Abstract
Plants produce specific structures constituting barriers, hindering the penetration of pathogens, while they also produce substances inhibiting pathogen growth. These compounds are secondary metabolites, such as phenolics, terpenoids, sesquiterpenoids, resins, tannins and alkaloids. Bioactive compounds are secondary metabolites from trees and shrubs and are used in medicine, herbal medicine and cosmetology. To date, fruits and flowers of exotic trees and shrubs have been primarily used as sources of bioactive compounds. In turn, the search for new sources of bioactive compounds is currently focused on native plant species due to their availability. The application of such raw materials needs to be based on knowledge of their chemical composition, particularly health-promoting or therapeutic compounds. Research conducted to date on European trees and shrubs has been scarce. This paper presents the results of literature studies conducted to systematise the knowledge on phenolic compounds found in trees and shrubs native to central Europe. The aim of this review is to provide available information on the subject and to indicate gaps in the present knowledge.
1. Introduction
Tree stands are exposed to the action of stress factors, both abiotic and biotic. The former include weather anomalies, UV radiation, intensive lighting, water deficits, substrate salinity, high temperature amplitudes and the presence of heavy metals. In turn, biotic factors include pest insects, pathogenic fungi, bacteria and viruses. Trees counter stressors by initiating defence mechanisms to minimise or eliminate disturbances in growth and development. They are related to the consumption of energy and assimilates, the limited production of biomass, its disadvantageous allocation, as well as reduced reproduction. The action of biotic stressors is mainly connected with trees and woody plants entering into symbiosis with antagonists of pathogens and insects, etc.
Plants produce specific structures constituting barriers, hindering the penetration of pathogens, e.g., resin canals and the presence of waxes and resins on their surface, while they also produce substances inhibiting pathogen growth and reducing the attractiveness of needles, etc. These compounds are secondary metabolites, such as phenolics, terpenoids, sesquiterpenoids, resins, tannins and alkaloids. A considerable number of secondary metabolites protect against the adverse effect of herbivorous insects [1,2], pathogenic fungi [3,4,5,6] and bacteria [7,8]. These compounds differ in their chemical structure and are found both on the surface of plants and inside their tissues. Most of them are located in vacuoles and cell walls of peripheral tissues. They are also contained in resins secreted from bark and fruits.
Studies on plant responses to the action of various abiotic and biotic stresses clearly show that they are processes related to the uncontrolled increase in levels of reactive oxygen species (ROS), also referred to as free radicals, and H2O2 [9,10]. When found in excessive amounts, they readily enter chemical reactions with cellular components.
During exposure to the action of stressors, one of the most important defence mechanisms is connected with the production of chemical compounds. This phenomenon involves two types of mechanisms: non-enzymatic and enzymatic. In the case of the former, we observe the action of free radical scavengers, which, when reacting with free radicals, protect cells against adverse reactions. These include ascorbic acid (vitamin C), A-tocopherol (vitamin E), b-carotene and flavonoids. The former type comprises mechanisms related to the formation of specialised enzymes eliminating free radicals and preventing their formation. The enzymatic system includes superoxide dismutase (SOD E.C.), catalysing the dismutation of the superoxide anion radical, and catalase (CAT E.C.), degrading hydrogen peroxide to water [11].
The penetration of a pathogen into a plant triggers defence mechanisms connected with the production of secondary metabolites (phytoalexins), defence proteins, i.e., glycine, serine-rich proteins and GSRP, (Golgi-localized SR-containing protein) being structural components of plant cell walls, pathogenesis-related proteins (PR)m as well as the accumulation of phenols [12,13,14,15]. The chemical defence mounted by plants requires considerable energy expenditure, which may have a negative effect on their growth and development. Thus, a considerable body of research has been based on the growth−differentiation balance hypothesis and the trade-off principle. According to this approach, differentiation is understood as the increased production of secondary metabolites involved in plant defence at the expense of primary metabolites directly related to plant growth and development [16,17]. In this respect, plant species are divided into those characterised by considerable and rapid growth—thus, to a limited extent, investing in chemical defence—and plants with limited, slow growth, but investing in secondary metabolites at a level ensuring effective protection against pathogens. Typically, only one type of compound predominates in plants: alkaloids, phenolic compounds or terpenoids. For example, the main defence compounds in oak leaves are condensed tannins [18], while in coniferous trees these compounds are terpenoids [19].
Many species of trees and shrubs occurring in Europe are used in folk medicine and industry.
Tree and shrub species are widespread in Europe and there are many that can be used in folk medicine as well as industry. Deciduous forests in the temperate zone are characterised by a relatively small number of tree species. The dominant trees are oak, beech and hornbeam. Elm, maple, linden and ash are of great importance. Forests in Northern Europe are dominated by two main species, Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies L. Karsten). In Poland, outside the mountainous areas, where species composition is observed, the share of spruce, fir and beech is higher in most of the country stands, while pine, as the dominant species, predominates. In addition, tree and shrub species that were not native but introduced species, which spread on a larger scale, e.g., bird cherry, were selected for the literature analysis.
