Analysis of Association between Intake of Red Wine Polyphenols and Oxidative Stress Parameters in the Liver of Growing Male Rats
Abstract
1. Introduction
2. Materials and Methods
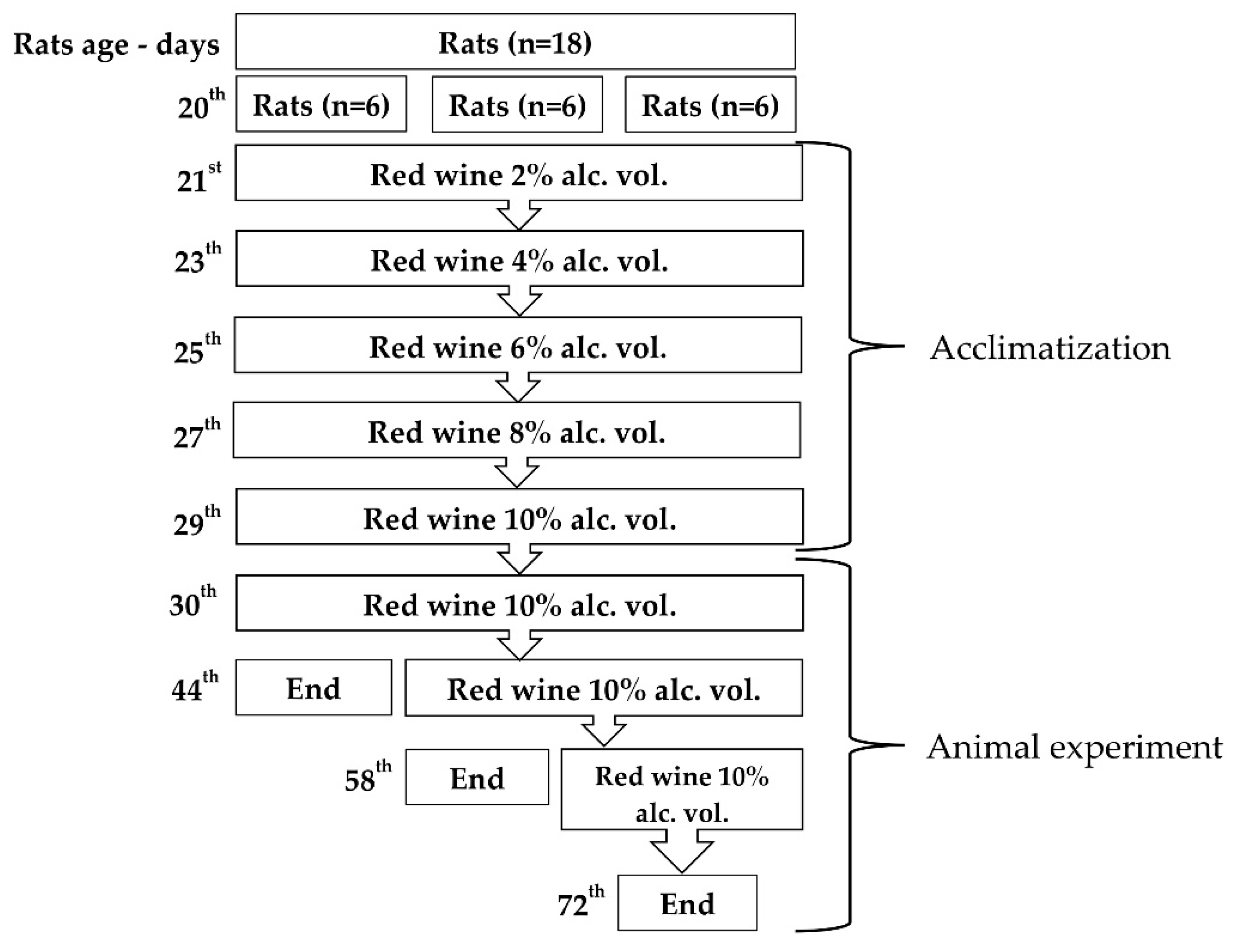
2.1. Study Design
2.2. Analytical Methods and Measurements
2.2.1. Liver Homogenate Preparation
2.2.2. Liver Homogenate Analysis
2.2.3. Red Wine Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spear, L.P. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol. Behav. 2015, 148, 122–130. [Google Scholar] [CrossRef]
- Green, K.M.; Doherty, E.E.; Zebrak, K.A.; Ensminger, M.E. Association between adolescent drinking and adult violence: Evidence from a longitudinal study of Urban African Americans. J. Stud. Alcohol Drugs 2011, 72, 701–710. [Google Scholar] [CrossRef]
- Latvala, A.; Rose, R.J.; Pulkkinen, L.; Dick, D.M.; Korhonen, T.; Kaprio, J. Drinking, smoking, and educational achievement: Cross-lagged associations from adolescence to adulthood. Drug Alcohol Depend. 2014, 137, 106–113. [Google Scholar] [CrossRef]
- Marshall, E.J. Adolescent alcohol use: Risks and consequences. Alcohol Alcohol. 2014, 49, 160–164. [Google Scholar] [CrossRef]
- Squeglia, L.M.; Jacobus, J.; Tapert, S.F. The effect of alcohol use on human adolescent brain structures and systems. Handb. Clin. Neurol. 2014, 125, 501–510. [Google Scholar] [CrossRef]
- Spear, L.P. Effects of adolescent alcohol consumption on the brain and behaviour. Nat. Rev. Neurosci. 2018, 19, 197–214. [Google Scholar] [CrossRef]
- Rosa, R.C.; Rodrigues, W.F.; Miguel, C.B.; Cardoso, F.A.G.; Espindula, A.P.; Oliveira, C.J.F.; Volpon, J.B. Chronic consumption of alcohol adversely affects the bone of young rats. Acta Ortop. Bras. 2019, 27, 321–324. [Google Scholar] [CrossRef]
- Block, G.D.; Yamamoto, M.E.; Mallick, A.; Styche, A.J. Effects on pubertal hormones by ethanol abuse in adolescents. Alcohol Clin. Exp. Res. 1993, 17, 505. [Google Scholar]
- Clark, D.B.; Lynch, K.G.; Donovan, J.E.; Block, G.D. Health problems in adolescents with alcohol use disorders: Self-report, liver injury, and physical examination findings and correlates. Alcohol. Clin. Exp. Res. 2001, 25, 1350–1359. [Google Scholar] [CrossRef]
- Petit, G.; Kornreich, C.; Verbanck, P.; Cimochowska, A.; Campanella, S. Why is adolescence a key period of alcohol initiation and who is prone to develop long-term problem use?: A review of current available data. Socioaffect. Neurosci. Psychol. 2013, 3, 21890. [Google Scholar] [CrossRef]
- The ESPAD Group. ESPAD Report 2015. Results from the European School Survey Project on Alcohol and Other Drugs; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Adger, H.; Saha, S. Alcohol use disorders in adolescents. Pediatr. Rev. 2013, 34, 103–114. [Google Scholar] [CrossRef][Green Version]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, V.; Bucciantini, M.; Stefani, M. Calabrese Healthy effects of plant polyphenols: Molecular mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef]
- Bazal, P.; Gea, A.; Martínez-González, M.; Salas-Salvadó, J.; Asensio, E.; Muñoz-Bravo, C.; Fiol, M.; Muñoz, M.-A.; Lapetra, J.; Serra-Majem, L.; et al. Mediterranean alcohol-drinking pattern, low to moderate alcohol intake and risk of atrial fibrillation in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 676–683. [Google Scholar] [CrossRef]
