Abstract
Chlamydomonas reinhardtii is a green microalgae used as a model organism associated with biotechnological applications, yet its nutritional value has not been assessed. This study investigates the nutritional capacity of C. reinhardtii as an additional value for this species beyond its known potential in biofuels and bio-products production. The composition of key nutrients in C. reinhardtii was compared with Chlorella and Spirulina, the species widely regarded as a superfood. The results revealed that the protein content of C. reinhardtii (46.9%) was comparable with that of Chlorella (45.3) and Spirulina (50.4%) on a dry weight basis. C. reinhardtii contained all the essential amino acids with good scores based on FAO/WHO values (0.9–1.9) as in Chlorella and Spirulina. Unsaturated fatty acids predominated the total fatty acids profile of C. reinhardtii were ~74 of which ~48% are n-3 fatty acids. Alpha-linolenic acid (ALA) content in C. reinhardtii (42.4%) was significantly higher than that of Chlorella (23.4) and Spirulina (0.12%). For minerals, Spirulina was rich in iron (3.73 mg/g DW) followed by Chlorella (1.34 mg/g DW) and C. reinhardtii (0.96 mg/g DW). C. reinhardtii, unlike the other two species, consisted of selenium (10 µg/g DW), and had a remarkably lower heavy metal load. Moreover, C. reinhardtii contained relatively high concentrations of chlorophyll (a + b) and total carotenoids (28.6 mg/g DW and 6.9 mg/g DW, respectively) compared with Chlorella (12.0 mg/g DW and 1.8 mg/g DW, respectively) and Spirulina (8.6 mg/g DW and 0.8 mg/g DW, respectively). This study confirms that, based on its nutrient credentials, C. reinhardtii has great potential as a new superfood or ingredient for a food supplement.
1. Introduction
The world’s ever-growing population is anticipated to reach over 9.7 billion by 2050. Therefore, demands on our limited natural resources will also increase to meet the energy and nourishment requirements for such high population [,]. Currently, the world’s food production capacity is highly influenced by many challenges including the growing competition for land, clean water and energy, as well as the overexploitation of fisheries. Climate change due to the use of fossil fuels poses another threat and with it comes a requirement for food and energy production with less impact on the environment [,].
Microalgae, microscopic photosynthetic organisms, are essential for life on Earth. Their photoautotrophic growth mechanism consumes the atmospheric carbon dioxide and produces almost half of the atmospheric oxygen and constitutes the base of the food chain for aquaculture species []. With enormous biodiversity, microalgae can synthesise, accumulate, and secrete a wide range of primary and secondary metabolites []. Many of which are valuable substances with potential applications in various industries such as food, feed, cosmetics, pharmaceutical and nutraceuticals. Yet, microalgae are considered as under-exploited “food crops” [].
Studies suggest that most microalgae species can synthesise high-quality protein characterised by favourable amino acid profiles, which competes well with the quality of conventional protein sources []. Large-scale microalgae production as a potential solution for the predicted shortage of protein supply in the world has been proposed, and the National Aeronautics and Space Administration (NASA) has agreed that microalgae make great, compact food for astronauts, while the WHO recognised microalgae as a “Super Food” [].
The lipid content and fatty acids profile of microalgae are of key interest. In the biofuel field, an optimal balance between saturated and unsaturated fatty acids is sought to be achieved. In contrast, essential polyunsaturated fatty acids (PUFA) which mammals are unable to synthesise are the target for food and feed purposes []. It is well agreed that for the algal biotechnology to achieve its potential, it is necessary to investigate more species and characterise their chemical and physical properties as well as culturing conditions and downstream processing [].
Microalgae produce relatively high amounts of lipids and, therefore, they are regarded as the third-generation feedstock for biofuels with the potential to override the 1st generation (crop-based) and second-generation (lignocellulosic-based) biofuels. Due to their high lipid content and ability to grow all year round, microalgae can be considered one of the best sources for biodiesel production. They can produce 58,700–136,900 L/ha/year of oil compared with 172–5950 L/ha/year of oil from the terrestrial oilseed crops []. Hence, producing biodiesel from microalgae may be the only way to produce a sufficient amount of fuel to replace current petro-diesel usage in the transportation sector. Cultivating microalgae for food and bioenergy does not compete with the terrestrial food crops for resources and/or space as marginal and non-arable land is adequate to set up a large scale open or closed system for production []. Reducing land use and carbon and water footprints is an essential element for sustainable biofuel production [,].
C. reinhardtii is the most researched unicellular green microalgae with sophisticated genetic tools and has become the model organism to study a variety of cellular functions []. The chloroplasts of C. reinhardtii have been engineered to produce recombinant proteins intended for therapeutically use in either humans or animals []. For this purpose, C. reinhardtii has been proved to be an inexpensive and easy to scale up the system. C. reinhardtii is generally regarded as safe (GRAS) []. Oral vaccines engineered from the chloroplast of this microalgae could be available with reduced cost and improved easiness to use and handle []. In feed applications, notably delivery of dietary enzymes with no need for protein purification, algal phytases have been expressed replacing the use of microbial phytases, widely used as feed additives to increase phytate phosphorus utilisation and to reduce faecal phytates and inorganic phosphate (ip) outputs []. The engineering of recombinant protein in C. reinhardtii has also been used to increase its content of high-value nutrients; for example, the expression of human selenoprotein to combat Se deficiency, reducing the risk of toxicity associated with the direct consumption of inorganic Se []. For carotenoids, C. reinhardtii is one of the Chlorophyceae microalgae species currently used for commercial production of carotenoid pigments [].
Currently, Chlorella (green microalgae) and Spirulina (cyanobacteria) are the dominant microalgae species produced and promoted as food supplements to boost good health and wellbeing. This is due to their well-established history of cultivation and safety for human consumption; they are also used as animal feed []. On the other hand, C. reinhardtii is well used as a model organism to investigate biological processes in photosynthetic eukaryotes and to elucidate metabolic processes in plants [].
Microalgae are grown using three modes of cultivation, namely; autotrophic, mixotrophic and heterotrophic. It was reported that using acetate in a mixotrophic condition was optimal for C. reinhardtii to produce the highest yield and the highest lipid content []. Hence, in this study, C. reinhardtii was cultivated under mixotrophic conditions using Tris-Acetate-Phosphate (TAP) medium. On the other hand, Chlorella and Spirulina were bought from a company called Naturya that sells a variety of superfoods. According to the company website (https://naturya.com/vegan-protein), those microalgae were grown autotrophically in open freshwater ponds located on an island in South China. They were harvested, washed with fresh water and fast dried using drying chamber (heat drying). In addition, Chlorella cells were milled to break down the hard cell wall to ensure better bioaccessibility by human gut [].