Enhanced biosynthesis of the above-mentioned bioactive compounds is an advantageous side effect of the action of stressors. Trees, in their anatomical parts most exposed to the action of pathogens, accumulate the greatest amounts of bioactive compounds, thanks to which shoots, fruits, leaves, needles and bark have become valuable sources of biologically active compounds. Secondary metabolites are most frequently synthesised via three metabolic pathways: terpenoid (mevalonate), phenolic (shikimate) and nitrogen metabolism (amino acids). For woody plants, the derivatives of the shikimate pathways are of greatest importance, e.g., phenolic compounds (phenols, alcohols and phenolic acids, phenylpropanoids, flavonoids, coumarins, tannins), hydroxamic acids and indole alkaloids [20,21,22,23].
The aim of this study was to review the available literature in terms of the content of phenolic compounds in trees and shrubs grown in Europe. The literature research was supplemented with the results of our own research on the various anatomical parts of selected trees and shrubs growing in Poland.
2. Materials and Methods
As part of this work, a bibliometric study was made of original scientific papers on bioactive compounds in Europe’s trees and shrubs available in databases. The following databases were used: Baidu Scholar, BASE (Pakistan Science, Mission Punjab, Pakistan, Baidu Technology, ParkBeijing, Chiny)(Bielefeld Academic Search Engine), CEON (Centrum Otwartej Nauki ICM UW Warszawa Polska) (Library of Science), CiteFactor(Directory Indexing of International Research Jurnals, New York, NY, USA) (Academic Scientific Journals), EBSCOhost (Academic Search Complete), Web of Science (New York, NY, USA), (ESCI, GIGA (Giga Inter Center, Catania, Italia), Information Centre, Google Scholar(Google, US) Index Copernicus International (Index Copernicus International S.A.,China Trading Hause, Hong Kong, China) InfoBase Index (Akshantak Enterprices, Mysure US), and OCLC WorldCat® (World Cat, New York, NY, USA).
Researching methods were based on a list of selected results and the unification of their units to make them easier to compare.
In addition to the results of the literature research, the work includes the results of our own research.
The research material consisted of samples of the following components: leaves (bird cherry Folium Prunus padus), dogwood Folium Cornus L.), fruit (elderberry Fructus Sambucus, dogwood Fructus Cornus, bird cherry Fructus Prunus padus.), bark (bird cherry Cortex Prunus padus) and needles: fir Abies Mill., Larix Mill., pine Pinus L, and spruce Picea Mill from the forests of northwest Poland, collected in 2018 and 2019. Samples weighing about 100 g were freeze-dried, ground and subjected to chromatographic analysis after prior acid and base hydrolysis according to the method described in [24].
HPLC Determination of Phenolic Acids and Flavonols.
Extracts were evaporated to dryness in a stream of nitrogen. Next, they were placed in sealed 17-mL culture test tubes, where first alkaline and then acid hydrolysis was run. In order to run alkaline hydrolysis, 1 mL distilled water and 4 mL 2-M aqueous sodium hydroxide were added to test tubes. Tightly sealed test tubes were heated in a water bath at 95 °C for 30 min. After cooling (approx. 20 min), test tubes were neutralised with 2 mL 6-M aqueous hydrochloric acid solution (pH = 2). Next, samples were cooled in water with ice. Flavonoids were extracted from the inorganic phase using diethyl ether (2 × 2 mL). Formed ether extracts were continuously transferred to 8-mL vials. Next acid hydrolysis was run. For this purpose, the aqueous phase was supplemented with 3 mL 6 M aqueous hydrochloric acid solution. Tightly sealed test tubes were heated in a water bath at 95 °C for 30 min. After being cooled in water with ice, the samples were extracted with diethyl ether (2 × 2 mL). The produced ether extracts were continuously transferred to 8-mL vials, after which they were evaporated to dryness in a stream of nitrogen. Prior to analyses, samples were dissolved in 1 mL methanol. Phenolic compound analysis was performed using an Acquity H class UPLC system equipped with a Acquity PDA detector (The ACQUITY UPLC Photodiode Array (PDA) Detector) (Waters Corp, Milford, MA, USA). Chromatographic separation was performed on an Acquity UPLC® BEH C18 column (100 mm × 2.1 mm, particle size—1.7 µm) (Watersy, Dublin, Ireland). Elution was carried out in a gradient using the following mobile phase composition: A: acetonitrile with 0.1% formic acid, B: 1% aqueous formic acid mixture (pH = 2). Concentrations of phenolic compounds were determined using an internal standard at wavelengths λ = 320 nm and 280 nm and finally expressed as mg/100 g dw of samples. Compounds were identified by comparing the retention time of the analysed peak with the retention time of the standard and by adding a specific amount of the standard to the analysed samples and repeating the analysis. The detection level was 1 µg/g [24].
3. Results
3.1. Phenolic Compounds
In the literature, polyphenols were the group of compounds considered to be the basic factor of the non-enzymatic plant immune mechanism.