- Popova, S.; Rehm, J.; Patra, J.; Zatonski, W. Comparing alcohol consumption in central and eastern Europe to other European countries. Alcohol Alcohol. 2007, 42, 465–473. [Google Scholar] [CrossRef]
- Sudhinaraset, M.; Wigglesworth, C.; Takeuchi, D.T. Social and cultural contexts of alcohol use. Alcohol Res. Curr. Rev. 2016, 38, 35–45. [Google Scholar]
- WHO. Alcohol in the European Union. Consumption, Harm and Policy Approaches; Anderson, P., Møllerand, L., Galea, G., Eds.; WHO Regional Office for Europe: Copenhagen, Denmark, 2012. [Google Scholar]
- Sieri, S.; Agudo, A.; Kesse-Guyot, E.; Klipstein-Grobusch, K.; San-José, B.; Welch, A.; Krogh, V.; Luben, R.N.; Allen, N.; Overvad, K.; et al. Patterns of alcohol consumption in 10 European countries participating in the European prospective investigation into cancer and nutrition (EPIC) project. Public Health Nutr. 2002, 5, 1287–1296. [Google Scholar] [CrossRef]
- Bräker, A.B.; Soellner, R. Alcohol drinking cultures of European adolescents. Eur. J. Public Health 2016, 26, 581–586. [Google Scholar] [CrossRef]
- Salucci, S.; Burattini, S.; Giordano, F.M.; Lucarini, S.; Diamantini, G.; Falcieri, E. Further highlighting on the prevention of oxidative damage by polyphenol-rich wine extracts. J. Med. Food 2017, 20, 410–419. [Google Scholar] [CrossRef]
- Fernandes, I.; Pérez-Gregorio, M.; Soares, S.; Mateus, N.; De Freitas, V. Wine flavonoids in health and disease prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis viniferaL.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 17, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Champ, C.E.; Kundu-Champ, A. Maximizing polyphenol content to uncork the relationship between wine and cancer. Front. Nutr. 2019, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Iuchi, A.; Harada, H.; Hashimoto, S. Potential beneficial effects of wine flavonoids on allergic diseases. Diseases 2019, 7, 8. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Pavlidou, E.; Mantzorou, M.; Fasoulas, A.; Tryfonos, C.; Petridis, D.; Giaginis, C. Wine: An aspiring agent in promoting longevity and preventing chronic diseases. Diseases 2018, 6, 73. [Google Scholar] [CrossRef]
- Osna, N.A.; Donohue, T.M.; Kharbanda, K.K. Alcoholic liver disease: Pathogenesis and current management. Alcohol Res. 2017, 38, 147–161. [Google Scholar]
- Beery, A.K.; Holmes, M.M.; Lee, W.; Curley, J.P. Stress in groups: Lessons from non-traditional rodent species and housing models. Neurosci. Biobehav. Rev. 2020, 113, 354–372. [Google Scholar] [CrossRef]
- Becker, H.C.; Lopez, M.F.; Doremus-Fitzwater, T.L. Effects of stress on alcohol drinking: A review of animal studies. Psychopharmacology 2011, 218, 131–156. [Google Scholar] [CrossRef]
- Ramirez, H.E.; Esperon, L.; Peris, J. Effects of an enrichment device on voluntary alcohol consumption on single-housed rats. J. Am. Assoc. Lab. Anim. Sci. 2008, 47, 24–29. [Google Scholar]
- Holgate, J.Y.; Shariff, M.; Mu, E.W.H.; Bartlett, S.E. A rat drinking in the dark model for studying ethanol and sucrose consumption. Front. Behav. Neurosci. 2017, 11, 29. [Google Scholar] [CrossRef]
- Li, J.; Chen, P.; Han, X.; Zuo, W.; Mei, Q.; Bian, E.Y.; Umeugo, J.; Ye, J.H. Differences between male and female rats in alcohol drinking, negative affects and neuronal activity after acute and prolonged abstinence. Int J. Physiol Pathophysiol. Pharmacol. 2019, 11, 163–176. [Google Scholar] [PubMed]