It is well proved that the growth conditions (including; light, carbon source, nutrients, temperature, pH, etc.) affect the composition and the characteristics of microalgae []. Therefore, it is generally recommended to grow different microalgae species under similar conditions for accurate comparison. However, this study aims to establish the nutritional capacity of C. reinhardtii in comparison with the samples already available in the food supplement market as they sold and consumed. Hence, the commercially available Chlorella and Spirulina samples were obtained from a trusted commercial source. The nutritional composition (macro and micronutrients) of lab-cultivated C. reinhardtii and the commercial Chlorella and Spirulina samples were analysed and compared in our labs.
2. Materials and Methods
2.1. Preparation of Stock Solutions
C. reinhardtii was grown in Tris-Acetate-Phosphate (TAP) []. Culturing was performed aseptically using a laminar flow (Microflow Peroxide Advanced Biosafety Cabinet, class II) and a sterilised (autoclaved) TAP media. The cultures were grown mixotrophically on an orbital shaker incubator with a photon irradiance. The growth condition was adjusted at 100 rpm with ambient CO2 level, 23 °C and 16:8 h alternating light: dark cycle, with a photon irradiance of 100 ± 5 µmol/m2/s for 7 days.
2.2. Growth Curve Establishment
The algae growth rate was measured at regular intervals using two methods simultaneously. A volume of 5 mL C. reinhardtii culture was taken from three independent flasks after being mixed thoroughly and measured for its optical density at 680 nm with Spectrophotometer (T80 UV/VIS Spectrometer). Appropriate dilution with TAP medium was applied once needed. Another three lots were used for counting the cells in known volume under an optical Microscope (25 × objectives, Zeiss Axiopian) using a haemocytometer []. Prior to counting under a microscope, samples were diluted by 1:4 with iodine solution (0.25 g iodine in 100 mL 95% ethanol) to immobilize the cells. The number of cells/mL, growth rates and doubling time was calculated using a haemocytometer [].
2.3. Sample Preparations for Transmission Electron Microscope (TEM)
TEM (EI Tecnai G2 12 Biotwin, ThermoFisher Scientific, Massachusetts, USA) was used to visualise morphology and localisation of the intracellular cell organelles. Samples (1 mL) were prepared as described by Gedi []. Briefly, samples were fixed with 3% glutaraldehyde in 0.1 M cacodylate buffer. They were then transferred into an Eppendorf tube and centrifuged at 500 rcf for 5 min (ROTINA 380R centrifuge, Tuttlingen, Germany). The supernatant was removed while pellets were suspended in a 1% osmium tetroxide (prepared in 0.1 M cacodylate buffer) and left for one h then centrifugation at 500 rcf for 5 min. Samples were then washed twice by re-suspending the pellets in distilled followed by centrifugation at 500 rcf for 5 min. Clean pellets were then dehydrated using 50, 70 and 90% ethanol solutions (2 changes with each concentration, 15 min for each change), then 100% ethanol (3 changes, 20 min for each change) and finally 100% propylene oxide (2 changes, 15 min for each change).
A resin in the ratio of 1:3 and 1:1 (resin: propylene oxide) was prepared. Initially, 1 mL of the resin 1:3 was added into the samples and left for 3 h. Samples were then centrifuged at 6000 rcf (ROTINA 380R centrifuge, Tuttlingen, Germany), supernatant resins removed and a new resin (1:1) was added. Tubes were then left overnight under the fume hood with the lids off. Finally, samples were suspended in a pure resin (3 changes, 2.5 h) then left in the oven (60 °C) for 48 h. Thin sections of the samples (90 nm) were cut with ultra-microtome (Leica EM, Leica Biosystems, Wetzlar, Germany) using a diamond knife. Thin sections of each sample in the nanoscale were then visualised under the TEM at X9900, X20500 and X48000 magnification, respectively.
2.4. Microalgae Biomass Preparation
C. reinhardtii batch cultures were grown as in Section 2.1, harvested during early stationary phase, lyophilised and stored at −20 °C for further analysis. Spirulina (Organic Spirulina Powder, 200 g) and Chlorella (Organic Chlorella Powder, 200 g) samples were grown on an island in South China, then manufactured and packaged by Naturya Superfood based at Bath, UK.
2.5. Ash and Residual Moisture
Three samples (0.5 g) of each microalgae species were dried overnight in a drying oven at 105 °C then weighed every two hours until three constant readings were obtained. The residual moisture content was measured as % weight loss.
Ash was determined by weighing 0.5 g of the microalgae samples in pre weighed crucibles then placed in a muffle furnace (CARBOLITE-ES3133) with a temperature ramping for 8 h (ignition at 550 °C). On the second day, crucibles were placed in desiccator and the weight was recorded. Ash content was calculated gravimetrically as % weight loss.
2.6. Total Lipid
Total lipid was extracted using Folch, et al. [] method, with slight modifications. Dried microalgae (Chlorella or Spirulina or C. reinhardtii) powder (0.1 g) was mixed with 2.4 mL chloroform: methanol (2:1). The mixtures were vortexed for 1 min and then sonicated for 15 min at 40 kHz, 150 W. To this, 0.6 mL of 0.9% NaCl in water was added and vortexed for 1 min. The mixtures were then centrifuged at 1300 rcf for 10 min at 4 °C (ROTINA 380R centrifuge, Tuttlingen, Germany). The lower, lipid-containing, chloroform layer was then collected. The pellets were extracted two more sequences by repeating the same procedure except for the Chlorella samples, which were further homogenised with a mini bead beater using steel beads to break down the rigid cell wall and allow maximum lipid extraction. Pooled extracts were combined and filtered through a 0.45 µm PTFE syringe and dried under a gentle flow of nitrogen gas. The content of total lipid was calculated gravimetrically.
2.7. Fatty Acids Profile Analysis
Fatty acids composition was determined using a TRACE GC Ultra Gas Chromatography-Mass Spectroscopy (GC-MS) equipped with a CTS Analytics PAL system autosampler (Thermo Fisher, Loughborough, UK). Initially, 2 mL of chloroform was added to each lipid extract from Section 2.6, followed by addition of 100 µL of methyl pentadecanoate (10 mg/mL) as an internal standard, and 200 µL of trimethylsulfonium hydroxide as methylation agent. The lipid extracts were then left for 10 min to achieve complete conversion to fatty acid methyl esters (FAMEs) and then filtered through 0.45 µm syringe filter into amber vials. Column and GC-MS conditions were based on Dron, et al. [].
2.8. Chlorophyll and Carotenoids Analysis
The total lipid extracted from 0.1 g sample (as in Section 2.6) was dried with N2 then dissolved in 1 mL acetone (100%) then further diluted to reach a final dilution of 1:1000 (lipid: acetone). Using glass cuvette and pure acetone as blank, the absorbance was measured with a spectrophotometer (CARY 50 Probe UV-visible) on the following wavelength, λ = 661.6 nm for chlorophyll α, λ = 644.8 nm for chlorophyll b, λ = 470 nm for carotenoids. The concentration of chlorophyll and carotenoids were calculated using published equations [].