Polyphenols are secondary plant metabolites varying greatly in terms of their structure and molecular mass, as well as their physical, biological and chemical properties. They are found in all plant parts, i.e., flowers, fruits, seeds, leaves, roots, bark and lignified parts [25]. Polyphenols are based on an aromatic group of phenols. For the purpose of this study, polyphenols were defined and classified into subclasses according to their structural features [26].
This polyphenols database categorises polyphenols into four classes (Figure 1).
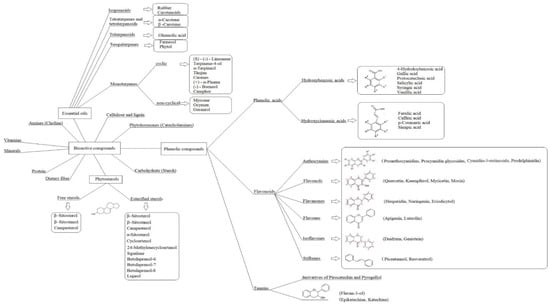
Figure 1.
Polyphenols.
Woody plants very often synthesise phenolic compounds via the shikimate pathway.
This pathway plays an important role in the synthesis of many aromatic compounds in plants. In this pathway, aromatic amino acids are formed, i.e., phenylalanine, tyrosine and tryptophan, and they are used by higher plants as structural components of proteins and as precursors of secondary metabolites. In this process, aromatic phenolic acids are produced, contained in the complex structures of secondary metabolites, e.g., lignin [27,28]. (Figure 2) They are synthesised in the reaction of phosphoenolpyruvate with erythrose-4-phosphate.
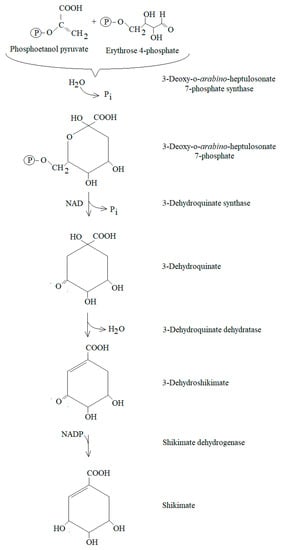
Figure 2.
The shikimate pathway [27,28].
Phenolic compounds, in terms of the structure of the basic carbon skeleton, may be divided into phenolic acids, flavonoids, tannins (hydrolysable and non-hydrolysable tannins—proanthocyanidins) and stilbenes [29].
3.1.1. Phenolic Acids
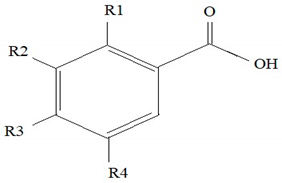
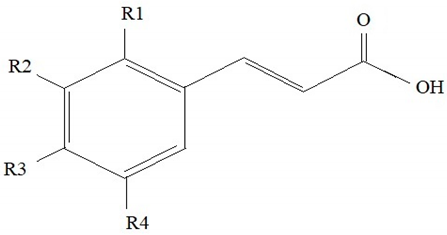
Phenolic acids, in their structure, contain a hydroxyl and a carboxyl group. Hydroxyl derivatives of benzoic and cinnamic acids are common in the plant world (Table 1).

Table 1.
The structures of phenolic acids [30,31].
In plant tissues, other complexes of phenolic acids were also identified, e.g., complexes with flavonoids, fatty acids, sterols and polymers of cell walls. Phenolic acids may also be components of anthocyanins or flavones [30,31]. A separate group is composed of depsides, being a complex of two or more molecules of phenolic acids. In plant organisms, including trees, they are formed mainly in the reaction of the so-called shikimate pathway or malate acid.
Tyrosine and phenylalanine are precursors of most phenolic acids, from which, as a result of deamination, cinnamic acid and its hydroxy derivatives are formed [32].
In plants, phenolic acids are mostly found in the bound form as esters and glycosides contained in lignins and hydrolysed tannins. Examples in this respect may be provided by hydroxycinnamic acids found in ester complexes with carboxylic acids or with glucose. They appear in ester complexes with the following acids: malonic, tartaric, α-hydroxy-hydrocaffeic, hydroxycinnamic, tartronic, shikimic, galacturonic, glucaric (as caffeic acid glucuronide), gluconic (as feruloylgluconic acid, which main isomer is 2-O-feruloyl gluconic acid) and 4-methoxyaldaric (as 2-O-feruloyl-4-methoxyaldaric acid). In turn, hydroxybenzoic acids are primarily found as glycosides. In plant tissues, other complexes of phenolic acids were also identified, e.g., complexes with flavonoids, fatty acids, sterols and polymers of cell walls [30,31]. Phenolic acids are found in wood of oak, pine, spruce, fir, walnut, willow, birch (leaves), and in the fruits of bird cherry (Table 2).