- Priddy, B.M.; Carmack, S.A.; Thomas, L.C.; Vendruscolo, J.C.; Koob, G.F.; Vendruscolo, L.F. Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol. Biochem. Behav. 2017, 152, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Spoelder, M.; Dourojeanni, J.P.F.; De Git, K.C.G.; Baars, A.M.; Lesscher, H.M.B.; Vanderschuren, L.J. Individual differences in voluntary alcohol intake in rats: Relationship with impulsivity, decision making and Pavlovian conditioned approach. Psychopharmacology 2017, 234, 2177–2196. [Google Scholar] [CrossRef]
- Carnicella, S.; Ron, R.; Barak, S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol 2014, 48, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Krohn, T.C.; Sørensen, D.B.; Ottesen, J.L.; Hansen, A.K. The effects of individual housing on mice and rats: A review. Anim. Welf. 2006, 15, 343–352. [Google Scholar]
- Kołota, A.; Głąbska, D.; Oczkowski, M.; Gromadzka-Ostrowska, J. Oxidative stress parameters in the liver of growing male rats receiving various alcoholic beverages. Nutrients 2020, 12, 158. [Google Scholar] [CrossRef]
- Kołota, A.; Głąbska, D.; Oczkowski, M.; Gromadzka-Ostrowska, J. Influence of alcohol consumption on body mass gain and liver antioxidant defense in adolescent growing male rats. Int. J. Environ. Res. Public Health 2019, 16, 2320. [Google Scholar] [CrossRef]
- Chaloupka, F.J. The effects of price on alcohol use, abuse, and their consequences. In Reducing Underage Drinking: A Collective Responsibility; Bonnie, R.J., O’Connell, M.E., Eds.; National Academies Press: Washington, WA, USA, 2004. [Google Scholar]
- Milat, A.M.; Mudnić, I.; Grković, I.; Ključević, N.; Grga, M.; Jerčić, I.; Jurić, D.; Ivanković, D.; Benzon, B.; Boban, M. Efects of white wine consumption on weight in rats: Do polyphenols matter? Oxid. Med. Cell. Longev. 2017, 2017, 8315803. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Reznick, A.Z.; Packer, L. [38] Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar] [CrossRef]
- Levine, R.L.; Williams, J.A.; Stadtman, E.P.; Shacter, E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994, 233, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. HPLC method for screening of flavonoids and phenolic acids in berries. J. Sci. Food Agric. 1998, 77, 543–551. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Farello, G.; Altieri, C.; Cutini, M.; Pozzobon, G.; Verrotti, A. Review of the literature on current changes in the timing of pubertal development and the incomplete forms of early puberty. Front. Pediatr. 2019, 7, 147. [Google Scholar] [CrossRef]
- Guney, E.; Ceylan, M.F.; Tektas, A.; Alisik, M.; Ergin, M.; Göker, Z.; Dinc, G.S.; Ozturk, O.; Korkmaz, A.; Eker, S. Oxidative stress in children and adolescents with anxiety disorders. J. Affect. Disord. 2014, 156, 62–66. [Google Scholar] [CrossRef]
- Tauman, R.; Shalitin, S.; Lavie, L. Oxidative stress in obese children and adolescents with and without type 2 diabetes mellitus is not associated with obstructive sleep apnea. Sleep Breath. 2019, 23, 117–123. [Google Scholar] [CrossRef]
- Túri, S.; Friedman, A.; Bereczki, C.; Papp, F.; Kovács, J.; Karg, E.; Németh, I. Oxidative stress in juvenile essential hypertension. J. Hypertens. 2003, 21, 145–152. [Google Scholar] [CrossRef]