2.9. Total Protein Content Analysis
Sample of each microalgae powder and two standards (Sulphanilamide STD) (3 mg) were separately weighed in tin capsules. They were then run on an Organic Elemental Analyser (Flash 200, Thermo Fisher Scientific Inc, Loughborough, UK). The Nitrogen values were automatically calculated in percentage. The element nitrogen values were converted to protein using a conversion factor of 4.78, recommended for marine microalgae [,].
2.10. Amino Acids Analysis
The amino acids composition was determined using the oxidative–hydrolysis method by the AOAC Official Method 994.12 []. Samples (0.5 g) of each species were oxidised with a 5 mL of chilled hydrogen peroxide/formic acid/phenol mixture for 16–18 h. The mixture was prepared at two steps; (a) mixing 735 mL formic acid with 111 mL deionised water then adding 4.73 g phenol, (b) dispensing 10 mL of 30% hydrogen peroxide into a 100 mL volumetric flask then making up to volume with the formic acid/phenol solution. Subsequently, 0.84 g of sodium metabisulfite and 50 mL of the hydrolysis reagent (6 M HCl) were added to each sample and then placed in the oven at 110 °C for 24 h with loosened bottles’ lids to prevent the gas pressure building up. The pH of each sample was adjusted to 2.20 using 1 N sodium hydroxide and 4 mL of internal standard (norleucine) was added. Samples were then rinsed using tri-sodium citrate (pH 2.20) and the flask volume was filled to 200 mL. Diluted hydrolysate (20 mL) was centrifuged (Biofuge stratos) at 3000 rcf for 2 min. The supernatant was filtered with a 0.22 µm filter syringe. Subsequently, amino acids were separated by ion-exchange chromatography (Pharmacia Biochrom, Cambridge, UK) and determined by reaction with ninhydrin using photometric detection at 570 nm (440 nm for proline).
Essential amino acid score (EAAS) was calculated according to the Equation (1):
where eaa1 is the concentration of one essential amino acid (mg) per g protein of the sample and EAA1 is the concentration of the same essential amino acid (mg) per g of the reference protein. An essential amino acid score of > 0.95 defines a “high” quality protein, a score of 0.86–0.95 implies a “good” quality protein, a score of 0.75–0.86 indicates a “useful” protein and a score of < 0.75 means an “inadequate” protein according to the FAO/WHO/UNU [].
2.11. Minerals Analysis
Acid digestion was conducted by adding 6 mL of 2% trace analysis grade HNO3 in Teflon vessels to 0.2 g of dry microalgae samples. The vessels were then heated up using microwave digestion system (Multiwave PRO, Anton Paar, Graz, Austria) for heating up to 140 °C in 10 min and held for 20 min then cooled down to 55 °C for 15 min. Milli-Q® water was then added to the samples to reach a final volume of 20 mL. Subsequent 1:10 dilution was followed, and the diluted samples were then stored at 4 °C ready for conducting the analysis. Multi-element analysis of diluted solutions was carried out using inductively coupled plasma mass spectrometry (ICP-MS) (ICAP-Q; Thermo Fisher Scientific, Bremen, Germany). For analytical quality control, blanks, duplicates, internal standards and certified reference materials were analysed in all instances of the experiment. The certified reference materials were tomato leaves (1573A) from the National Institute of Standards and Technology, Gaithersburg, MD, USA.
2.12. Statistical Analysis
All experiments were carried out in triplicates. The values presented herein are expressed as means ± SD of each sample in triplicate. Statistical analyses were performed using one-way analysis of variance (ANOVA) using Minitab software with a 5% significance level. Where significant differences were observed, treatments means were differentiated using pairwise multiple comparison procedures (Tukey post hoc test).
3. Results and Discussion
3.1. Growth and Ultrastructure of C. reinhardtii
The growth kinetics of C. reinhardtii was determined during 30 days of cultivation for quality monitoring of C. reinhardtii batch cultures to achieve a stable and steady quality of the biomass used in subsequent analysis (Figure 1A). Figure 1A shows cell growth number reached 1.68 × 107 cell/mL with a doubling time of 9.35 h. It was reported that the doubling time of C. reinhardtii ranges between 7 and 17 h and it depends on many factors including strain type and growth condition (light duration and intensity, temperature, media nutrient, etc.) [,].
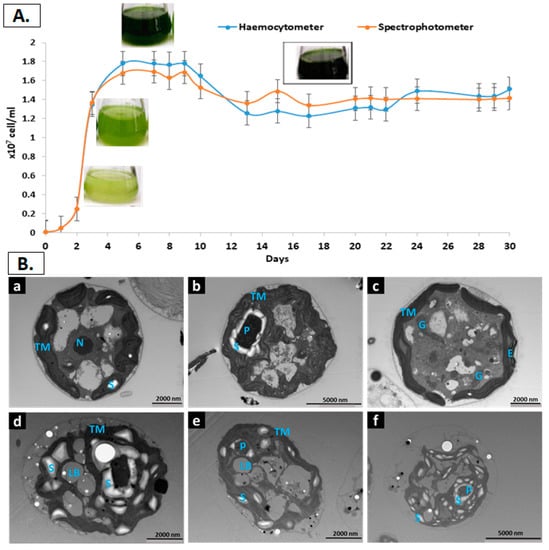
Figure 1.
(A) Growth curve of C. reinhardtii population grown in mixotrophic condition and measured using either a haemocytometer or spectrophotometer. Data representative of triplicate measurements (SD = 3). (B) TEM images of C. reinhardtii grown in TAP and imaged at different time and conditions, a to c are the images of the 14 days old cells while d to f are the images of the 30 days old cells. Letters in blue inside the images refer to the following: P: Pyrenoid; LB: Lipid Bodies; S: Starch granule; TM: Thylakoid Membrane; E: Eyespots; G: Golgi bodies.
Four distinct phases were recognised during C. reinhardtii growth, which resembles the expected microalgae growth curve when total cells are measured. The first phase is the lag or induction phase, which is characterised by a slight increase in cell density due to the time required for the cells’ metabolism to adapt to the new environment such as enzymes level and metabolites required for cell division and photosynthesis. This phase lasted for around 2 days when cell number of C. reinhardtii reached 0.2 × 107 cells/mL. The second phase is the logarithmic (exponential) phase when cell density increment follows a logarithmic function. This phase lasted for around 3 days with a maximum cell number of 1.8 × 107 cells/mL. The third phase is the early stationary phase, which starts when the media limiting factors and the cell division rate are balanced, resulting in a relatively constant cell density. The cells ranged from1.8 × 107 to 1.2 × 107 cells/mL during the 5 days of this phase. The fourth phase is the late stationary or aged culture, which comprised the last 20 days of the cultivation. During this phase, the change in chemical and physical factors limits the growth and slows down the cell division pace. The cell number slightly decreased to a range between 1.2 × 107 and 1.4 × 107 (Figure 1A). Similar results for cell count was obtained throughout the growth curve using haemocytometer and spectrophotometer. This confirms the accuracy of the results as well as the methods used.