A particularly interesting active compound from the group of hydroxybenzoic acids is ellagic acid, a dimer of gallic acid, found in plants in the free form and (more frequently) in an ester complex with glucose, forming hydrolysable tannins (ellagotannins) [33,34]. It is found in the wood of oak, walnut and sweet chestnut, as well as in berry fruits, such as in strawberries and raspberries [35,36], as well as in the loosestrife family (Lythraceae), particularly pomegranate [10,18,37], and in certain nut seeds [38] and Muscadine grapes. Ellagic acid exhibits, e.g., anti-cancer properties, thanks to which it may inhibit cell division and induce apoptosis in cancer cells [39,40]. Moreover, its anti-inflammatory and antioxidant action [41,42] were investigated and confirmed. Ellagic acid found in Cornelian cherry fruit exhibits immunostimulatory, immunomodulatory, antimicrobial, antioxidative and anti-cancer action. It inhibits the adverse effect of UVB radiation (Ultraviolet B), protects skin against degradation and exhibits anti-inflammatory action [43,44,45]. Ellagic acid is also found in the ester form, bound with glucose, forming hydrolysable tannins (so-called ellagitannins).
Salicylic acid, i.e., 2-hydrobexybenzoic acid, whose natural source is willow, is another compound of particular interest. Willow bark contains a biologically active substance referred to as salicin [46]. Salicin is a β-glucoside of saligenin [47], which, in vivo, undergoes a two-stage transformation consisting of deglycolisation and oxidation to salicylic acid [48,49]. Thanks to the rapid development of chemical synthesis in the late 19th century, this acid has become a direct precursor of other drugs of similar structure, the so-called salicylates, and non-steroid anti-inflammatory drugs. They include, e.g., non-acetylated derivatives of salicylic acid such as sodium salicylate, methyl salicylate, diflunisal, phenyl salicylate (salol), choline salicylate, ethylene glycol salicylate, salicylamide, salsalate, benorylate and diethylamine salicylate [50,51]. In turn, the acetylated derivative of this acid, i.e., aspirin, is an anti-inflammatory, analgesic, antipyretic and antirheumatic drug.

Table 2.
Phenolic acids found in trees and shrubs.
Table 2.
Phenolic acids found in trees and shrubs.
| Trees and Shrubs | Phenolic Acids | Literature |
|---|---|---|
| Scots pine Pinus sylvestris L. | Caffeic acid, salicylic, ferulic, vanillic, gallic, sinapic, p-coumaric, protocatechuic acids | [52,53,54,55,56,57] |
| Norway spruce Picea abies H. Karst in the roots, wood, mature seeds | Shikimic acid, galusic acid, p-coumaric acid, protocatechuic acid, ferulic, vanillic, syringic, sinapic, salicylic, quinic acids, protocatechuic, gallic acids | [52,58,59,60,61,62] |
| Silver fir Abies alba Mill. Wood and bark | Gallic acid, homovanillic acid protocatehuic acid, p-hydroxybenzoic acid, vanillic and p-coumaric acids | [63] |
| European beech Fagus sylvatica L.-leaves | Caffeic acid, ferulic acid, chlorogenic acid syringic, gallic, abscisic and cinnamic acids | [52,58,64] |
| Oak Quercus robus L. | Ellagic acid, gallic acid, gentisic acid, p-hydroxybenzoic acid, protocatechuic acid syringic acid vanillic acid, p-coumaric acid, caffeic acid, ferulic acid sinapic acid | [52,58,65,66,67] |
| Walnut Juglans regia L. | Ellagic acid, caffeic acid, p-coumaric acid, galusic acid | [52,58,68] |
| Willow Salix spp. | Ferulic, caffeic, salicylic, vanillic, syringic, α-resorcylic, m and p-hydroxybenzoic, p-coumaric, cinnamic acids | [58,69] |
| Salix alba L. | Salicylic and p-coumaric acid | [70] |
| Salix babylonica L.-leaves | Caffeic and p-coumaric acids | [71] |
| Salix capitata L.-leaves | Protocatechuic acid | [72,73] |
| Silver birch Betula pendula Roth-leaves | Chlorogenic, p-hydroxybenzoic, caffeic, gallic, coumaric, p-hydroxycinnamic acids | [74] |
| Hawthorn Crataegus L. | Chlorogenic, caffeic acid | [58,75] |
| Rowan Sorbus aucuparia L. | Neochlorogenic, chlorogenic, protocatechuic, caffeic and p-hydroxybenzoic acids | [76] |
| White poplar Populus alba L.-buds | Benzoic, ferulic, caffeic acids, cinnamic, cis-p-coumaric and trans-p-coumaric acids | [58,77] |
| Bird cherry Prunus padus L. fruits | Caffeic acid, ferulic, coumaric, chlorogenic, elagic, gallic acids | [58,78] |
| Prunus serotina Ehrh. | Gallic acid, caffeic and p-hydroxybenzoic acids, p-coumaric, ferulic, cinnamic acids | [79] |
To summarise, phenolic acids protect plants against the action of microorganisms and insects, while, in combination with polysaccharides, they make cell walls more rigid. In the human organism, they exhibit diverse biological activity, e.g., scavenging free radicals, chelating metal ions, modifying enzyme activity and protein availability. They prevent cardiovascular disease, cancer and diabetes. Additionally, they protect against photooxidative skin damage [80].