- Loperena, R.; Harrison, D.G. Oxidative stress and hypertensive diseases. Med. Clin. N. Am. 2017, 101, 169–193. [Google Scholar] [CrossRef]
- Nasca, M.M.; Zhang, R.; Super, D.M.; Hazen, S.L.; Hall, H.R. Increased oxidative stress in healthy children following an exercise program: A pilot study. J. Dev. Behav. Pediatr. 2010, 31, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Avloniti, A.; Chatzinikolaou, A.; Deli, C.K.; Vlachopoulos, D.; Gracia-Marco, L.; Leontsini, D.; Draganidis, D.; Jamurtas, A.Z.; Mastorakos, G.; Fatouros, I.G. Exercise-induced oxidative stress responses in the pediatric population. Antioxidants 2017, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.D.; Padmavathi, P.; Hymavathi, R.; Maturu, P.; Varadacharyulu, N. Alcohol-induced oxidative stress in rat liver microsomes: Protective effect of Emblica officinalis. Pathophysiology 2014, 21, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Moreno, M.; Gutiérrez-Reyes, G. Papel del estrés oxidativo en el desarrollo de la enfermedad hepática alcohólica. Rev. Gastroenterol. Mex. 2014, 79, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Gris, E.; Mattivi, F.; Ferreira, E.; Vrhovsek, U.; Filho, D.; Pedrosa, R.; Bordignon-Luiz, M.T. Phenolic profile and effect of regular consumption of Brazilian red wines on in vivo antioxidant activity. J. Food Compos. Anal. 2013, 31, 31–40. [Google Scholar] [CrossRef]
- Auberval, N.; Dal, S.; Maillard, E.; Bietiger, W.; Peronet, C.; Pinget, M.; Schini-Kerth, V.; Sigrist, S. Beneficial effects of a red wine polyphenol extract on high-fat diet-induced metabolic syndrome in rats. Eur. J. Nutr. 2016, 56, 1467–1475. [Google Scholar] [CrossRef]
- Becker, H.C.; Ron, R. Animal models of excessive alcohol consumption: Recent advances and future challenges. Alcohol 2014, 48, 205–208. [Google Scholar] [CrossRef]
- Hernández, J.A.; López-Sánchez, R.C.; Rendón-Ramírez, A. Lipids and oxidative stress associated with ethanol-induced neurological damage. Oxid. Med. Cell. Longev. 2016, 2016, 1543809. [Google Scholar] [CrossRef]
- Ceni, E.; Mello, T.; Galli, A. Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World J. Gastroenterol. 2014, 20, 17756–17772. [Google Scholar] [CrossRef]
- Majumdar, A.; Patere, S.; Saraf, M.N. Exacerbation of alcohol-induced oxidative stress in rats by polyunsaturated fatty acids and iron load. Indian J. Pharm. Sci. 2011, 73, 152–158. [Google Scholar] [CrossRef]
- Dey, A.; Cederbaum, A.I. Alcohol and oxidative liver injury. Hepatology 2006, 43, S63–S74. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.M.P.; Lopes, K.S.; Santana, L.N.S.; Fontes-Júnior, E.A.; Ribeiro, C.H.M.A.; Silva, M.C.F.; Paraense, R.S.D.O.; Crespo-López, M.E.; Gomes, A.R.Q.; Lima, R.R.; et al. Repeated cycles of binge-like ethanol intake in adolescent female rats induce motor function impairment and oxidative damage in motor cortex and liver, but not in blood. Oxid. Med. Cell. Longev. 2018, 2018, 3467531. [Google Scholar] [CrossRef]
- Duchnowicz, P.; Broncel, M.; Podsędek, A.; Koter, M. Hypolipidemic and antioxidant effects of hydroxycinnamic acids, quercetin, and cyanidin 3-glucoside in hypercholesterolemic erythrocytes (in vitro study). Eur. J. Nutr. 2011, 51, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Balasubashini, M.S.; Rukkumani, R.; Viswanathan, P.; Menon, V.P. Ferulic acid alleviates lipid peroxidation in diabetic rats. Phytother. Res. 2004, 18, 310–314. [Google Scholar] [CrossRef]