Representative ultrastructure of TEM images are also presented in Figure 1B. The images revealed different cell sections with a detailed morphology and localisation of each intracellular cell organelles. The round-shaped images resulted from a section across the width, while the oval-like images represent a section across the length []. The sheet-like thylakoid membranes (TM) are the main component of the chloroplast, the organelle that occupies two-thirds of the cells grown under standard conditions, which is clear in Figure 1Bb, where chloroplasts occupy a significant part of the cell volume. The eyespot, as it appears in Figure 1Bc, is attached to the inner plastid membrane. In cells grown in nutrients replete media to stationary phase (Figure 1Ba–c), all the cell organelles are observable in different sections. In addition, few starch granules were detected at the stationary phase, while no lipid bodies were identified. Conversely, both storage entities (starch granules and lipid bodies) are abundant at 30-days old cells, while TM are less noticeable (Figure 1Bd–f). It is well established that lipid accumulation as TAG in microalgae increases as the cells age, and generally as a result of nutrient consumption during the stationary growth and is accompanied by a cessation in cell division [].
3.2. Macronutrient Content
The composition of the whole intact biomass of C. reinhardtii, Spirulina and Chlorella was analyzed and calculated as a percentage of dry weight (DW) (Figure 2). The moisture content of 1.1% for C. reinhardtii, 1.8% for Spirulina, and 1.88% for Chlorella are within the range of the recommended limit of <10% for microalgae powder quality []. The protein content of C. reinhardtii (46.9%) was slightly higher than that of Chlorella (45.3%) and slightly lower than that of Spirulina (50.4%), however, there were no significant differences between the three species (p ≥ 0.05).
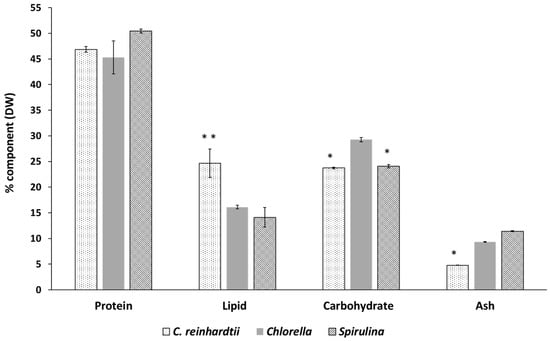
Figure 2.
Macronutrient content of Chlamydomonas reinhardtii, Spirulina and Chlorella on dry weight (DW) basis. Results are the means of triplicate determinations ± SD. Values with two asterisks (**) are significantly higher within the same component and those with one asterisk (*) are significantly lower within the same component at (p ≤ 0.05) using Tukey’s test. Carbohydrate content was calculated by difference: Carbohydrate = 100−(Protein + Lipid + Ash).
Protein sources of “not animal origin” are generally divided into two groups, conventional such as legumes and non-conventional proteins such as single-cell protein including microalgae []. The protein contents of the three studied species were superior to that of high-protein plant foods such as soybean (37%) and milk (26%) [].
Kent et al. [] reported that the protein content (calculated as a sum of amino acids) was 51.56% for Spirulina, and 39% for Chlorella, which is in close agreement with the values obtained in this study. Similarly, primary metabolism and the biomass composition of C. reinhardtii as % of DW under different energy inputs were measured and it was found that the protein content ranged between 37 and 42% []. Likewise, differences in the basic composition of C. reinhardtii under different growth conditions, where the protein content under mixotrophic growing system reached 38.1% DW was reported [].
The ash content of the three microalgae species in this study varied significantly. Ash content in microalgae is directly correlated with the concentration of inorganic compounds and salts in the water environment, where the microalgae were grown []. On a dry weight basis, C. reinhardtii exhibited the lowest ash content (4.8%), followed by Chlorella (9.3%) and Spirulina (11.4%). The three values are well situated within the wide range of 8–40% ash content observed in microalgae []. The ash contents of the three species were lower than the maximum value allowed in algal products sold in the USA (45% DW), and were comparable with that of land vegetables, ranging between 5–10% DW []. The lower ash content in C. reinhardtii may help to explain its relatively high content of lipids and pigments (chlorophyll and carotenoids) compared with the commercial species Chlorella and Spirulina.
In Figure 2, C. reinhardtii showed a lipid content of 24.7% DW, which is higher than that of Chlorella (16.1%) and Spirulina (14.1%). Lipid content varies even among the same microalgae species and is highly affected by the growing parameters, more specifically the growing stress conditions. Numerous studies have been conducted to boost the total lipid level in C. reinhardtii by applying different techniques of cell stressing. Reducing the nitrogen content was one of the most common stressing techniques which increased neutral lipid accumulation, as TAG (triacylglycerol) droplets (from <1 μg up to 11 μg per million cells DW basis), accompanied with reduced photosynthetic efficiency and decreased growth. The stressing technique was extensively studied to boost TAG content for biofuel production from microalgae [].
C. reinhardtii had lower carbohydrate content compared with either Spirulina or Chlorella products (Figure 2). Total carbohydrates in microalgae vary with the species, cultivation conditions and cultivation time during the cell’s life cycle. Generally, green microalgae contain about 20% (DW) carbohydrates, half of which is starch []. Algal carbohydrates can offer dietary fibre functionality; some are also claimed to have other benefits to human health such as antioxidant, anticoagulant and antiviral properties []. Briefly, the macronutrients composition of C. reinhardtii was comparable with that of commercial Spirulina and Chlorella, paving the way for further detailed analysis to assess its micronutrients content, digestibility and potential use as a source of healthy food.
3.3. Amino Acids Profile
Quantitative and qualitative determination of amino acid are shown in Table 1. The total amino acids composition represents both protein constituents, free amino acids and/or amino acid salts. Protein quality is valued based on 8 essential amino acids, namely, methionine, leucine, isoleucine, lysine, phenylalanine, threonine, tryptophan and valine []. The essential amino acids score (EEAS) “determines the effectiveness with which absorbed dietary nitrogen can meet the indispensable amino acids requirement at a safe level of protein intake” []. The quantity of each essential amino acid in each microalgae is compared with the level of the amino acids required for pre-school children (2–5 years old) []. Additionally, according to the report of the joint FAO/WHO expert consultation (1989) this scoring pattern is robust enough to be used for all ages and adults, except for infants [].

Table 1.
Amino acids composition of C. reinhardtii, Spirulina and Chlorella, expressed as mg/g DW.
Sample for each amino acid determination was analysed in triplicate and reported as a mean ± SD value using one-way Anova. Means that do not share a superscript letter are significantly different based on Tukey Simultaneous 95% Cis.
†: The pattern requirement (g/100 g protein) for pre-school children (2–5 years) recommended by the Food and Agriculture Organisation (FAO), World Health Organisation (WHO), and United Nations University (UNU).