Based on our own conducted research on a representative number of plant material samples of wild-growing trees and shrubs in north-western Poland, it was found that spruce needles had the highest content of phenolic acids, and pine needles the lowest (Table 3). Among the analysed acids, we found in fir benzoic and vanillic, in larch caffeic and coumaric acids, in pine caffeic and ferulic, and, in spruce, 4 hydroxybenzoic, caffeic and chlorogenic, which were found in the highest concentration in the needles tested. Salicylic acid and rozmaric acid, on the other hand, were present at a very low concentrations in all tested samples, except for larch for the latter acid. The samples varied considerably in terms of the content of phenolic acids (Table 3).

Table 3.
Concentration range of phenolic acids (mg/kg d.w. needles) in pine, fir, larch and spruce needles.
Elderberry, bird cherry and dogwood fruits and dogwood leaves were characterised by high phenolic acid content, and the samples were particularly rich in benzoic acid (especially dogwood), p-coumaric acid and chlorogenic acid. The highest variation was found in the case of benzoic acid, where, in the dogwood fruit sample, it was present in concentrations of 655 µg/g d.w. and 17 µg/g d.w. for bird cherry bark. Protocatechuic, 4-hydroxybenzoic, vanillic, caffeic, salicylic and rozmaric acids were present in low concentrations in almost all samples (Table 4).

Table 4.
Concentration range of phenolic acids (mg/kg d.w.) in elderberry, bird cherry dogwood fruits; bird cherry and dogwood leaves and bird cherry bark.
3.1.2. Flavonoids
The chemical structure of all flavonoids is based on the hydrocarbon skeleton of flavone (Figure 1). They differ in the number and type of substituents, while differences between these compounds result primarily from a different structure in only one basic ring. Chalcone formed via biosynthesis from phenylalanine is a precursor of flavonoids. Its synthesis starts with shikimic acid. Flavonoids are found not only as free molecules (aglycones), but also much more frequently in the bound form with sugars (glycosides). To date, over 7000 various flavonoids have been identified, which, in terms of their chemical structure, are divided into flavones, flavonols (3-hydroxyflavones), flavanones, flavanols (flavan-3-oles), flavanonoles, anthocyanidins, isoflavones and neoflavonoids (Figure 1, Table 5). Thanks to their unique structure, flavonoids may protect the cell against reactive oxygen species (ROS) generated in the organism [81,82].

Table 5.
Division of flavonoids depending on their chemical structure [83].
Flavonoids are phytoalexins, i.e., substances serving protective functions, formed as a result of the plant’s contact with a pathogen, frequently inducing the expression of several genes encoding enzymes of the phenolic biosynthesis pathway [84]. Isoflavonoids are highly toxic towards fungal pathogens, which is particularly evident in such compounds as pterocarpanes, isoflavanes, isoflavones and isoflavonones. The mechanism of their action consists of the inhibition of spore development and mycelium growth as well as the damage of fungal cell membrane structure [85,86,87,88]. Flavonoids are compounds that are commonly found in plants; therefore, they constitute an everyday part of the average human diet (approx. 1 g/day). They are, among others, found in fruits (chokeberry, citrus fruits, blueberries, blueberries, grapes, cherries) and vegetables (onions, tomatoes, peppers, soybeans, broccoli) and in trees and shrubs (Table 6).

Table 6.
Flavonoids found in trees and shrubs.
Based on our own conducted research on plant material samples, it was found that fir needles and elderberry fruit had the highest content in flavonoids, and spruce needles and bird cherry leaves the lowest (Table 7 and Table 8). Catechin and naringenin were present in the highest concentration in the needles tested.

Table 7.
Concentration range of flavonoids (mg/kg d.w. Needles) in pine, fir, larch and spruce needles.

Table 8.
Concentration range of flavonoids (mg/kg d.w.) in elderberry, bird cherry dogwood fruits; bird cherry and dogwood leaves and bird cherry bark.
The highest concentration of flavonoids found in elderberry fruits turned out to be quercetin. A high concentration of catechins was observed in samples of the bark and fruit of bird cherry. Among the analysed flavonoids, vitexin, rutin, quercetin, apigenin, kaempferol, and luteolin were present in very low concentrations in all sample needles. Samples of the fruit, bark and leaves of the examined trees and shrubs contained low concentrations of luteolin, vitexin and kaempferol (Table 8).
Experiments in vitro and in vivo show the varied attributes of these compounds, including their antioxidant, anti-inflammatory, anticancer, antiatherosclerotic and anti-aggregational properties, as well as their capacity for plugging vessels and detoxification. The multidirectional spectrum of the functions of flavonoids suggests a wide range of prospective applications for these compounds, not only in the prevention of many diseases, but also in their therapy (e.g., cancers, cardiovascular disease, atherosclerosis, diabetes, etc.) [111,112,113,114].