- Shen, Y.; Song, X.; Li, L.; Sun, J.; Jaiswal, Y.; Huang, J.; Liu, C.; Yang, W.; Williams, L.; Zhang, H.; et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 2019, 111, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Zych, M.; Wojnar, W.; Borymski, S.; Szałabska, K.; Bramora, P.; Kaczmarczyk-Sedlak, I. Effect of rosmarinic acid and sinapic acid on oxidative stress parameters in the cardiac tissue and serum of type 2 diabetic female rats. Antioxidants 2019, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Deng, Q.; Xu, J.; Wang, X.; Hu, C.; Tang, H.; Huang, F. Sinapic acid and resveratrol alleviate oxidative stress with modulation of gut microbiota in high-fat diet-fed rats. Food Res. Int. 2019, 116, 1202–1211. [Google Scholar] [CrossRef]
- Lafay, S.; Gueux, E.; Rayssiguier, Y.; Mazur, A.; Rémésy, C.; Scalbert, A. Caffeic acid inhibits oxidative stress and reduces hypercholesterolemia induced by iron overload in rats. Int. J. Vitam. Nutr. Res. 2005, 75, 119–125. [Google Scholar] [CrossRef]
- Montlahuc, C.; Julia, C.; Touvier, M.; Fezeu, L.; Hercberg, S.; Galan, P.; Kesse-Guyot, E.; Chevret, S. Association between dietary polyphenols intake and an oxidative stress biomarker: Interest of multiple imputation for handling missing covariates and outcomes. BMC Nutr. 2016, 2, 71. [Google Scholar] [CrossRef][Green Version]
- Tsuda, T.; Horio, F.; Osawa, T. The role of anthocyanins as an antioxidant under oxidative stress in rats. BioFactors 2000, 13, 133–139. [Google Scholar] [CrossRef]
- Estruch, R.; Sacanella, E.; Mota, F.; Chiva-Blanch, G.; Antúnez, E.; Casals, E.; Deulofeu, R.; Rotilio, D.; Andres-Lacueva, C.; Lamuela-Raventós, R.M. Moderate consumption of red wine, but not gin, decreases erythrocyte superoxide dismutase activity: A randomised cross-over trial. Nutr. Metab. Cardiovasc. Dis. 2009, 21, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Zemser, M.; Weisz, M.; Halevy, S.; Martín-Belloso, O.; Trakhtenberg, S. The influence of alcohol-containing and alcohol-free beverages on lipid levels and lipid peroxides in serum of rats. J. Nutr. Biochem. 1998, 9, 682–686. [Google Scholar] [CrossRef]
- Pazzini, C.E.F.; Colpo, A.C.; Poetini, M.R.; Pires, C.F.; De Camargo, V.B.; Mendez, A.S.L.; Azevedo, M.L.; Soares, J.C.M.; Folmer, V. Effects of red wine tannat on oxidative stress induced by glucose and fructose in erythrocytes in vitro. Int. J. Med. Sci. 2015, 12, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Montilla-López, P.; Barcos, M.; Muñoz, M.; Muñoz-Castañeda, J.R.; Bujalance, I.; Túnez, I. Protective effect of Montilla-Moriles appellation red wine on oxidative stress induced by streptozotocin in the rat. J. Nutr. Biochem. 2004, 15, 688–693. [Google Scholar] [CrossRef]
- Schrieks, I.C.; Berg, R.V.D.; Sierksma, A.; Beulens, J.W.; Vaes, W.H.; Hendriks, H.F. Effect of red wine consumption on biomarkers of oxidative stress. Alcohol Alcohol. 2012, 48, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Zorraquín-Peña, I.; Esteban-Fernández, A.; De Llano, D.G.; Bartolomé, B.; Moreno-Arribas, M.V. Wine-derived phenolic metabolites in the digestive and brain function. Beverages 2019, 5, 7. [Google Scholar] [CrossRef]