The results exhibited no significant difference between the total sum of the essential amino acids per g protein of C. reinhardtii and Spirulina (458.4 and 449.8 mg/g, respectively), however, it was a bit higher in Chlorella (468.9 mg/g). For their EAAS i.e., C. reinhardtii (1.49) Chlorella (1.55) and Spirulina (1.48) the three species can be labelled as a source of “high-quality” protein and their EAA composition meets the FAO requirements of pre-school children. However, this conclusion should be further supported by in-vitro and in-vivo digestibility trials of various microalgae species and be compared with another protein source.
The essential amino acid with the lowest score represents the “most limiting amino acid”, hence, the limiting amino acids in the three species was lysine. This is in good agreement with the general trend of most microalgae protein to have tryptophan and lysine as limiting amino acids []. Among the essential amino acids, leucine exhibited the highest content in the three species.
Glutamic acid was the most dominant amino acid in the three species. They comprised of high concentrations of glutamic acid, ranging between 120 to 137 mg/g (Table 1). Glutamic acid contributes to the fifth flavour “Umami” or “Savoury”, and increasing attention is being paid to its natural sources, such as microalgae, which contain high amounts of glutamic acid [].
3.4. Fatty Acids Profile of C. reinhardtii
In this study, total fatty acid (TFA) profile of C. reinhardtii as well as Chlorella and Spirulina was analysed using GC-MS (Table 2). C. reinhardtii contained the highest amount of TFA compared with Spirulina and Chlorella, which is consistent with its higher concentration of the total lipids. The fatty acid profile of C. reinhardtii is marked by a high amount of α-linolenic (ALA) (C18:3 n-3), accounting for 42.4% of its TFA. Palmitic acid (C16:0) was the second predominant fatty acid with 23.8%, followed by oleic acid (C18:1) with 14.7% of its TFA content. In contrast to C. reinhardtii, the fatty acid profile of Chlorella showed that linoleic acid (LA) (C18:2 n-6) was the dominant fatty acid (31.4% of the TFA), followed by ALA (23.4% of the TFA) and palmitic acid (22.2%). Despite this clear difference, the proportion of unsaturated fatty acids (USFAs) to saturated fatty acids (SFAs) was nearly the same in both C. reinhardtii and Chlorella with USFAs constituting above 70% of TFAs.

Table 2.
Fatty acid profile of C. reinhardtii, Spirulina and Chlorella.
Spirulina, on the other hand, showed a different trend, where SFAs, mainly palmitic acid (57.88% of the TFA) outweighed the USFAs. Unlike C. reinhardtii and Chlorella, gamma-linolenic (GLA) (C18:3 n-6) (19.45% of the TFA) is the main USFA in Spirulina followed by LA (18.9% of the TFA). Fatty acid profile is used in many studies as a chemotaxonomic marker to define taxa in algae generally. For example, eukaryotes, including green microalgae, produce ALA and PUFAs as major fatty acids stored as acyl lipids located mainly in the chloroplasts []. Likewise, cyanobacteria or photosynthetic bacteria, as well as non-photosynthetic bacteria, exclusively produce saturated and monounsaturated fatty acids. However, some genera like Spirulina, can synthesis GLA which is usually linked to the galactolipids []. Several studies investigated the potential of cultivating Spirulina platensis under various growing conditions to produce GLA which can reach up to 32.6% TFA with the advantage of being easy to purify compared to other sources. Gamma-linolenic acid (GLA) is an n-6 fatty acid and, once consumed by humans, is a precursor for monoenoic prostaglandins such as PGE1, an important biologically active compound necessary for reducing chronic inflammation and blood pressure [].
C. reinhardtii grown under favourable conditions is rich in membrane lipids, which are characterised by their high content of PUFAs (60 mol%), of which, 80% are C18:3 (n-3), C16: 4 (9 n-3), C18: 4 (n-3), and C18:3 (n-6) []. The n-3 PUFAs also proved to be present in both plastidic and extraplastidic membrane lipids such as monogalactosyldiacylglycerol (MGDG) and phosphatidylethanolamine (PtdEtn) []. Many studies have confirmed that C. reinhardtii accumulates triacylglycerol (TAG) once grown in a media limited with nitrogen, sulphur and phosphorous. In these cases, TAGs rich in C16:0, C18:1 and C18:2 accumulate in oil bodies in parallel with an almost 80% reduction in the major plastidial membrane lipids and hence a reduction in n-3 PUFAs []. Unlike plants, where there are distinct genes for plastidial and extraplastidial n-3 fatty acid desaturases, only one putative n-3 desaturase seems encoded in C. reinhardtii genome. Several explanations have been suggested, first, the existence of a mechanism to export n-3 acyls from their site of biogenesis to other membranes. Second, the presence of a dual localisation of the n-3 desaturase homolog (plastid and endoplasmic reticulum) [].
The ratio n-6:n-3 in C. reinhardtii was the lowest followed by Chlorella, while it was undefined in Spirulina since the amount of n-3 was extremely low (Table 2). Epidemiological studies have been conducted to investigate the many health benefits of n-3 PUFAs. The positive effects of n-3 PUFAs on cardiovascular disease, diabetes, cancer, Alzheimer’s disease, dementia, depression, visual and neurological development, and maternal and child health were summarised in a recent review []. The n-3 and n-6 fatty acids compete in several enzyme systems and thus n-6:n-3 FAs ratio may be of value in interpreting biomarker data and in making nutritional recommendations. The most beneficial ratio of n-6:n-3 in the human diet was recommended to be 1:1 up to 4:1, however, the ratio in the Western diet is 15:1–16.7:1 []. The study also linked the high n-6: n-3 ratio, common in the Western diet, with pathogenesis of several diseases, namely cardiovascular, inflammatory, cancer and autoimmune Alzheimer’s disease [].
3.5. Chlorophylls and Total Carotenoids
Chlorophylls, carotenoids (oxygenated and non-oxygenated), and phycobilins are the three major groups of photosynthetic pigments in microalgae. Extraction of pigments from microalgae can be done either by using a solvent or supercritical CO2 method []. In this study, solvent extraction was employed to extract the pigments followed by a spectrophotometric method to determine the concentrations of chlorophyll a and b, as well as total carotenoids in the lipid extract of the studied species (Figure 3).
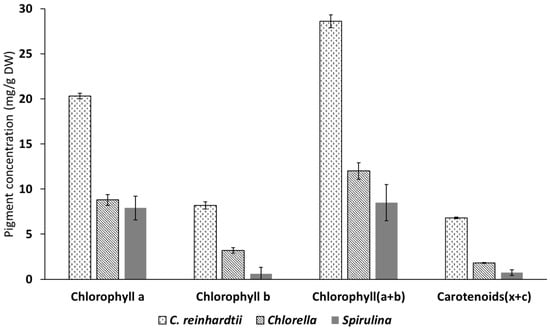
Figure 3.
Pigment concentration of Chlamydomonas reinhardti, Spirulina and Chlorella expressed as mg/g DW. Results are the means of triplicate determinations ± SD.