3.1.3. Tannins
Tannins are a group of organic chemical compounds, derivatives of phenols, which are naturally produced by plants. Tannins are usually divided into two basic groups: hydrolysable and non-hydrolysable (condensed) tannins [115,116,117].
In the centre of the hydrolysable tannin molecule is a monosaccharide (glucose or other polyols, e.g., branched sugar–hamamelose, shikimic, quinic acid, and even pectin), whose hydroxyl groups, partially or completely, are esterified with gallic acid residues, e.g., m-digalus acid. These tannins are easily hydrolysed by weak acids and bases or enzymes to monomeric products. Depending on the type of resulting products, gallotannins and ellagitannins are distinguished. Gallotanins are the simplest tannins, containing, in their molecule, glucose and ester in association with gallic acid. Another example of such compounds is tannic acid (C76H52O46); see Figure 3 and Figure 4.
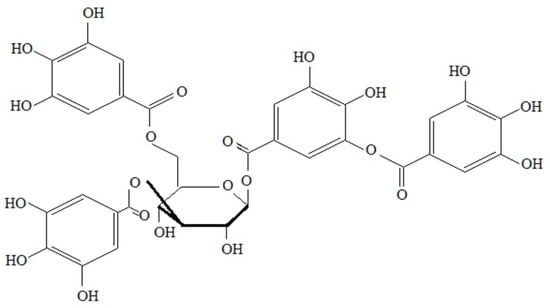
Figure 3.
Tannic acid.
Proanthocyanidins (PA) (the condensed tannins–non-hydrolysable tannins) are oligomers or polymers of flavonoid structures, mainly flavan-3-ols, (-)—epicatechin and (+)—catechin (Figure 1), flavan-3,4-diols or a mixture of both. In contrast to hydrolysable tannins, they do not contain sugar units. Some authors distinguish an additional third group—catechin tannins—examples of which are the polyphenols found in green tea leaves. They have a characteristic carbon-condensed tannin and do not contain sugar residues. These include monomeric flavan-3-ols, e.g., (+)—catechin, (-)—epicatechin, (+)—gallocatechin and (-)—epigallocatechin or their ester derivatives, e.g., 3-gallates (-) -epicatechin and (-)—epigallocatechin. The latter group of compounds is easily enzymatically hydrolysed under the influence of tannase (4). The flavan-3-ol units are linked mainly through the C4→C8 bond (Figure 4), but the C4→C6 bond also exists (both called B-type). The flavan-3-ol units can also be doubly linked by an additional ether bond at C2→O7 (A-type). The size of PA molecules can be described by their degree of polymerisation (114). Three common flavan-3-ols, which differ in their hydroxylation patterns, are found in PAs. Proanthocyanidins, consisting exclusively of (epi)catechin, are called procyanidins (PCs). Proanthocyanidins, containing (epi) afzelechin or (epi)gallocatechin as subunits, are named propelargonidins (PPs) or prodelphinidins (PDs), respectively. Propelargonidins and PDs are less common in nature than procyanidins [115].
The basic route for the synthesis of all tannins is the pathway associated with sugar catabolism, leading to shikimic acid. Gallic acid is formed from shikimic or quinic acid, which is a substrate in various types of condensation, resulting in the synthesis of tannins [115].
Proanthocyanidins (also called condensed or non-hydrolysable tannins) are also found in leaves, lignified parts of plants, as well as flowers and fruits [118]. Inflorescences of hawthorn (Crataegi inflorescentia, Crataegus sp. Rosaceae) are well-known sources of proanthocyanidin that have been used in herbal medicine for years [119,120]. Recently, intensive studies have been conducted on an extract from the bark of maritime pine Pinus pinaster (Pinaceae), patented as Pycnogenol, whose proanthocyanidin content is 85%. This preparation exhibits, e.g., strong antioxidant properties (Table 9) [121,122,123].

Table 9.
Proanthocyanidins (condensed tannins) found in trees and shrubs.
A considerable body of data indicates that a diet rich in anthocyanins plays a significant role in the prevention of cardiovascular disease and cancer [135]. It was shown that plant extracts rich in anthocyanins may exert a protective effect on the function of blood vessel walls, preventing endothelial dysfunction and the loss of its regulatory activity [124,136,137]. The antioxidant properties of these compounds may be used in the prevention of cancer, both of the alimentary tract and internal organs [137]. They also prevent the oxidation of the LDL cholesterol fraction (Low-density lipoprotein) [138,139].
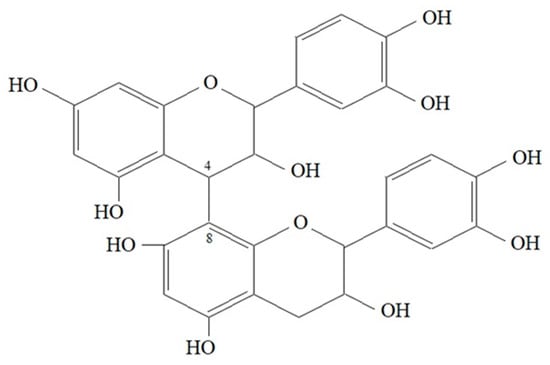
Figure 4.
Dimeric 4→8 B type proanthocyanidin chemical structure [140,141].
3.1.4. Stilbenes
Stilbenes are metabolites of the phenylpropanoid pathway, activated under biotic and abiotic stress. They are compounds with a 1,2-diphenylethylene skeleton. Only some unrelated plant species are capable of synthesising and accumulating stilbenes. The enzyme facilitating this synthesis is stilbene synthase (STS). In plants, stilbenes serve several functions, among which the most significant is related to strong antimicrobial properties; thus, they are classified as phytoalexins [142]. Other known functions also include their repellent action against herbivores, as well as their allelopathic and antioxidant properties. Stilbenes are produced in small amounts; however, biosynthesis is activated primarily post infection, while it is also triggered by wounding, UV radiation, ozone and aluminium ions. Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is one of the most extensively described stilbenes [142,143,144].
Resveratrol is found in the form of two isomeric forms: cis and trans (Figure 1). Transresveratrol is a phenol stilbene found in many plants, e.g., the grape family (Vitaceae), and is particularly common in grape vines (Vitis vinifera). The other form of resveratrol, i.e., cis, is formed as a result of the isomerisation of trans-resveratrol and after the decomposition of resveratrol polymer molecules during the fermentation of grape skins, due to the action of UV radiation, and at high pH [144]. Resveratrol was first isolated in 1940 from the roots of Veratum gradiflorum [145]. Its highest concentration was recorded in the roots of Japanese knotweed (Polygonum cuspidatum). In folk medicine, this plant was successfully used to treat pyoderma, mycoses and venereal diseases [146]. Moreover, resveratrol has been applied in cancer prevention and treatment thanks to its ability to effectively inhibit each stage of neoplasia, i.e., the initiation, promotion and progression of the disease [147,148].
Stilbenes are secondary metabolites that are relatively rarely found in nature (Table 10). To date, they have been reported in almost 70 unrelated plant species belonging to approx. 30 genera and 12 families. The greatest stilbene contents are detected in plants from the pine family (Pinaceae), the grape family (Vitaceae), the beech family (Fagaceae), the mulberry family (Moraceae) and the grass family (Poaceae) [141].

Table 10.
Stilbenes found in trees and shrubs.
The presented literature sources indicate that phenolic substances of plant origin, particularly those obtained from trees and shrubs growing in a temperate climate zone, exhibit a beneficial effect on human health. Thanks to the presence of bioactive compounds in those plants, they have found applications as detoxicants, vitamin supplements, as well as preparations boosting immunity and adjunctive medication in the treatment of various diseases. There is a considerable body of data indicating that a diet rich in bioactive compounds plays a significant role in the prevention of cardiovascular diseases and cancer. In view of the fact that the treatment of chronic pain, cancer, cardiovascular disease and a number of other diseases requires a combination of several therapeutic methods, alternative therapies using plant origin preparations are gaining popularity. It also needs to be stressed that molecular mechanisms of action, in the case of active substances contained in plant preparations, have not been fully elucidated and require further research.
The review of the literature presented in this paper presents the potential of trees and shrubs native to temperate zones as sources of phenolic substances.
Author Contributions
L.S.-M., K.S.-S., A.P.-B. designed and conducted the study; all authors contributed to the manuscript preparation according to their professional experience. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Polish Ministry of Science and Higher Education program ‘Regional Initiative of Excellence’ in years 2019-2022, Project No. 005/RID/2018/19.
Conflicts of Interest
There are no conflicts of interest.
References
- Kaplan, I.; Halitschke, R.; Kessler, A.; Sardanelli, S.; Denno, R.F. Constitutive and induced defenses to herbivory in above- and belowground plant tissues. Ecology 2008, 89, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Carmona, D.; Lajeunesse, M.J.; Johnson, M.T. Plant traits that predict resistance to herbivores. Funct. Ecol. 2010, 25, 358–367. [Google Scholar] [CrossRef]
- Rayner, A.D.M.; Boddy, L. Fungal Decomposition of Wood—Its Biology and Ecology; John Wiley and Sons: Chichester, UK; New York, NY, USA; Brisbane, Australia; Toronto, ON, Canada; Singapore, 1988; pp. 1–428. [Google Scholar]
- Kozłowska, M. Phenolic composition of red raspberry canes in relation to Didymella applanata (Niessl) Sacc. response. Acta Physiol. Plant 1994, 16, 211–215. [Google Scholar]