- Martins, L.A.M.M.; Coelho, B.P.; Behr, G.; Pettenuzzo, L.; De Souza, I.C.C.; Moreira, J.C.F.; Borojevic, R.; Gottfried, C.; Guma, F.C.R. Resveratrol induces pro-oxidant effects and time-dependent resistance to cytotoxicity in activated hepatic stellate cells. Cell Biophys. 2013, 68, 247–257. [Google Scholar] [CrossRef]
- Soobrattee, M.; Neergheen, V.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hartzfeld, P.W.; Riechel, T.L. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kılıç, M.; Sharifi-Rad, J.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Man, A.W.; Li, H.; Xia, N. Resveratrol and the interaction between gut microbiota and arterial remodelling. Nutrients 2020, 12, 119. [Google Scholar] [CrossRef]
- Weiskirchen, S.; Weiskirchen, R. Resveratrol: How much wine do you have to drink to stay healthy? Adv. Nutr. 2016, 7, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Sun, Z.Y. Phenolic compounds, total antioxidant capacity and volatile components of Cabernet Sauvignon red wines from five different wine-producing regions in China. Food Sci. Technol. 2018, 39, 735–746. [Google Scholar] [CrossRef]
- Xiang, L.; Xiao, L.; Wang, Y.; Li, H.; Huang, Z.; He, X. Health benefits of wine: Don’t expect resveratrol too much. Food Chem. 2014, 156, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, G.; Straniero, S.; Donati, A.; Bergamini, E. Resveratrol requires red wine polyphenols for optimum antioxidant activity. J. Nutr. Health Aging 2016, 20, 540–545. [Google Scholar] [CrossRef]
- Zenebe, W.; Pechánová, O.; Bernátová, I. Protective effects of red wine polyphenolic compounds on the cardiovascular system. Exp. Clin. Cardiol. 2001, 6, 153–158. [Google Scholar]
- Carbonell, T.; Alva, N.; Sánchez-Nuño, S.; Dewey, S.; Gomes, A.V. Subnormothermic perfusion in the isolated rat liver preserves the antioxidant glutathione and enhances the function of the ubiquitin proteasome system. Oxid. Med. Cell. Longev. 2016, 2016, 9324692. [Google Scholar] [CrossRef]
- Alva, N.; Bardallo, R.G.; Basanta, D.; Palomeque, J.; Carbonell, T. Preconditioning-like properties of short-term hypothermia in isolated perfused rat Liver (IPRL) system. Int. J. Mol. Sci. 2018, 19, 1023. [Google Scholar] [CrossRef]
- Bellini, M.I.; Yiu, J.; Nozdrin, M.; Papalois, V. The effect of preservation temperature on liver, kidney, and pancreas tissue ATP in animal and preclinical human models. J. Clin. Med. 2019, 8, 1421. [Google Scholar] [CrossRef]
- Piotrowska-Kempisty, H.; Nowicki, M.; Jodynis-Liebert, J.; Kurpik, M.; Ewertowska, M.; Adamska, T.; Oszmiański, J.; Kujawska, M. Assessment of hepatoprotective effect of chokeberry juice in rats treated chronically with carbon tetrachloride. Molecules 2020, 25, 1268. [Google Scholar] [CrossRef]
- Mohamadin, A.M.; Hammad, L.N.A.; El-Bab, M.F.; Gawad, H.S.A. Attenuation of oxidative stress in plasma and tissues of rats with experimentally induced hyperthyroidism by caffeic acid phenylethyl ester. Basic Clin. Pharmacol. Toxicol. 2006, 100, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Noeman, S.A.; Hamooda, H.E.; Baalash, A.A. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol. Metab. Syndr. 2011, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Freitas, I.; Boncompagni, E.; Tarantola, E.; Gruppi, C.; Bertone, V.; Ferrigno, A.; Milanesi, G.; Vaccarone, R.; Tira, M.E.; Vairetti, M. In situevaluation of oxidative stress in rat fatty liver induced by a methionine- and choline-deficient diet. Oxid. Med. Cell. Longev. 2016, 2016, 9307064. [Google Scholar] [CrossRef]