The results obtained show that C. reinhardtii was a superior source of chlorophyll a and b as well as for total carotenoids when compared with both Chlorella and Spirulina. The two main chlorophylls (a and b) were also present at high concentrations in Chlorella, although extra steps of cell wall disruption using a mini bead beater were needed to allow the full extraction of the green pigments (this was assessed visually). This step was not necessary for C. reinhardtii and Spirulina because Chlorella has cellulosic cell wall which is more robust and needed mechanical disruption to be permeable. These results are supported by the literature that chlorophyll a and b are the most dominant in green microalgae []. However, the ratio of these pigments is highly affected by the growing conditions.
Spirulina contained the lowest amount of Chlorophyll b and total carotenoids. Spirulina, however, contains a high amount of the blue pigment phycocyanin which has important application in food colouring and as potent antioxidant, immune system enhancer and having anti-viral properties [].
The importance of these pigments comes from their many biological values, such as pro-vitamins and antioxidant, and is a natural alternative to the synthesised colouring agents []. Total chlorophyll and carotenoid levels for various leafy vegetables were assessed within our group []. The study found that spinach leaves from a local supermarket contained 7.8 mg/g DW of chlorophyll (a and b) and 2.2 mg/g DW of total carotenoids. Comparing to spinach, C. reinhardtii could be considered a super-rich source for both chlorophyll (28. 6 mg/g DW) and carotenoids (6.8 mg/g DW).
3.6. Minerals and Heavy Metals Composition
Minerals content of microalgae is highly correlated with the growing environment. It also varies with microalgae species, harvesting time, growth phase and growing site. These minerals are not only essential for their growth but can also be a valuable source of a wide variety of macrominerals and trace elements for humans and animals [].
The mineral composition as well as the heavy metals concentration of C. reinhardtii, Spirulina and Chlorella are shown in Table 3. Phosphorus (P) was the most abundant essential element in both Chlorella and C. reinhardtii, while potassium (K+) was the dominant one in Spirulina and the second most abundant in Chlorella. Spirulina and Chlorella had higher mineral content than C. reinhardtii for most studied minerals except for calcium (Ca2+), copper (Cu2+) and selenium (Se2+). Table 3 shows that C. reinhardtii had higher concentrations of Mn2+, Cu2+ and Fe3+ than the values reported for some land vegetables such as lettuce, cabbage, carrots, broccoli and spinach []. The three studied species contained higher amounts of Fe3+/Fe2+ than spinach, which is regarded as an iron-rich food []. Importantly, Spirulina is a very rich source of iron (3.73 mg/g DW), thus in theory, consumption of 2.15 g DW per day covers the daily recommendation of a male adult, while 8.4 g DW and 6 g DW of C. reinhardtii and Chlorella would be required, respectively, assuming all iron is bioavailable for absorption []. However, iron bioaccessibility and thus its bioavailability from microalgae generally needs to be further investigated.

Table 3.
Mineral composition of C. reinhardtii, Spirulina and Chlorella.
C. reinhardtii contained 0.01 mg/g DW selenium while Chlorella and Spirulina lacked such essential nutrient. A daily allowance of 55 µg (0.7 µmol) is recommended for adult men and women. To cover the daily recommendation of selenium, for example, 5.50 g of C. reinhardtii per day is theoretically adequate. Selenium (Se2+) is important for selenoprotein enzymes, which function as defence antioxidants (glutathione peroxidases (GPx)) []. A link between the micronutrient Se2+ deficiency and virulence of some contemporary RNA viruses was reported, and supplementation of Se-deficient virus-infected hosts with dietary Se2+ diminished viral mutation rates and improved immunocompetence []. It might be worth to note that the current pandemic Covid-19 is among the RNA viruses, and thus the adequate intake of Se2+ might have a positive effect on reducing its mutation rates, too.
Generally, many health benefits are associated with the intake of microalgae, however, there are also concerns to be considered. Microalgae are known for their ability to absorb and accumulate heavy metals, which have detrimental health effects on human and animals []. In this study, the amounts of arsenic, cadmium, silver and lead were analysed (Table 3). Spirulina and Chlorella contained higher amounts of cadmium (Cd2+), arsenic (As2+), lead (Pb2+) and silver Ag+ than C. reinhardtii.
There are several examples of heavy metals’ adverse effects on human health. For instance, arsenic-related cancer, as well as peripheral vascular diseases and renal tubular dysfunction are associated with exposure to cadmium and reduced mental development associated with mercury []. Limits for presumed safe intakes of contaminants, known as the provisional tolerable weekly intake (PTWI) for all the heavy metals have been set by the FAO/WHO Joint Expert Committee of Food Additives (JECFA) []. PTWI is “an estimate of the amount of a substance in air, food, soil or drinking water that can be assimilated weekly per unit body weight (bw) over a lifetime without appreciable health risk” []. The FAO/WHO has established a PTWI of 5 µg/kg, 5.6 µg/kg and 25 µg/kg body weight per week, respectively, for total mercury, cadmium and lead. Arsenic content of the algae analysed in this study was 0.83–0.88 mg/kg DW of Spirulina and Chlorella, respectively, while levels of cadmium were the highest in Chlorella 0.19 mg/kg DW. Lead content of both Spirulina (2.97 mg/kg DW) and Chlorella (1.85 mg/kg DW) were particularly high which raises questions about the safety of these currently available microalgae supplements. However, these values do not pose any risk within the reasonable consumption of any microalgae supplement which is usually declared on the package and most of these supplements are served as tablets of 500 mg, which can be taken twice a day maximum. For example, to exceed the PTWI of lead, weekly consumption amount by an adult weight (60 Kg) of 0.5 Kg of Spirulina, 0.81 kg of Chlorella and 16.6 Kg of C. reinhardtii. The poisonous potential or effect of the heavy metal is also dependent on its physical state. While arsenic is most toxic in its inorganic form, mercury is most toxic in its organic form methylated mercury, MeHg [].
4. Conclusions
The chemical composition of C. reinhardtii was analysed and compared with Spirulina and Chlorella spp. Although both conventional food species, Spirulina and Chlorella spp., contained valuable nutrients, C. reinhardtii outperformed them in several nutritional factors. Taking into consideration that different growing conditions might affect the composition of the three studied species, nonetheless, C. reinhardtii surpassed both the reference species regarding the lipid content and the quality of its fatty acid profile which contains higher amounts of USFAs of which 48% are omega-3 fatty acids. In addition, C. reinhardtii compared well with Spirulina and Chlorella in terms of its protein content and the quality of the amino acids. Even though the three species showed a high concentration of pigments (chlorophyll a and b and total carotenoids), C. reinhardtii contained significantly higher amounts of these high-value chemicals. Furthermore, C. reinhardtii contained 10 µg/g of selenium, revealing a new source of such rare and vital nutritional element. Furthermore, C. reinhardtii contained significantly lower heavy metal load than the commercially grown Chlorella and Spirulina, which eliminates the risk of heavy metals accumulation imposed by high dosage of microalgae and seaweeds generally. The results obtained in this study introduces C. reinhardtii as a new valuable ingredient in feed, food and dietary supplement industries. This is very important as C. reinhardtii already has a wide exploitation in biotechnology and biofuel industries so that the end product can be altered based on the market needs. Moreover, they give a strong base and pilot data to design further experiments on C. reinhardtii digestibility using in vitro and in vivo models as well as sensory studies. This is to complete the picture in terms of its nutrient’s bioavailability upon human and animal consumption and establish the optimal dose and way of consumption.
