The Influence of Different Pre-Treatments on the Quality and Nutritional Characteristics in Dried Undersized Yellow Kiwifruit
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
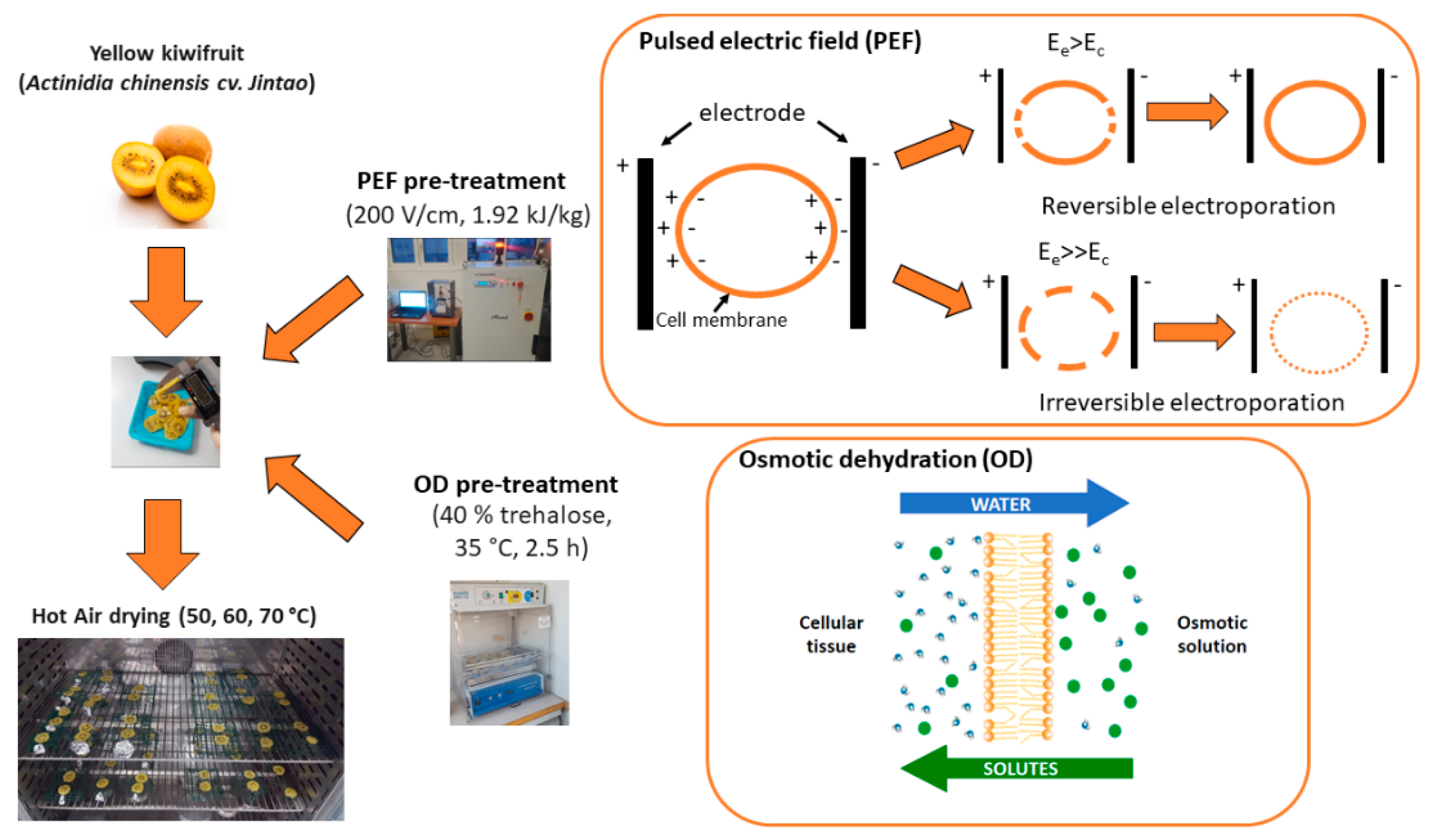
2.1. Sample Preparation
2.2. Physico-Chemical Analysis
2.2.1. Water Activity
2.2.2. Color
2.3. Functional Components Content and Antioxidant Activity
2.3.1. Total Polyphenols (TP)
2.3.2. Vitamin C Content
2.3.3. Antioxidant Activity (DPPH and ABTS Assay)
2.4. Statistical Analysis
3. Results
3.1. Color
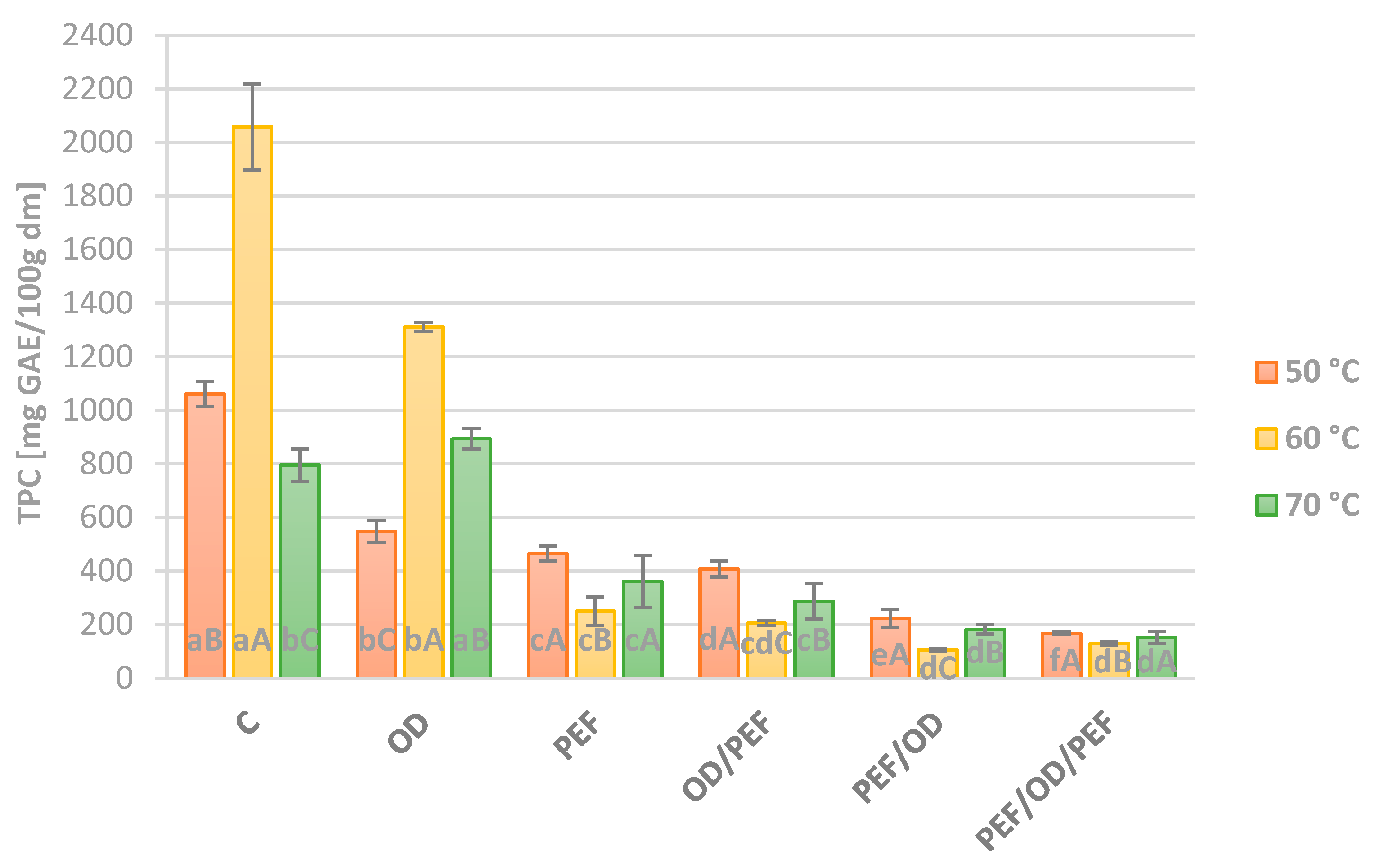
3.2. Total Polyphenols
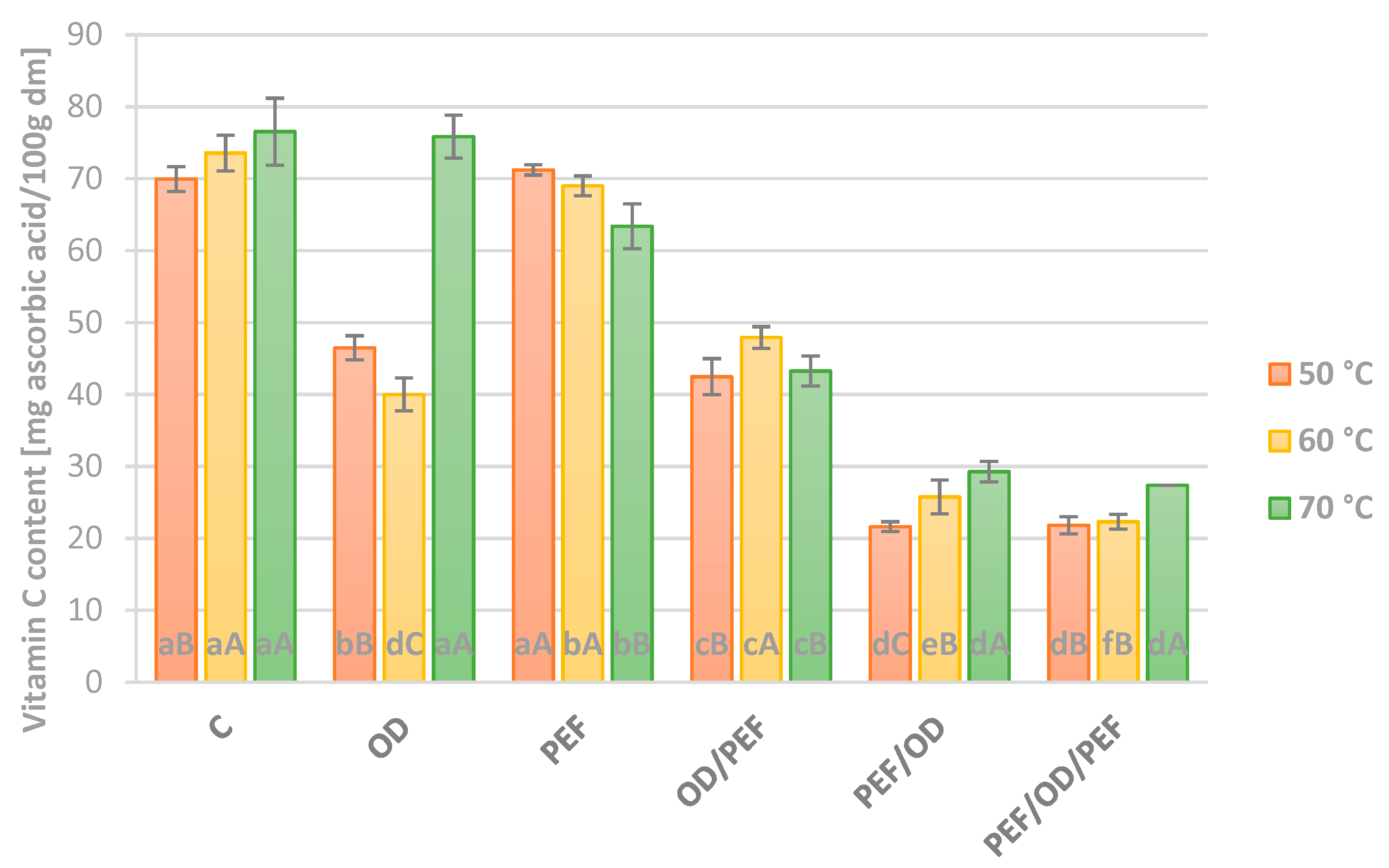
3.3. Vitamin C Content
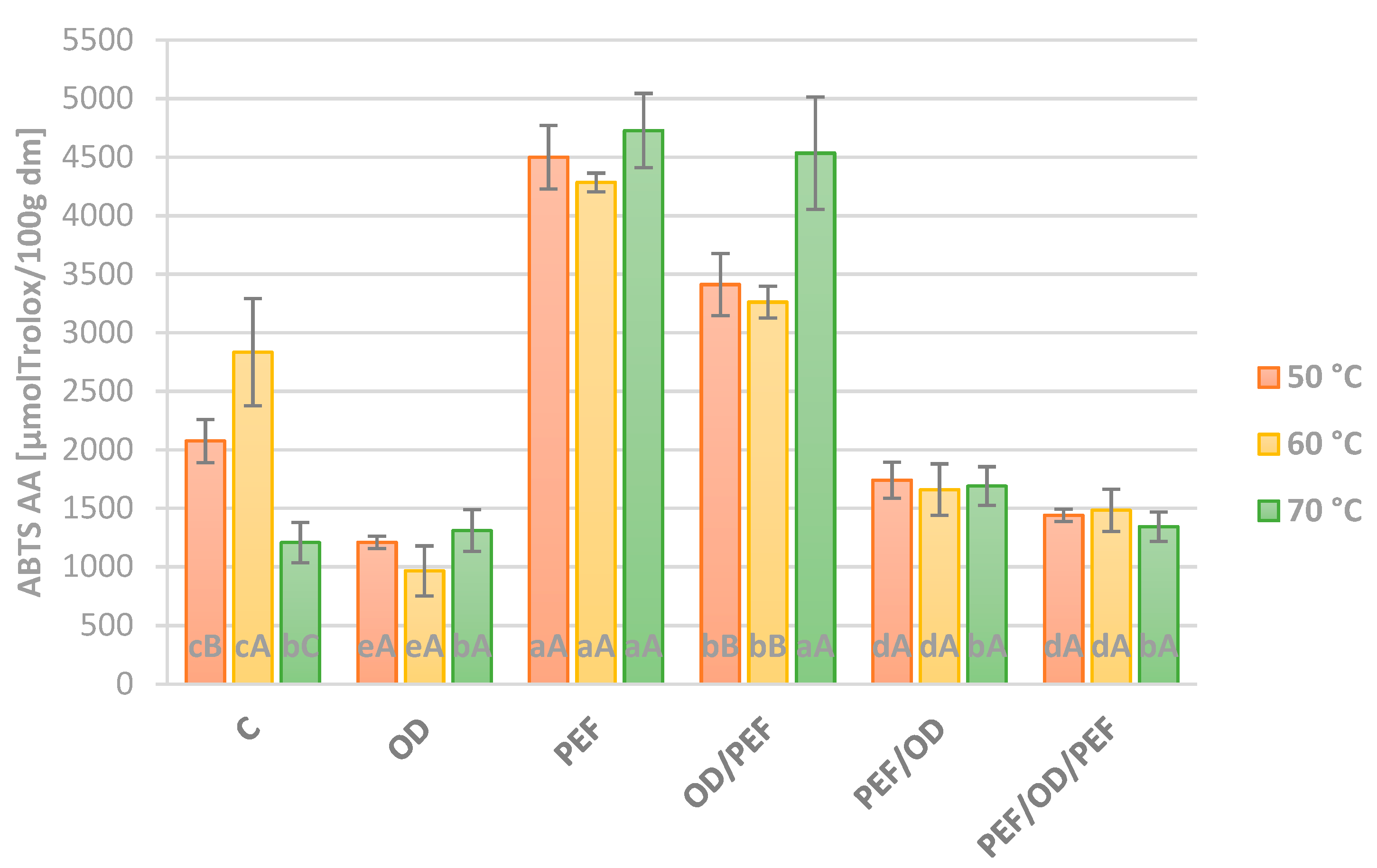
3.4. Antioxidant Activity (DPPH and ABTS Assay)
4. Discussion
5. Conclusions
Basic Mechanisms of the Pre-Treatments Applied
Author Contributions
Funding
Conflicts of Interest
References
- Betoret, E.; Betoret, N.; Vidal, D.; Fito, P. Functional foods development: Trends and technologies. Trends Food Sci. Technol. 2011, 22, 498–508. [Google Scholar] [CrossRef]
- Meynard, J.M.; Jeuffroy, M.H.; Le Bail, M.; Lefèvre, A.; Magrini, M.B.; Michon, C. Designing coupled innovations for the sustainability transition of agrifood systems. Agric. Syst. 2017, 157, 330–339. [Google Scholar] [CrossRef]
- Woodhouse, A.; Davis, J.; Pénicaud, C.; Östergren, K. Sustainability checklist in support of the design of food processing. Sustain. Prod. Consum. 2018, 16, 110–120. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 521–531. [Google Scholar] [CrossRef]