| Parameter | 2 Weeks | 4 Weeks | 6 Weeks | p-Value | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ANOVA | 2 vs. 4 | Duncan Test 2 vs. 6 | 4 vs. 6 | |
| Red wine intake (g/kg body weight) | 52.43 ± 13.7 a | 47.31 ± 9.13 a,b | 35.43 ± 5.89 b | <0.05 | NS | <0.05 | NS |
| Ethanol intake (g/kg body weight) | 5.46 ± 1.43 a | 4.93 ± 0.95 a,b | 3.69 ± 0.61 b | <0.05 | NS | <0.05 | NS |
| TPA (mg/kg body weight) | 0.78 ± 0.20 | 0.71 ± 0.14 | 0.53 ± 0.09 | <0.05 | NS | <0.05 | NS |
| CA (mg/kg body weight) | 0.017 ± 0.00 a | 0.015 ± 0.00 a,b | 0.011 ± 0.00 b | <0.05 | NS | <0.05 | NS |
| FA (mg/kg body weight) | 0.07 ± 0.02 a | 0.06 ± 0.01 a,b | 0.04 ± 0.01 b | <0.05 | NS | <0.05 | NS |
| HBA (mg/kg body weight) | 0.54 ± 0.14 a | 0.49 ± 0.09 a,b | 0.37 ± 0.06 b | <0.05 | NS | <0.05 | NS |
| SA (mg/kg body weight) | 0.009 ± 0.00 | 0.009 ± 0.00 | 0.006 ± 0.00 b | <0.05 | NS | <0.05 | NS |
| pCA (mg/kg body weight) | 0.15 ± 0.04 a | 0.13 ± 0.03 a,b | 0.09 ± 0.02 b | <0.05 | NS | <0.05 | NS |
| TA (mg/kg body weight) | 3.29 ± 0.98 a | 3.03 ± 0.65 a | 1.72 ± 0.32 b | <0.05 | NS | <0.05 | <0.01 |
| C (mg/kg body weight) | 0.07 ± 0.02 a | 0.06 ± 0.01 a,b | 0.04 ± 0.01 b | <0.05 | NS | <0.05 | NS |
| C3G (mg/kg body weight) | 0.39 ± 0.10 a | 0.35 ± 0.07 a,b | 0.26 ± 0.04 b | <0.05 | NS | <0.05 | NS |
| C3R (mg/kg body weight) | 0.06 ± 0.02 a | 0.05 ± 0.01 a,b | 0.04 ± 0.01 b | <0.05 | NS | <0.05 | NS |
| D (mg/kg body weight) | 0.16 ± 0.04 a | 0.15 ± 0.03 a,b | 0.11 ± 0.02 b | <0.05 | NS | <0.05 | NS |
| P (mg/kg body weight) | 2.62 ± 0.81 a | 2.41 ± 0.55 a | 1.26 ± 0.24 b | <0.01 | NS | <0.01 | <0.01 |
| Parameter | 2 Weeks | 4 Weeks | 6 Weeks | p-Value | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ANOVA | 2 vs. 4 | Duncan Test 2 vs. 6 | 4 vs. 6 | |
| Red wine intake (g) | 10.95 ± 2.49 | 9.67 ± 2.16 | 10.28 ± 1.50 | NS | NS | NS | NS |
| Ethanol intake (g) | 1.09 ± 0.25 | 0.97 ± 0.22 | 1.03 ± 0.15 | NS | NS | NS | NS |
| TPA (mg) | 0.16 ± 0.04 | 0.14 ± 0.03 | 0.15 ± 0.02 | NS | NS | NS | NS |
| CA (mg) | 0.0003 ± 0.0008 | 0.003 ± 0.001 | 0.003 ± 0.0005 | NS | NS | NS | NS |
| FA (mg) | 0.014 ± 0.03 | 0.012 ± 0.0027 | 0.013 ± 0.001 | NS | NS | NS | NS |
| HBA (mg) | 0.114 ± 0.025 | 0.100 ± 0.022 | 0.107 ± 0.02 | NS | NS | NS | NS |
| SA (mg) | 0.002 ± 0.0004 | 0.002 ± 0.0004 | 0.002 ± 0.0003 | NS | NS | NS | NS |
| pCA (mg) | 0.030 ± 0.007 | 0.027 ± 0.006 | 0.028 ± 0.004 | NS | NS | NS | NS |
| TA (mg) | 0.68 ± 0.17 | 0.61 ± 0.12 | 0.49 ± 0.08 | NS | NS | NS | NS |
| C (mg) | 0.014 ± 0.003 | 0.012 ± 0.003 | 0.013 ± 0.002 | NS | NS | NS | NS |
| C3G (mg) | 0.081 ± 0.02 | 0.072 ± 0.02 | 0.076 ± 0.01 | NS | NS | NS | NS |
| C3R (mg) | 0.013 ± 0.003 | 0.011 ± 0.002 | 0.012 ± 0.002 | NS | NS | NS | NS |
| D (mg) | 0.03 ± 0.008 | 0.03 ± 0.01 | 0.03 ± 0.004 | NS | NS | NS | NS |
| P (mg) | 0.54 ± 0.14 a | 0.49 ± 0.09 a,b | 0.37 ± 0.06 b | <0.05 | NS | <0.05 | NS |
| Parameter | 2 Weeks | 4 Weeks | 6 Weeks | p-Value | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ANOVA | 2 vs. 4 | Duncan Test 2 vs. 6 | 4 vs. 6 | |
| Final body weight (g) | 211.18 ± 19.4 b | 204.16 ± 19.7 b | 290.77 ± 7.2 a | <0.01 | NS | <0.01 | <0.01 |