Author Contributions
R.D. conceived, designed and performed the experiments, and wrote the first draft of the manuscript; M.A.G. and A.S.Z. contributed in designing the experiments, analyzing the data, and writing the manuscript; P.A. and H.A. collaborated in the lab work; D.A.G. initiated the idea, supervised the research and proofread the manuscript. All the authors discussed data, revised the manuscript and approved the final manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank Alison Smith and her research group at the University of Cambridge for providing essential training and materials. We also would like to thank Dongfang Li from the School of Biosciences and Denise McLean from the School of Life Science for technical support. The authors are grateful to the School of Biosciences at the University of Nottingham for funding and supporting this project.
Conflicts of Interest
The authors declare no conflict of interest
References
- Gedi, M.A.; Di Bari, V.; Ibbett, R.; Darwish, R.; Nwaiwu, O.; Umar, Z.; Agarwal, D.; Worrall, R.; Gray, D.; Foster, T. Upcycling and valorisation of food waste. In Routledge Handbook of Food Waste; Routledge: London, UK, 2020; pp. 413–427. [Google Scholar]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.S.; French, C.E.; Tucker, G.A.; Du, C. Improving the productivity of bioethanol production using marine yeast and seawater-based media. Biomass Bioenergy 2020, 139, 105615. [Google Scholar] [CrossRef]
- Monroig, Ó.; Tocher, D.R.; Navarro, J.C. Biosynthesis of polyunsaturated fatty acids in marine invertebrates: Recent advances in molecular mechanisms. Mar. Drugs 2013, 11, 3998–4018. [Google Scholar] [CrossRef] [PubMed]
- Becker, W. Microalgae in human and animal nutrition. In Handbook of Microalgal Culture; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 312–351. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. Environ. Biol. Fishes 2016, 29, 949–982. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Taelman, S.E.; De Meester, S.; Van Dijk, W.; Da Silva, V.; Dewulf, J. Environmental sustainability analysis of a protein-rich livestock feed ingredient in The Netherlands: Microalgae production versus soybean import. Resour. Conserv. Recycl. 2015, 101, 61–72. [Google Scholar] [CrossRef]
- Greetham, D.; Zaky, A.S.; Du, C. Exploring the tolerance of marine yeast to inhibitory compounds for improving bioethanol production. Sustain. Energy Fuels 2019, 3, 1545–1553. [Google Scholar] [CrossRef]
- Zaky, A.S.; Greetham, D.; Tucker, G.A.; Du, C. The establishment of a marine focused biorefinery for bioethanol production using seawater and a novel marine yeast strain. Sci. Rep. 2018, 8, 12127. [Google Scholar] [CrossRef]
- Harris, E.H. Chlamydomonasas amodelorganism. Annu. Rev. Plant. Biol. 2001, 52, 363–406. [Google Scholar] [CrossRef] [PubMed]
- Almaraz-Delgado, A.L.; Flores-Uribe, J.; Pérez-España, V.H.; Manjarrez, E.S.; Badillo-Corona, J.A. Production of therapeutic proteins in the chloroplast of Chlamydomonas reinhardtii. AMB Express 2014, 4, 57. [Google Scholar] [CrossRef] [PubMed]
- Becker, E. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Dreesen, I.A.; Hamri, G.C.-E.; Fussenegger, M. Heat-stable oral alga-based vaccine protects mice from Staphylococcus aureus infection. J. Biotechnol. 2010, 145, 273–280. [Google Scholar] [CrossRef]
- Yoon, S.-M.; Kim, S.Y.; Li, K.F.; Yoon, B.H.; Choe, S.; Kuo, M.M.-C. Transgenic microalgae expressing Escherichia coli AppA phytase as feed additive to reduce phytate excretion in the manure of young broiler chicks. Appl. Microbiol. Biotechnol. 2011, 91, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Qiu, S.; Liu, Q.; Tian, J.; Hu, Z.; Ni, J. Selenoprotein-Transgenic Chlamydomonas reinhardtii. Nutrients 2013, 5, 624–636. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef]
- Davies, J.P.; Grossman, A.R. The use of chlamydomonas (chlorophyta: Volvocales) as a model algal system for genome studies and the elucidation of photosynthetic processes. J. Phycol. 1998, 34, 907–917. [Google Scholar] [CrossRef]
- Taghavi, N.; Robinson, G. Improving the optimum yield and growth of Chlamydomonas reinhardtii CC125 and CW15 using various carbon sources and growth regimes. Afr. J. Biotechnol. 2016, 15, 1083–1100. [Google Scholar]
- Naturya.com. Chlorella and Spirulina. Available online: https://naturya.com/vegan-protein (accessed on 17 September 2020).
- Zhou, W.; Lu, Q.; Han, P.; Li, J. Chapter 3—Microalgae cultivation and photobioreactor design. In Microalgae Cultivation for Biofuels Production; Yousuf, A., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 31–50. [Google Scholar] [CrossRef]
- Gorman, D.S.; Levine, R.P. Photosynthetic electron transport chain of chlamydomonas reinhardi VI. Electron transport in mutant strains lacking either cytochrome 553 or plastocyanin. Plant. Physiol. 1966, 41, 1648–1656. [Google Scholar] [CrossRef]
- Godoy-Hernández, G.; Vázquez-Flota, F.A. Growth measurements: Estimation of cell division and cell expansion. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 41–48. [Google Scholar] [CrossRef]
- Godoy-Hernández, G.; Vázquez-Flota, F.A. Growth measurements: Estimation of cell division and cell expansion. Methods Mol Biol 2006, 318, 51–58. [Google Scholar]
- Gedi, M.A. Nutrient Composition and Digestibility of Chloroplast Rich Fractions from Green Leaf Materials. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2017. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Dron, J.; Linke, R.; Rosenberg, E.; Schreiner, M. Trimethylsulfonium hydroxide as derivatization reagent for the chemical investigation of drying oils in works of art by gas chromatography. J. Chromatogr. A 2004, 1047, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; De-Paula, J.C.; Pereira, L.O.d.S.; Marquez, U.M.L. Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycol. Res. 2002, 50, 233–241. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; Lavin, P.; Marquez, U.M.L.; Aidar, E. Distribution of intracellular nitrogen in marine microalgae: Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 2004, 39, 17–32. [Google Scholar] [CrossRef]
- Windham, W. AOAC official method 994.12, amino acids in feeds, alternative III, acid hydrolysis method. In Official Methods of Analysis of AOAC International; 1995; Available online: https://ci.nii.ac.jp/naid/10018325012/ (accessed on 25 September 2020).
- FAO/WHO/UNU. Expert Consultation on Energy and Protein Requirements (1981: Rome, Italy), Food and Agriculture Organisation of the United Nations, World Health Organisation and United Nations University. Energy and Protein Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation [held in Rome from 5 to 17 October 1981]. World Health Organisation; FAO/WHO/UNU, 1985; Available online: https://apps.who.int/iris/handle/10665/39527 (accessed on 25 September 2020).
- Damodaran, S.P.; Eberhard, S.; Boitard, L.; Rodriguez, J.G.; Wang, Y.; Bremond, N.; Baudry, J.; Bibette, J.; Wollman, F.A. A Millifluidic Study of Cell-to-Cell Heterogeneity in Growth-Rate and Cell-Division Capability in Populations of Isogenic Cells of Chlamydomonas reinhardtii. PLoS ONE 2015, 10, e0118987. [Google Scholar] [CrossRef]
- Oldenhof, H.; Zachleder, V.; Ende, H.V.D. The cell cycle ofChlamydomonas reinhardtii: The role of the commitment point. Folia Microbiol. 2007, 52, 53–60. [Google Scholar] [CrossRef]
- Rochaix, J.D.; Tzafrir, I.; McElver, J.A.; Liu, C.M.; Yang, L.J.; Wu, J.Q.; Martinez, A.; Patton, D.A.; Meinke, D. Assembly, function, and dynamics of the photosynthetic machinery in chlamydomonas reinhardtii. Plant. Physiol. 2001, 127, 1394–1398. [Google Scholar] [CrossRef][Green Version]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant. J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional evaluation of Australian microalgae as potential human health supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef] [PubMed]
- Kliphuis, A.M.J.; Klok, A.J.; Martens, D.E.; Lamers, P.P.; Janssen, M.; Wijffels, R.H. Metabolic modeling of Chlamydomonas reinhardtii: Energy requirements for photoautotrophic growth and maintenance. Environ. Biol. Fishes 2011, 24, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Boyle, N.R.; Morgan, J.A. Flux balance analysis of primary metabolism in Chlamydomonas reinhardtii. BMC Syst. Biol. 2009, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Siaut, M.; Cuiné, S.; Cagnon, C.; Fessler, B.; Nguyen, H.M.; Carrier, P.; Beyly-Adriano, A.; Beisson, F.; Triantaphylidès, C.; Li-Beisson, Y.; et al. Oil accumulation in the model green alga Chlamydomonas reinhardtii: Characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol. 2011, 11, 7. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Dempster, T.A.; Jones, H.D.T.; Wolfrum, E.; Van Wychen, S.; McAllister, J.S.P.; Rencenberger, M.; Parchert, K.J.; Gloe, L.M. Algal Biomass constituent analysis: Method uncertainties and investigation of the underlying measuring chemistries. Anal. Chem. 2012, 84, 1879–1887. [Google Scholar] [CrossRef]
- Pellett, P.L.; Young, V.R. Commentary: Joint FAO/WHO expert consultation on protein quality evaluation Bethesda, MD, USA, 4–8 December 1989. Ecol. Food Nutr. 1990, 24, 297–303. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal proteins: Extraction, application, and challenges concerning production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Lang, I.; Hodač, L.; Friedl, T.; Feussner, I. Fatty acid profiles and their distribution patterns in microalgae: A comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant. Biol. 2011, 11, 124. [Google Scholar] [CrossRef]
- Quoc, K.P.; Dubacq, J.P. Effect of growth temperature on the biosynthesis of eukaryotic lipid molecular species by the cyanobacterium Spirulina platensis. Biochim. Biophys. Acta 1997, 1346, 237–246. [Google Scholar] [CrossRef]
- Ronda, S.R.; Lele, S. Culture Conditions stimulating high γ-Linolenic Acid accumulation by Spirulina platensis. Braz. J. Microbiol. 2008, 39, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Cuiné, S.; Beyly-Adriano, A.; Légeret, B.; Billon, E.; Auroy, P.; Beisson, F.; Peltier, G.; Li-Beisson, Y. The green microalga chlamydomonas reinhardtii has a single ω-3 fatty acid desaturase that localizes to the chloroplast and impacts both plastidic and extraplastidic membrane lipids. Plant. Physiol. 2013, 163, 914–928. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Casas, L.; Serrano, C.M.; Rodríguez, M.R.; De La Ossa, E.J.M.; Lubián, L.M. Extraction of carotenoids and fatty acids from microalgae using supercritical technology. Am. J. Anal. Chem. 2012, 3, 877–883. [Google Scholar] [CrossRef]
- Masojıdek, J.; Koblızek, M.; Torzillo, G. Photosynthesis in microalgae. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; p. 20. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118567166.ch2 (accessed on 25 September 2020).
- Gedi, M.A.; Briars, R.; Yuseli, F.; Zainol, N.; Darwish, R.; Salter, A.M.; Gray, D.A. Component analysis of nutritionally rich chloroplasts: Recovery from conventional and unconventional green plant species. J. Food Sci. Technol. 2017, 54, 2746–2757. [Google Scholar] [CrossRef]
- Tabarsa, M.; Rezaei, M.; Ramezanpour, Z.; Waaland, J.R. Chemical compositions of the marine algae Gracilaria salicornia (Rhodophyta) and Ulva lactuca (Chlorophyta) as a potential food source. J. Sci. Food Agric. 2012, 92, 2500–2506. [Google Scholar] [CrossRef]
- Campbell, S. Dietary reference intakes: Water, potassium, sodium, chloride, and sulfate. Clin. Nutr. Insight 2004, 30, 1–4. [Google Scholar]
- Monsen, E.R. Dietary reference intakes for the antioxidant nutrients: Vitamin C, vitamin E, selenium, and carotenoids. J. Am. Diet. Assoc. 2000, 100, 637–640. [Google Scholar] [CrossRef]
- Nelson, H.K.; Shi, Q.; Van Dael, P.; Schiffrin, E.J.; Blum, S.; Barclay, D.; Levander, O.A.; Beck, M.A.; Brough, G.H.; Wu, S.; et al. Host nutritional selenium status as a driving force for influenza virus mutations. FASEB J. 2001, 15, 1846–1848. [Google Scholar] [CrossRef]
- Jarup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- FAO/WHO. FAO/WHO Expert Committee on Food Additives. Meeting ( 61st: 2003, Rome, Italy), International Programme on Chemical Safety. In Safety Evaluation of Certain Food Additives and Contaminants/Prepared by the Sixty-First Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA); World Health Organization, 2004; Available online: https://apps.who.int/iris/handle/10665/43038 (accessed on 25 September 2020)ISBN 9241209224.
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).