- The Publications Office of the European Union. COMMISSION REGULATION (EC) No 1673/2004 of 24 September 2004 Laying Down the Marketing Standard Applicable to Kiwifruit; The Publications Office of the European Union: Luxembourg, 2004; pp. 5–10. [Google Scholar]
- Ma, T.; Sun, X.; Zhao, J.; You, Y.; Lei, Y.; Gao, G.; Zhan, J. Nutrient compositions and antioxidant capacity of kiwifruit (Actinidia) and their relationship with flesh color and commercial value. Food Chem. 2017, 218, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Amami, E.; Khezami, L.; Vorobiev, E.; Kechaou, N. Effect of pulsed electric field and osmotic dehydration pretreatment on the convective drying of carrot tissue. Dry. Technol. 2018, 26, 231–238. [Google Scholar] [CrossRef]
- Tylewicz, U.; Nowacka, M.; Rybak, K.; Drozdzal, K.; Dalla Rosa, M.; Mozzon, M. Design of Healthy Snack Based on Kiwifruit. Molecules 2020, 25, 3309. [Google Scholar] [CrossRef]
- Aguilera, J.M.; Chiralt, A.; Fito, P. Food dehydration and product structure. Trends Food Sci. Technol. 2003, 14, 432–437. [Google Scholar] [CrossRef]
- Chou, S.K.; Chua, K.J. New hybrid drying technologies for heat sensitive foodstuffs. Trends Food Sci. Technol. 2001, 12, 359–369. [Google Scholar] [CrossRef]
- Tylewicz, U.; Tappi, S.; Mannozzi, C.; Romani, S.; Dellarosa, N.; Laghi, L.; Ragni, L.; Rocculi, P.; Dalla Rosa, M. Effect of pulsed electric field (PEF) pre-treatment coupled with osmotic dehydration on physico-chemical characteristics of organic strawberries. J. Food Eng. 2017, 213, 2–9. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.; Zachariou, I.; Andreou, V.; Taoukis, P.S. Effect of pulsed electric fields on mass transfer and quality of osmotically dehydrated kiwifruit. Food Bioprod. Process. 2016, 100, 535–544. [Google Scholar] [CrossRef]