- Evensen, P.C.; Solheim, H.; Høiland, K.; Stenersen, J. Induced resistance of Norway spruce, variation of phenolic compounds and their effects on fungal pathogens. For. Pathol. 2000, 30, 97–108. [Google Scholar] [CrossRef]
- Przybył, K.; Karolewski, P.; Oleksyn, J.; Łabędzki, A.; Reich, P. Fungal Diversity of Norway Spruce Litter: Effects of Site Conditions and Premature Leaf Fall Caused by Bark Beetle Outbreak. Microb. Ecol. 2007, 56, 332–340. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, H.; Min, H.; Park, E.; Lee, K.; Ahn, Y.H.; Cho, Y.; Pyee, J. Antibacterial and antifungal activity of pinosylvin, a constituent of pine. Fitoterapia 2005, 76, 258–260. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. REACTIVE OXYGEN SPECIES: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Shiliang, L.; Rongjie, Y. Regulations of reactive oxygen species in plants abiotic stress: An integrated overview. Plant Life Under Chang. Environ. 2020, 323–353. [Google Scholar]
- Vertuani, S.; Angusti, A.; Manfredini, S. The antioxidants and pro-antioxidants network: An overview. Curr. Pharm. Des. 2004, 10, 1677–1694. [Google Scholar] [CrossRef] [PubMed]
- Darvill, A.G.; Albersheim, P. Phytoalexins and their elicitors—A defense against microbial infection in plants. Annu. Rev. Plant Physiol. 1984, 35, 243–275. [Google Scholar] [CrossRef]
- Van Loon, L.C. Pathogenesis-related proteins. Plant Mol. Biol. 1985, 4, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.P.; Klessig, D.F. The Pathogenesis-Related Proteins of Plants. In Genetic Engineering: Principles and Methods; Setlow, J.K., Ed.; Plenum Press: New York, NY, USA, 1989; Volume 11, pp. 65–109. [Google Scholar]
- Dixon, R.A.; Harrison, M.J.; Lamb, C.J. Early events in the activation of plant defense responses. Ann. Rev. Phytopathol. 1994, 32, 479–501. [Google Scholar] [CrossRef]
- Agrawal, A.A. Macroevolution of plant defense strategies. Trends Ecol. Evol. 2007, 22, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.E.; Koricheva, J. The Ontogeny of Plant Defense and Herbivory: Characterizing General Patterns Using Meta-Analysis. Am. Nat. 2010, 175, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Forkner, R.E.; Marquis, R.J.; Lill, J.T. Feeny revisited: Condensed tannins asanti-herbivore defences in leaf−chewing herbivore communities of Quercus. Ecol. Entomol. 2004, 29, 174–187. [Google Scholar] [CrossRef]
- Manninen, A.-M.; Vuorinen, M.; Holopainen, J.K. Variation in Growth, Chemical Defense, and Herbivore Resistance in Scots Pine Provenances. J. Chem. Ecol. 1998, 24, 1315–1331. [Google Scholar] [CrossRef]
- Harborne, J.B. The comparative biochemistry of phytoalexin induction in plants. Biochem. Syst. Ecol. 1999, 27, 335–367. [Google Scholar] [CrossRef]
- Leszczyński, B. Rola allelozwiązków w oddziaływaniach owady-rośliny. In Biochemiczne Oddziaływania Środowiskowe [Biochemical Environmental Interactions]; Oleszek, W., Głowniak, K., Leszczyński, B., Eds.; Akademia Medyczna: Lublin, Poland, 2001; pp. 61–85. [Google Scholar]
- Malinowski, H. Strategie obronne roślin drzewiastych przed szkodliwymi owadami. Defensive strategies of woody plants against harmful insects. Lesne Pr. Badaw. For. Res. Pap. 2008, 69, 165–173. [Google Scholar]
- Dziedziński, M.; Kobus-Cisowska, J.; Szymanowska, D.; Stuper-Szablewska, K.; Baranowska, M.; Baranowska, M. Identification of Polyphenols from Coniferous Shoots as Natural Antioxidants and Antimicrobial Compounds. Molecules 2020, 25, 3527. [Google Scholar] [CrossRef] [PubMed]
- Hermann, K. Review on nonessential constituents of vegetables. III. Carrots, celery, pars-nips, beets, spinach, lettuce, endives, chicory, rhubarb, and artichokes. Lebensm. Unters. Forsch. 1978, 167, 262–273. [Google Scholar]
- Kolesnikov, M.P.; Gins, V.K. Characteristics of the accumulation of phenolic compounds in amaranth leaves under the effect of growth stimulators Prikl. Biokhim. Mikrobiol. 2001, 37, 616–620. [Google Scholar]
- Rothwell, J.A.; Pérez-Jiménez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Herrmann, K.M. The Shikimate Pathway: Early Steps in the Biosynthesis of Aromatic Compounds. Plant Cell 1995, 7, 907–919. [Google Scholar] [CrossRef]
- Schmid, J.; Amrhein, N. Molecular organization of the shikimate pathway in higher plants. Phytochemistry 1995, 39, 737–749. [Google Scholar] [CrossRef]
- Swanson, P.G. Tannins and Polyphenols. Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2003; pp. 5729–5733. [Google Scholar]
- Robbins, R.J. Phenolic Acids in Foods: An Overview of Analytical Methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Jeszka, M.; Flaczek, E.; Kobus-Cisowska, J.; Dziedzic, K. Związki fenolowe—Charakterystyka i znaczenie w technologii żywności. Nauka Przyr. Technol. 2010, 4, 1–13. [Google Scholar]
- Castellucio, C.; Paganga, G.; