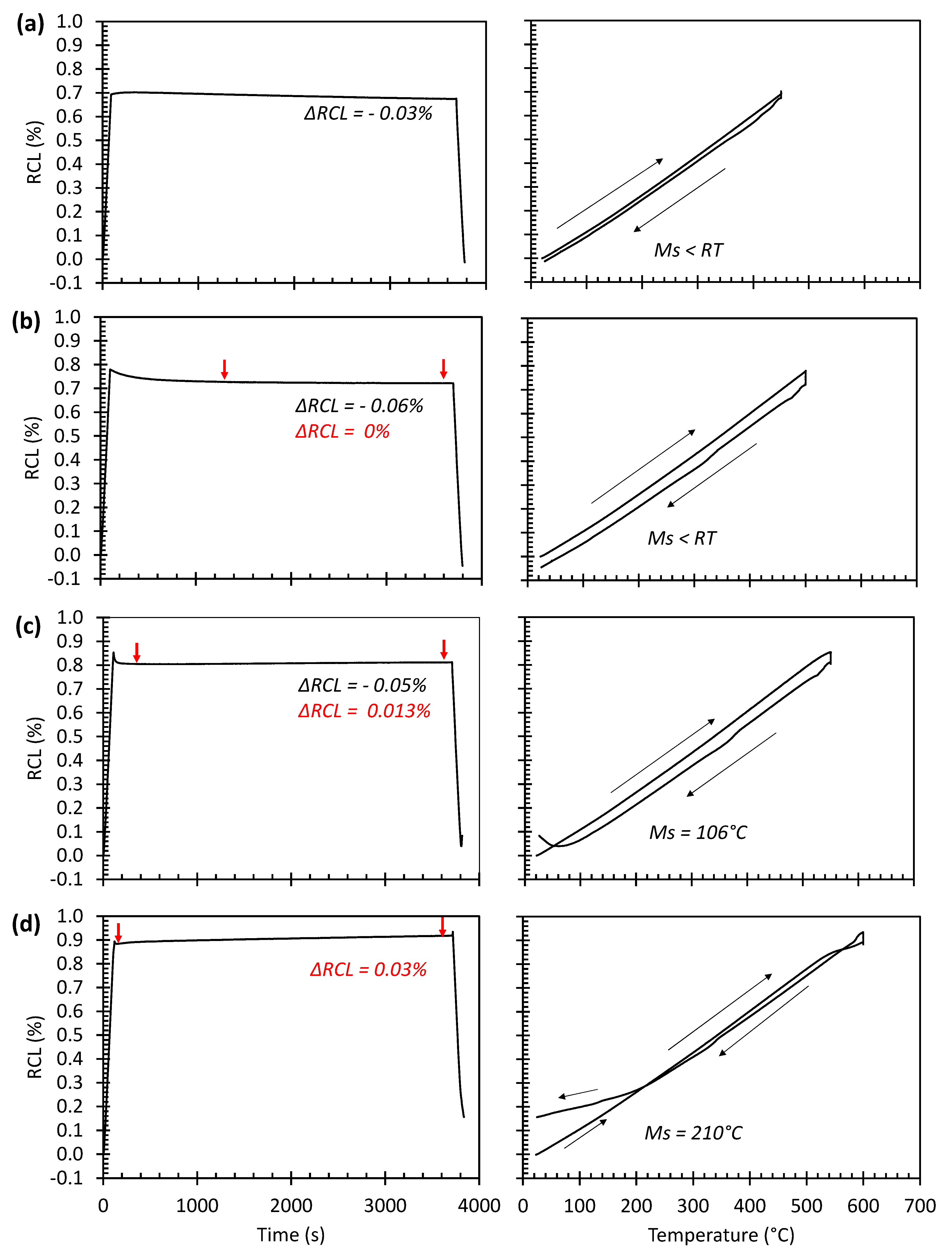
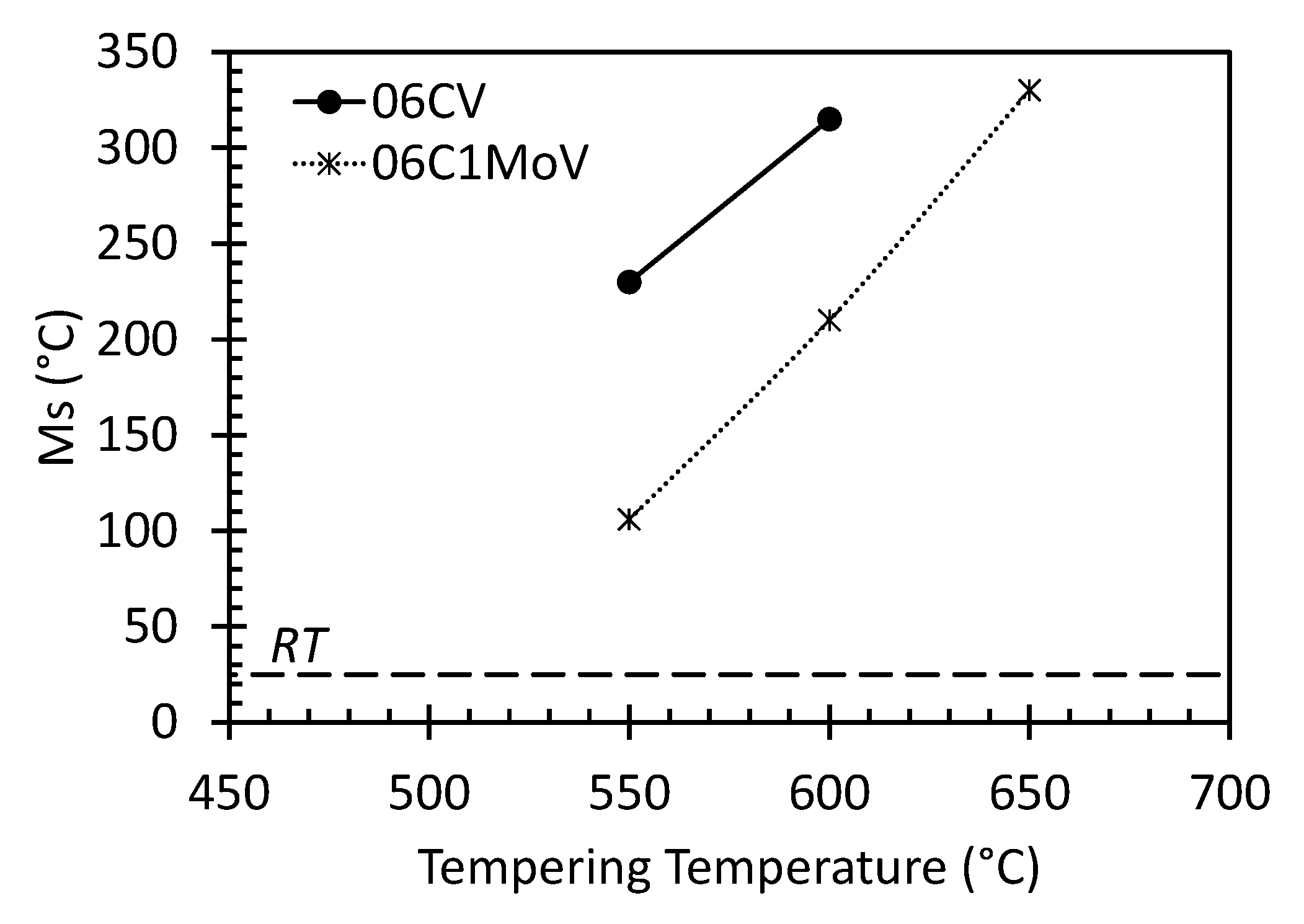
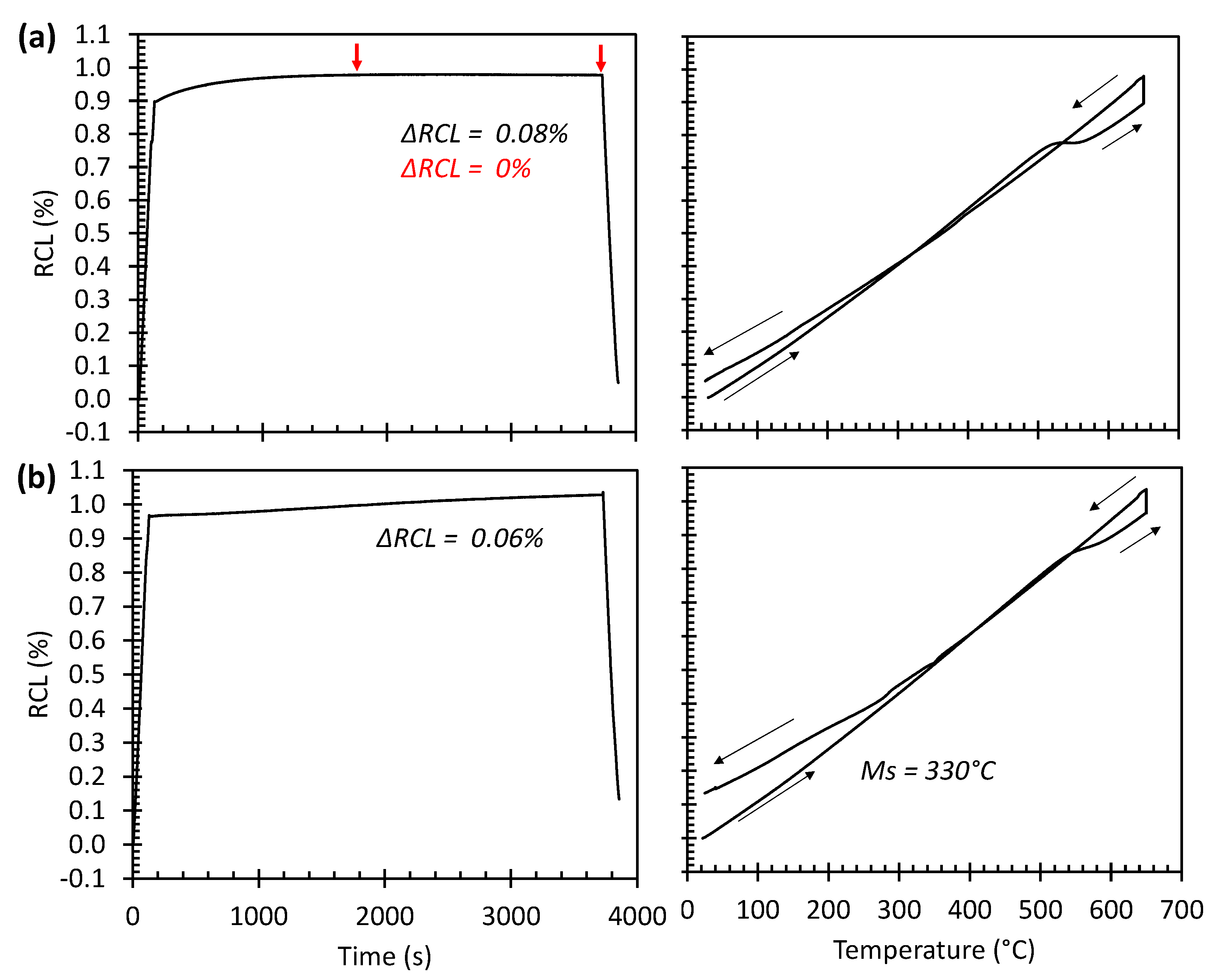
3.3. Dilatometric Study of the Microstructural Evolution during Tempering
- Retained austenite decomposes into a mixture of ferrite and cementite, . These calculations took into account the fact that the austenite retained in the bainitic microstructure has two distinctive morphologies that are characterized by containing very different levels of C in solid solution [34,35,36]. Therefore, was varied between that of the bulk (no C enrichment) and 2.5 wt %, which is a typical C enrichment detected for thin films of retained austenite [34,35,36].
- Before the decomposition described above, it could occur that part of the austenite goes through an intermediate state where it releases part of its C saturation due to the precipitation of the cementite particles [13,14], which are mostly rich in Fe but also with a certain amount of Cr and Mo: . The calculations for this scenario are made from the XRD results shown in Table 4 for the 06C1MoV alloy.
- The excess of C in bainitic ferrite can be released as cementite precipitates, leading to the progressive loss of tetragonality of the bainitic ferrite and finally reaching its equilibrium as a bcc phase, . The initial C content of bainitic ferrite, , is assumed to be 0.2 wt %, which is in accordance with both the XRD results presented in Table 4 and previous experiences with similar microstructures [37,38,39,40].
- Finally, the possibility is considered that some of the C from the supersaturated bainitic ferrite could be transferred to the retained austenite in a similar way to what happens during Q&P or Q + T processes. The calculations for this scenario are made from the XRD results shown in Table 4 for the 06C1MoV alloy. In this case, it must be considered that there are no changes in the fractions of the phases involved: .
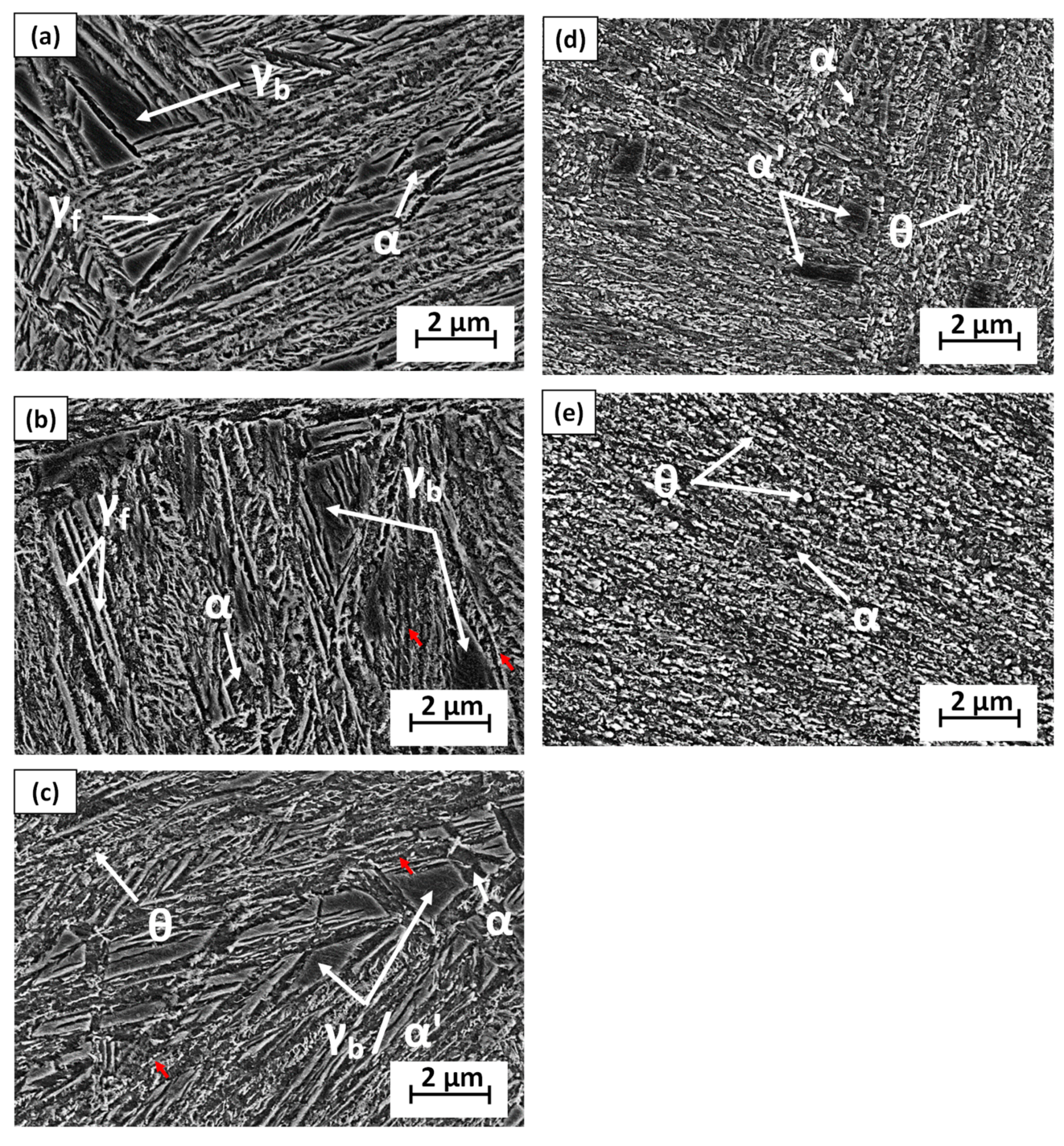
3.4. Microstructural Characterization of Tempered Microstructures by XRD and SEM
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Perez, M.; Sidoroff, C.; Vincent, A.; Esnouf, C. Microstructural evolution of martensitic 100Cr6 bearing steel during tempering: From thermoelectric power measurements to the prediction of dimensional changes. Acta Mater. 2009, 57, 3170–3181. [Google Scholar] [CrossRef]
- Sourmail, T.; Caballero, F.G.; Moudian, F.; De Castro, D.; Benito, M. High hardness and retained austenite stability in Si-bearing hypereutectoid steel through new heat treatment design principles. Mater. Des. 2018, 142, 279–287. [Google Scholar] [CrossRef]
- Hasan, H.S.; Peet, M.J.; Bhadeshia, H.K.D.H. Severe tempering of bainite generated at low transformation temperatures. Int. J. Mater. Res. 2012, 103, 1319–1324. [Google Scholar] [CrossRef]
- Wang, D.; Chen, C.-W.; Dalton, J.C.; Yang, F.; Sharghi-Moshtaghin, R.; Kahn, H.; Ernst, F.; Williams, R.E.A.; McComb, D.W.; Heuer, A.H. “Colossal” interstitial supersaturation in delta ferrite in stainless steels—I. Low-temperature carburization. Acta Mater. 2015, 86, 193–207. [Google Scholar] [CrossRef]
- Liu, D.; Bai, B.; Fang, H.; Zhang, W.; Gu, J.; Chang, K. Effect of tempering temperature and carbide free bainite on the mechanical characteristics of a high strength low alloy steel. Mater. Sci. Eng. A 2004, 371, 40–44. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Tempering of Bainite. Bainite in Steels; IOM Communications: Geneva, Switzerland, 2001; pp. 91–116. [Google Scholar]
- Peet, M.J.; Babu, S.S.; Miller, M.K.; Bhadeshia, H.K.D.H. Tempering of low-temperature bainite. Metall. Mater. Trans. A 2017, 48, 3410–3418. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G. Bainitic steels: Tempering. In Encyclopedia of Iron, Steel, and Their Alloys; Taylor & Francis: Milton Park, Abingdon, UK, 2016; pp. 1–14. ISBN 1-4665-1104-4. [Google Scholar]
- Caballero, F.G.; Miller, M.K.; Garcia-Mateo, C. The approach to equilibrium during tempering of a bulk nanocrystalline steel: An atom probe investigation. J. Mater. Sci. 2008, 43, 3769–3774. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Peet, M.; Caballero, F.G.; Bhadeshia, H.K.D.H. Tempering of hard mixture of bainitic ferrite and austenite. Mater. Sci. Technol. 2004, 20, 814–818. [Google Scholar] [CrossRef]
- Hulme-Smith, C.N.; Lonardelli, I.; Peet, M.J.; Dippel, A.C.; Bhadeshia, H.K.D.H. Enhanced thermal stability in nanostructured bainitic steel. Scr. Mater. 2013, 69, 191–194. [Google Scholar] [CrossRef]
- Santajuana, M.A.; Rementeria, R.; Kuntz, M.; Jimenez, J.A.; Caballero, F.G.; Garcia-Mateo, C. Low-Temperature bainite: A thermal stability study. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2018, 49, 2026–2036. [Google Scholar] [CrossRef]
- Saha Podder, A.; Bhadeshia, H.K.D.H. Thermal stability of austenite retained in bainitic steels. Mater. Sci. Eng. A 2010, 527, 2121–2128. [Google Scholar] [CrossRef]
- Saha Podder, A. Tempering of a Mixture of Bainite and Retained Austenite. Ph.D. Thesis, University of Cambridge, Cambridge, UK, January 2011. [Google Scholar]
- Kuntz, M.; Garcia-Mateo, C.; Garcia Caballero, F.; Ruiz-Jimenez, V.; Sourmail, T.; Lille, S.; Wicks, G.; Allain, S.; Denis, S.; Geandier, G.; et al. Design of New Economic Secondary Precipitating Steels for Fatigue Resistance at Elevated Service Temperatures (STEELSECO-RFCS-754070); EUROPEAN COMMISSION Directorate G—Industrial Technologies, Unit, G.5—Research Fund for Coal and Steel, European Commission: Luxembourg, 2020. [Google Scholar]
- Baker, R.G. The tempering of 2.25 Cr%-1% Mo steel after quenching and normalizing. J. Iron Steel Inst. 1959, 192, 257–268. [Google Scholar]
- Irvine, K.J.; Pickering, F.B. Low-carbon bainitic steels. J. Iron Steel Inst. 1957, 187, 292–309. [Google Scholar]
- Siwecki, T.; Eliasson, J.; Lagneborg, R.; Hutchinson, B. Vanadium Microalloyed Bainitic Hot Strip Steels. ISIJ Int. 2010, 50, 760–767. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Sourmail, T.; Caballero, F.G.; Smanio, V.; Kuntz, M.; Ziegler, C.; Leiro, A.; Vuorinen, E.; Elvira, R.; Teeri, T. Nanostructured steel industrialisation: Plausible reality. Mater. Sci. Technol. 2014, 30, 1071–1078. [Google Scholar] [CrossRef]
- Sourmail, T.; Smanio, V.; Ziegler, C.; Heuer, V.; Kuntz, M.; Caballero, F.G.; Garcia-Mateo, C.; Cornide, J.; Elvira, R.; Leiro, A.; et al. Novel Nanostructured Bainitic Steel Grades to Answer the Need for High-Performance Steel Components (Nanobain); RFSR-CT-2008-00022; EUROPEAN COMMISSION Directorate G—Industrial Technologies, Unit, G.5—Research Fund for Coal and Steel, European Commission: Luxembourg, 2013; ISBN 978-92-79-29234-7. [Google Scholar]
- Garcia-Mateo, C.; Caballero, F.G.; Sourmail, T.; Smanio, V.; De Andres, C.G. Industrialised nanocrystalline bainitic steels. Design approach. Int. J. Mater. Res. 2014, 105, 725–734. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in Steels. Theory and Practice; Maney Publishing: London, UK, 2015; ISBN 978-1-909662-74-2. [Google Scholar]
- Balzar, D.; Audebrand, N.; Daymond, M.R.; Fitch, A.; Hewat, A.; Langford, J.I.; Le Bail, A.; Louër, D.; Masson, O.; McCowan, C.N.; et al. Size–strain line-broadening analysis of the ceria round-robin sample. J. Appl. Crystallogr. 2004, 37, 911–924. [Google Scholar] [CrossRef]
- Cheng, L.; Brakman, C.M.; Korevaar, B.M.; Mittemeijer, E.J. The tempering of iron-carbon martensite; dilatometric and calorimetric analysis. Metall. Trans. A 1988, 19, 2415–2426. [Google Scholar] [CrossRef]
- Lee, S.J.; Lusk, M.T.; Lee, Y.K. Conversional model of transformation strain to phase fraction in low alloy steels. Acta Mater. 2007, 55, 875–882. [Google Scholar] [CrossRef]
- Stuart, H.; Rindley, N. Thermal expansion of cementite and other phases. J. Iron Steel Inst. 1966, 204, 711–717. [Google Scholar]
- García De Andrés, C.; Caballero, F.G.; Capdevila, C.; Bhadeshia, H.K.D.H. Modelling of kinetics and dilatometric behavior of non-isothermal pearlite-to-austenite transformation in an eutectoid steel. Scr. Mater. 1998, 39, 791–796. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G.; Capdevila, C.; de Andres, C.G. Estimation of dislocation density in bainitic microstructures using high-resolution dilatometry. Scr. Mater. 2009, 61, 855–858. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; David, S.A.; Vitek, J.M.; Reed, R.W. Stress induced transformation to bainite in Fe–Cr–Mo–C pressure vessel steel. Mater. Sci. Technol. 1991, 7, 686–698. [Google Scholar] [CrossRef]
- Dyson, D.J.; Holmes, B. Effect of alloying additions on the lattice parameter of austenite. J. Iron Steel Inst. 1970, 208, 469–474. [Google Scholar]
- Moyer, J.M.; Ansell, G.S. The volume expansion accompanying the martensite transformation in iron-carbon alloys. Metall. Trans. A 1975, 6, 1785–1791. [Google Scholar] [CrossRef]
- Materials, A.S. Annual Book of ASTM Standards: Vol. 03.01, Metals-Mechanical Testing; Elevated and Low Temperature Tests, Metallography; Metals Test Methods and Analytical Procedures; American Society for Testing and Materials: West Conshohocken, PA, USA, 1999. [Google Scholar]
- Laboratory, N.P. MTDATA; National physical Laboratory: Teddington, Middlesex, UK, 2003. [Google Scholar]
- Bhadeshia, H.K.D.H.; Edmonds, D.V. The mechanism of bainite formation in steels. Acta Metall. 1980, 28, 1265–1273. [Google Scholar] [CrossRef]
- Guo, L.; Bhadeshia, H.K.D.H.; Roelofs, H.; Lembke, M.I. In situ synchrotron X-ray study of bainite transformation kinetics in a low-carbon Si-containing steel. Mater. Sci. Technol. (U. K.) 2017, 33, 2147–2156. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G.; Miller, M.K.; Jimenez, J.A. On measurement of carbon content in retained austenite in a nanostructured bainitic steel. J. Mater. Sci. 2012, 47, 1004–1010. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Jimenez, J.A.; Yen, H.-W.; Miller, M.K.; Morales-Rivas, L.; Kuntz, M.; Ringer, S.P.; Yang, J.-R.; Caballero, F.G. Low temperature bainitic ferrite: Evidence of carbon super-saturation and tetragonality. Acta Mater. 2015, 91, 162–173. [Google Scholar] [CrossRef]
- Hulme-Smith, C.N.; Peet, M.J.; Lonardelli, I.; Dippel, A.C.; Bhadeshia, H.K.D.H. Further evidence of tetragonality in bainitic ferrite. Mater. Sci. Technol. (U. K.) 2015, 31, 254–256. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Carbon in cubic and tetragonal ferrite. Philos. Mag. 2013, 93, 3714–3725. [Google Scholar] [CrossRef]
- Jang, J.H.; Bhadeshia, H.K.D.H.; Suh, D.W. Solubility of carbon in tetragonal ferrite in equilibrium with austenite. Scr. Mater. 2013, 68, 195–198. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Edmonds, D.V. Bainite in silicon steels: New composition–property approach Part 1. Met. Sci. 1983, 17, 411–419. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Edmonds, D.V. The bainite transformation in a silicon steel. Metall. Trans. A 1979, 10, 895–907. [Google Scholar] [CrossRef]
- Sandvik, B.P.J. The Bainite reaction in Fe-Si-C Alloys: The primary stage. Metall. Trans. A 1982, 13, 777–787. [Google Scholar] [CrossRef]
- Tanino, M.; Nishida, T. On the secondary hardening on tempering in vanadium steels. Trans. Jpn. Inst. Met. 1968, 9, 103–110. [Google Scholar] [CrossRef]
- Lagneborg, R.; Siwecki, T.; Zajac, S.; Hutchinson, B. Role of vanadium in microalloyed steels. Scand. J. Metall. 2001, 28, 58–59. [Google Scholar]
- Baker, T.N. Processes, microstructure and properties of vanadium microalloyed steels. Mater. Sci. Technol. 2009, 25, 1083–1107. [Google Scholar] [CrossRef]
- Podder, A.S.; Lonardelli, I.; Molinari, A.; Bhadeshia, H.K.D.H. Thermal stability of retained austenite in bainitic steel: An in situ study. Proc. R. Soc. A Math. Phys. Eng. Sci. 2011, 467, 3141–3156. [Google Scholar] [CrossRef]
- Hasan, H.S.; Peet, M.J.; Avettand-Fènoël, M.-N.; Bhadeshia, H.K.D.H. Effect of tempering upon the tensile properties of a nanostructured bainitic steel. Mater. Sci. Eng. A 2014, 615, 340–347. [Google Scholar] [CrossRef]
- Stewart, J.W.; Thomson, R.C.; Bhadeshia, H.K.D.H. Cementite precipitation during tempering of martensite under the influence of an externally applied stress. J. Mater. Sci. 1994, 29, 6079–6084. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Theoretical analysis of changes in cementite composition during tempering of bainite. Mater. Sci. Technol. 1989, 5, 131–137. [Google Scholar] [CrossRef]
- Bhadeshia, H.; Honeycombe, R. Tempering of martensite. In Steels: Microstructure and Properties; Elsevier: Amsterdam, The Netherlands, 2017; pp. 237–270. ISBN 9780081002704. [Google Scholar]
- Yamasaki, S.; Bhadeshia, H.K.D.H. Modelling and characterisation of V4C3 precipitation and cementite dissolution during tempering of Fe—C—V martensitic steel. Mater. Sci. Technol. 2003, 19, 1335–1343. [Google Scholar] [CrossRef]
- Sourmail, T.; Smanio, V.; Caballero, F.G.; Cornide, J.; Capdevilla, C.; Garcia-Mateo, C. Evolution of microstructure and mechanical properties during tempering of continuously cooled bainitic steels. In Materials Science Forum—Thermec 2011; Trans Tech Publications Ltd.: Kapellweg, Switzerland, 2012; pp. 706–709. ISBN 9783037853030. [Google Scholar]
- Grajcar, A.; Morawiec, M.; Jimenez, J.A.; Garcia-Mateo, C. Dilatometric and microstructural study of martensite tempering in 4% mn steel. Materials 2020, 13, 4442. [Google Scholar] [CrossRef] [PubMed]
- Caballero, F.G.; Miller, M.K.; Garcia-Mateo, C.; Capdevila, C.; Babu, S.S. Redistribution of alloying elements during tempering of a nanocrystalline steel. Acta Mater. 2008, 56, 188–199. [Google Scholar] [CrossRef]
- Caballero, F.G.; Miller, M.K.; Garcia-Mateo, C. Atom Probe Tomography Analysis of Precipitation during Tempering of a Nanostructured Bainitic Steel. Metall. Mater. Trans. A 2011, 42, 3660–3668. [Google Scholar] [CrossRef]
- Caballero, F.G.; Miller, M.K.; Clarke, A.J.; Garcia-Mateo, C. Examination of carbon partitioning into austenite during tempering of bainite. Scr. Mater. 2010, 63, 442–445. [Google Scholar] [CrossRef]
- Caballero, F.G.; Garcia-Mateo, C.; García De Andrés, C. Dilatometric study of reaustenitisation of high silicon bainitic steels: Decomposition of retained austenite. Mater. Trans. 2005, 46, 581–586. [Google Scholar] [CrossRef]
- Talebi, S.; Ghasemi-Nanesa, H.; Jahazi, M.; Melkonyan, H. In situ study of phase transformations during non-isothermal tempering of bainitic and martensitic microstructures. Metals 2017, 7, 346. [Google Scholar] [CrossRef]
- Talebi, S.; Jahazi, M.; Melkonyan, H. Retained austenite decomposition and carbide precipitation during isothermal tempering of a medium-carbon low-alloy bainitic steel. Materials 2018, 11, 1441. [Google Scholar] [CrossRef]
- Caballero, F.G.; Miller, M.K.; Babu, S.S.; Garcia-Mateo, C. Atomic scale observations of bainite transformation in a high carbon high silicon steel. Acta Mater. 2007, 55, 381–390. [Google Scholar] [CrossRef]
- Kalish, D.; Cohen, M. Structural changes and strengthening in the strain tempering of martensite. Mater. Sci. Eng. A 1970, 6, 156–166. [Google Scholar] [CrossRef]
- He, S.H.; He, B.B.; Zhu, K.Y.; Huang, M.X. Evolution of dislocation density in bainitic steel: Modeling and experiments. Acta Mater. 2018, 149, 46–56. [Google Scholar] [CrossRef]





| C | Si | Mn | Cr | Ni | Mo | Cu | V | |
|---|---|---|---|---|---|---|---|---|
| 06CV | 0.6 | 1.7 | 1.3 | 1.7 | 0.2 | 0.2 | 0.2 | 0.5 |
| 06C1MoV | 0.6 | 1.7 | 1.3 | 1.7 | 0.2 | 1.0 | 0.2 | 0.5 |
| Crystal Structure | Unit Cell Atomic Volume | Ref. | |
|---|---|---|---|
| (fcc) | [13] | ||
| (bct) | [27] | ||
| (bcc) | [13] | ||
| (Orthorhombic) | [28,29] |
| Lattice Parameter (Å) at Room T | Ref. | |
|---|---|---|
(fcc) | [30] | |
| or (bct) | [31] | |
(bcc) | [26] | |
| (Orthorhombic) | [28,29] |
| Steel | (±0.03) | (±0.06 wt %) | (±0.03) | (±0.03 wt %) | HV30 |
|---|---|---|---|---|---|
| 06CV | 0.23 | 0.90 | 0.77 | 0.18 | 596 ± 3 |
| 06C1MoV | 0.32 | 0.91 | 0.68 | 0.20 | 601 ± 2 |
| State | Phases | |
|---|---|---|
| Scenario 1 | ||
| Initial | = 0.6–2.5 | |
| Final | = 0.03 | |
| = 6.67 | ||
| Scenario 2 | ||
| Initial | = 0.91 | |
| Final | = 0.9–0.7 | |
| = 6.67 | ||
| Scenario 3 | ||
| Initial | = 0.2 | |
| Final | = 0.03 | |
| = 6.67 | ||
| Scenario 4 | ||
| Initial | = 0.91 = 0.32 | |
| = 0.2 = 1 − = 0.68 | ||
| Final | = | |
| = 0.2–0.1 = | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Jimenez, V.; Kuntz, M.; Sourmail, T.; Caballero, F.G.; Jimenez, J.A.; Garcia-Mateo, C. Retained Austenite Destabilization during Tempering of Low-Temperature Bainite. Appl. Sci. 2020, 10, 8901. https://doi.org/10.3390/app10248901
Ruiz-Jimenez V, Kuntz M, Sourmail T, Caballero FG, Jimenez JA, Garcia-Mateo C. Retained Austenite Destabilization during Tempering of Low-Temperature Bainite. Applied Sciences. 2020; 10(24):8901. https://doi.org/10.3390/app10248901
Chicago/Turabian StyleRuiz-Jimenez, Victor, Matthias Kuntz, Thomas Sourmail, Francisca G. Caballero, Jose A. Jimenez, and Carlos Garcia-Mateo. 2020. "Retained Austenite Destabilization during Tempering of Low-Temperature Bainite" Applied Sciences 10, no. 24: 8901. https://doi.org/10.3390/app10248901
APA StyleRuiz-Jimenez, V., Kuntz, M., Sourmail, T., Caballero, F. G., Jimenez, J. A., & Garcia-Mateo, C. (2020). Retained Austenite Destabilization during Tempering of Low-Temperature Bainite. Applied Sciences, 10(24), 8901. https://doi.org/10.3390/app10248901