- Thamkaev, G.; Gómez Galindo, F. Influence of pulsed and moderate electric field protocols on the reversible permeabilization and drying of Thai basil leaves. Innov. Food Sci. Emerg. Technol. 2020. [Google Scholar] [CrossRef]
- Wiktor, A.; Dadan, M.; Nowacka, M.; Rybak, K.; Witrowa-Rajchert, D. The impact of combination of pulsed electric field and ultrasound treatment on air drying kinetics and quality of carrot tissue. LWT-Food Sci. Technol. 2019, 110, 71–79. [Google Scholar] [CrossRef]
- Wiktor, A.; Nowacka, M.; Dadan, M.; Rybak, K.; Lojkowski, W.; Chudoba, T.; Witrowa-Rajchert, D. The effect of pulsed electric field on drying kinetics, color, and microstructure of carrot. Dry. Technol. 2016, 34, 1286–1296. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.; Chalkia, A.; Dimopoulos, G.; Taoukis, P. Combined effect of pulsed electric field and osmotic dehydration pre-treatments on mass transfer and quality of air dried goji berry. Innov. Food Sci. Emerg. Technol. 2018, 49, 106–115. [Google Scholar] [CrossRef]
- Khan, M.R. Osmotic dehydration technique for fruits preservation—A review. Pak. J. Food Sci. 2012, 22, 71–85. [Google Scholar]
- Crowe, J.H.; Crowe, L.M.; Oliver, A.E.; Tsvetkova, N.; Wolkers, W.; Tablin, F. The trehalose myth revisited. Introduction to a symposium on stabilization of cells in the dry state. Cryobiology 2001, 43, 89–105. [Google Scholar] [CrossRef]
- Galmarini, M.V.; Schebor, C.; Zamora, M.C.; Chirife, J. The effect of trehalose, sucrose and maltodextrin addition on physicochemical and sensory aspects of freeze—Dried strawberry puree. IJFST 2009, 44, 1869–1876. [Google Scholar] [CrossRef]