| Body weight gain (g) | 118.25 ± 11.6 b | 110.73 ± 17.9 b | 198.85 ± 8.9 a | <0.01 | NS | <0.001 | <0.001 |
| Diet intake (g/d) | 15.06 ± 1.15 | 16.18 ± 1.26 | 16.12 ± 1.09 | NS | NS | NS | NS |
| TBARS (µmol/mg protein) | 0.36 ± 0.07 | 0.41 ± 0.09 | 0.38 ± 0.04 | NS | NS | NS | NS |
| PCG (nmol/mL) | 21.49 ± 5.26 a | 12.11 ± 5.38 b | 26.71 ± 8.82 a | <0.01 | <0.05 | <0.001 | NS |
| Parameter | 2 Weeks | 4 Weeks | 6 Weeks | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Red Wine Intake | Ethanol Intake | TBARS | Red Wine Intake | Ethanol Intake | TBARS | Red Wine Intake | Ethanol Intake | TBARS | ||||||||||
| p | R | p | R | p | R | p | R | p | R | p | R | p | R | p | R | p | R | |
| Red wine intake | - | <0.05 | 0.95 | NS | - | <0.05 | 0.87 | NS | - | <0.001 | 0.99 | NS | ||||||
| Ethanol intake | <0.05 | 0.95 | - | <0.05 | 0.87 | <0.05 | 0.87 | - | NS | <0.001 | 0.99 | - | NS | |||||
| TPA | <0.05 | 0.95 | NS | <0.05 | 0.87 | <0.05 | 0.87 | NS | NS | <0.05 | 0.95 | NS | NS | |||||
| CA | <0.05 | 0.95 | NS | <0.05 | 0.87 | <0.05 | 0.87 | NS | NS | <0.001 | 0.99 | NS | NS | |||||
| FA | <0.05 | 0.95 | NS | <0.05 | 0.87 | <0.05 | 0.87 | NS | NS | <0.001 | 0.99 | NS | NS | |||||
| HBA | <0.05 | 0.95 | NS | <0.05 | 0.87 | <0.05 | 0.87 | NS | NS | <0.001 | 0.99 | NS | NS | |||||
| SA | <0.05 | 0.95 | NS | <0.05 | 0.87 | <0.05 | 0.87 | NS | NS | <0.001 | 0.99 | NS | NS | |||||
| pCA | <0.05 | 0.95 | NS | <0.05 | 0.87 | <0.05 | 0.87 | NS | NS | <0.001 | 0.99 | NS | NS | |||||
| TA | <0.05 | 0.88 | NS | NS | NS | NS | NS | <0.01 | 0.97 | NS | NS | |||||||
| C | <0.05 | 0.95 | NS | <0.05 | 0.83 | <0.05 | 0.87 | NS | NS | <0.001 | 0.99 | NS | NS | |||||
| C3G | <0.05 | 0.95 | NS | <0.05 | 0.87 | <0.05 | 0.87 | NS | NS | <0.001 | 0.99 | NS | NS | |||||
| C3R | <0.05 | 0.95 | NS | <0.05 | 0.87 | <0.05 | 0.87 | NS | NS | <0.001 | 0.99 | NS | NS | |||||
| D | <0.05 | 0.95 | NS | <0.05 | 0.87 | <0.05 | 0.87 | NS | NS | <0.001 | 0.99 | NS | NS | |||||
| P | <0.05 | 0.86 | NS | <0.05 | 0.81 | NS | NS | NS | <0.01 | 0.97 | NS | NS | ||||||
| TBARS | <0.05 | 0.87 | NS | - | NS | NS | - | NS | NS | - | ||||||||
| PCG | <0.05 | 0.85 | NS | NS | NS | NS | NS | NS | NS | NS | ||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kołota, A.; Głąbska, D.; Oczkowski, M.; Gromadzka-Ostrowska, J. Analysis of Association between Intake of Red Wine Polyphenols and Oxidative Stress Parameters in the Liver of Growing Male Rats. Appl. Sci. 2020, 10, 6389. https://doi.org/10.3390/app10186389
Kołota A, Głąbska D, Oczkowski M, Gromadzka-Ostrowska J. Analysis of Association between Intake of Red Wine Polyphenols and Oxidative Stress Parameters in the Liver of Growing Male Rats. Applied Sciences. 2020; 10(18):6389. https://doi.org/10.3390/app10186389
Chicago/Turabian StyleKołota, Aleksandra, Dominika Głąbska, Michał Oczkowski, and Joanna Gromadzka-Ostrowska. 2020. "Analysis of Association between Intake of Red Wine Polyphenols and Oxidative Stress Parameters in the Liver of Growing Male Rats" Applied Sciences 10, no. 18: 6389. https://doi.org/10.3390/app10186389
APA StyleKołota, A., Głąbska, D., Oczkowski, M., & Gromadzka-Ostrowska, J. (2020). Analysis of Association between Intake of Red Wine Polyphenols and Oxidative Stress Parameters in the Liver of Growing Male Rats. Applied Sciences, 10(18), 6389. https://doi.org/10.3390/app10186389