- Tylewicz, U.; Mannozzi, C.; Romani, S.; Castagnini, J.M.; Samborska, K.; Rocculi, P.; Dalla Rosa, M. Chemical and physicochemical properties of semi-dried organic strawberries enriched with bilberry juice-based solution. LWT–Food Sci. Technol. 2019, 114, 108377. [Google Scholar] [CrossRef]
- Ciancaglini, P.; Santos, H.L.; Daghastanli, K.R.P.; Thedei, G., Jr. Using a Classical Method of Vitamin C Quantification as a Tool for Discussion of Its Role in the Body. Biochem. Mol. Biol. Edu. 2001, 29, 110–114. [Google Scholar] [CrossRef]
- Amarowicz, R.; Naczk, M.; Shahidi, F. Antioxidant activity of various fractions of non-tannin phenolics of canola hulls. J. Agric. Food Chem. 2000, 48, 2755–2759. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Tylewicz, U.; Oliveira, G.; Alminger, M.; Nohynek, L.; Dalla Rosa, M.; Romani, S. Antioxidant and antimicrobial properties of organic fruits subjected to PEF-assisted osmotic dehydration. Innov. Food Sci. Emerg. Technol. 2020, 102341. [Google Scholar] [CrossRef]
- Wiktor, A.; Sledz, M.; Nowacka, M.; Rybak, K.; Chudoba, T.; Lojkowski, W.; Witrowa-Rajchert, D. The impact of pulsed electric field treatment on selected bioactive compound content and color of plant tissue. Innov. Food Sci. Emerg. Technol. 2015, 30, 69–78. [Google Scholar] [CrossRef]
- Nowacka, M.; Tylewicz, U.; Romani, S.; Dalla Rosa, M.; Witrowa-Rajchert, D. Influence of ultrasound-assisted osmotic dehydration on the main quality parameters of kiwifruit. Innov. Food Sci. Emerg. Technol. 2017, 41, 71–78. [Google Scholar] [CrossRef]
- Silva, A.M.; Pinto, D.; Fernandes, I.; Gonçalves Albuquerque, T.; Costa, H.S.; Freitas, V.; Rodrigues, F.; Oliveira, M.B.P.P. Infusions and decoctions of dehydrated fruits of Actinidia arguta and Actinidia deliciosa: Bioactivity, radical scavenging activity and effects on cells viability. Food Chem. 2019, 289, 625–634. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Q.; Lan, T.; Geng, T.; Gao, C.; Yuan, Q.; Zhang, Q.; Xu, P.; Sun, X.; Liu, X.; et al. Comparative Analysis of Physicochemical Characteristics, Nutritional and Functional Components and Antioxidant Capacity of Fifteen Kiwifruit (Actinidia) Cultivars—Comparative Analysis of Fifteen Kiwifruit (Actinidia) Cultivars. Foods 2020, 9, 1267. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, T.Z.; Fan, X.; Wu, J. Biochemical degradation and physical migration of polyphenolic compounds in osmotic dehydrated blueberries with pulsed electric field and thermal pretreatments. Food Chem. 2018, 239, 1219–1225. [Google Scholar] [CrossRef]
- Huang, H.; Wang, S.; Huang, R.; Jiang, Z.; Zhang, Z. ‘Jintao’, a novel, hairless, yellow-fleshed kiwifruit. HortScience 2002, 37, 1135–1136. [Google Scholar] [CrossRef]
- Piga, A.; Del Caro, A.; Corda, G. From plums to prunes: Influence of drying parameters on polyphenols and antioxidant activity. J. Agric. Food Chem. 2003, 51, 3675–3681. [Google Scholar] [CrossRef]
- Gamboa-Santosa, J.; Megías-Pérez, R.; Soria, A.C.; Olano, A.; Montilla, A.; Villamiel, M. Impact of processing conditions on the kinetic of vitamin C degradation and 2-furoylmethyl amino acid formation in dried strawberries. Food Chem. 2014, 153, 164–170. [Google Scholar] [CrossRef]
- Bozkir, H. Effects of hot air, vacuum infrared, and vacuum microwave dryers on the drying kinetics and quality characteristics of orange slices. J. Food Process Eng. 2020, e13485. [Google Scholar] [CrossRef]
- Del Caro, A.; Piga, A.; Vacca, V.; Agabbio, M. Changes of flavonoids, vitamin C and antioxidant capacity in minimally processed citrus segments and juices during storage. Food Chem. 2004, 84, 99–105. [Google Scholar] [CrossRef]
- Huang, W.; Feng, Z.; Aila, R.; Hou, Y.; Carne, A.; Bekhit, A.E.D.A. Effect of pulsed electric fields (PEF) on physico-chemical properties, β-carotene and antioxidant activity of air-dried apricots. Food Chem. 2019, 291, 253–262. [Google Scholar] [CrossRef]
- Osae, R.; Zhou, C.; Xu, B.; Tchabo, W.; Bonah, E.; Alenyorege, E.A.; Ma, H. Nonthermal pretreatments enhances drying kinetics and quality properties of dried ginger (Zingiber officinale Roscoe) slices. J. Food Process. Eng. 2019, 42. [Google Scholar] [CrossRef]
- Bialik, M.; Wiktor, A.; Witrowa-Rajchert, D.; Gondek, E. The Influence of Osmotic Dehydration Conditions on Drying Kinetics and Total Carotenoid Content of Kiwiberry (Actinidia Arguta). IJFE 2020, 16, 20180328. [Google Scholar] [CrossRef]
- Lenart, A.; Lewicki, P.P. Energy consumption during osmotic and convective drying of plant tissue. Acta Aliment. Pol. 1988, 1, 65–72. [Google Scholar]
- Dalla Rosa, M.; Giroux, F. Osmotic treatments (OT) and problems related to the solution management. J. Food Eng. 2001, 49, 223–236. [Google Scholar] [CrossRef]
- Tylewicz, U. How does pulsed electric field work? In Pulsed Electric Fields to Obtain Healthier and Sustainable Food for Tomorrow; Barba, F.J., Parniakov, O., Wiktor, A., Eds.; Elsevier: Cambridge, MA, USA, 2020; pp. 3–21. [Google Scholar]
- Donsì, F.; Ferrari, G.; Pataro, G. Applications of pulsed electric field treatments for the enhancement of mass transfer from vegetable tissue. Food Eng. Rev. 2010, 2, 109–130. [Google Scholar] [CrossRef]
- Tylewicz, U.; Panarese, V.; Laghi, L.; Rocculi, P.; Nowacka, M.; Placucci, G.; Dalla Rosa, M. NMR and DSC Water Study during Osmotic Dehydration of Actinidia deliciosa and A. chinensis kiwifruit. Food Biophys. 2011, 6, 327. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Pantelaiaki, K.; Andreou, V.; Katsaros, G.J.; Taoukis, P.S. Osmotic pretreatment for the production of novel dehydrated tomatoes and cucumbers. J. Food Process. Preserv. 2019, 43, e13968. [Google Scholar] [CrossRef]

| Treatment | Sample Code |
|---|---|
| Untreated | C |
| OD | OD |
| PEF | PEF |
| OD + PEF | OD/PEF |
| PEF + OD | PEF/OD |
| PEF + OD + PEF | PEF/OD/PEF |
| Drying Temperature [°C] | Drying Time [min] | |||||
|---|---|---|---|---|---|---|
| C | OD | PEF | PEF/OD | OD/PEF | PEF/OD/PEF | |
| 50 | 960 | 360 | 300 | 360 | 300 | 300 |
| 60 | 480 | 300 | 240 | 360 | 300 | 270 |
| 70 | 420 | 240 | 180 | 300 | 210 | 210 |
| C | OD | PEF | OD/PEF | PEF/OD | PEF/OD/PEF | |
|---|---|---|---|---|---|---|
| 50 °C | ||||||
| L* | 47.1 ± 2.1aA | 45.1 ± 4.0 aA | 43.9 ± 2.3 aA | 39.9 ± 4.5 abA | 38.7 ± 3.7 bAB | 42.9 ± 3.2 aA |
| h° | 79.1 ± 1.8 aA | 80.4 ± 1.5 aA | 78.3 ± 2.1 aA | 77.9 ± 2.1 aA | 77.7 ± 1.9 aA | 77.9 ± 1.9 aA |
| C* | 29.9 ± 3.5 a | 27.7 ± 2.8 abA | 26.7 ± 1.8 abcB | 22.8 ± 4.0 cB | 24.8 ± 1.9 bcA | 27.6 ± 4.1 bcA |
| 60 °C | ||||||
| L* | 43.5 ± 3.5 aB | 43.2 ± 4.8 aAB | 43.1 ± 2.7 aA | 39.8 ± 2.4 aA | 40.6 ± 2.5 aA | 39.7 ± 4.3 aB |
| h° | 77.4 ± 2.1 aB | 78.4 ± 1.3 aB | 78.4 ± 1.1 aA | 78.5 ± 1.9 aA | 78.3 ± 2.7 aA | 78.0 ± 2.2 aA |
| C* | 29.3 ± 2.4 aA | 27.7 ± 3.9 abA | 25.4 ± 1.9 abB | 24.7 ± 2.4 bAB | 24.6 ± 2.3 bA | 24.5 ± 4.7 bB |
| 70 °C | ||||||
| L* | 43.3 ± 5.8 aB | 42.5 ± 2.2 aB | 42.7 ± 2.3 aA | 39.5 ± 2.2 abA | 36.9 ± 1.8 bB | 42.8 ± 3.2 aA |
| h° | 77.4 ± 1.5 aB | 78.9 ± 1.3 aB | 77.3 ± 1.8 aA | 77.2 ± 1.3 aA | 77.7 ± 1.9 aA | 78.2 ± 2.5 aA |
| C* | 29.8 ± 3.9 aA | 26.4 ± 3.8 abA | 28.5 ± 1.7 aA | 25.9 ± 2.3 abA | 22.8 ± 1.2 bB | 28.9 ± 4.1 aA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannozzi, C.; Tylewicz, U.; Tappi, S.; Dalla Rosa, M.; Rocculi, P.; Romani, S. The Influence of Different Pre-Treatments on the Quality and Nutritional Characteristics in Dried Undersized Yellow Kiwifruit. Appl. Sci. 2020, 10, 8432. https://doi.org/10.3390/app10238432
Mannozzi C, Tylewicz U, Tappi S, Dalla Rosa M, Rocculi P, Romani S. The Influence of Different Pre-Treatments on the Quality and Nutritional Characteristics in Dried Undersized Yellow Kiwifruit. Applied Sciences. 2020; 10(23):8432. https://doi.org/10.3390/app10238432
Chicago/Turabian StyleMannozzi, Cinzia, Urszula Tylewicz, Silvia Tappi, Marco Dalla Rosa, Pietro Rocculi, and Santina Romani. 2020. "The Influence of Different Pre-Treatments on the Quality and Nutritional Characteristics in Dried Undersized Yellow Kiwifruit" Applied Sciences 10, no. 23: 8432. https://doi.org/10.3390/app10238432
APA StyleMannozzi, C., Tylewicz, U., Tappi, S., Dalla Rosa, M., Rocculi, P., & Romani, S. (2020). The Influence of Different Pre-Treatments on the Quality and Nutritional Characteristics in Dried Undersized Yellow Kiwifruit. Applied Sciences, 10(23), 8432. https://doi.org/10.3390/app10238432










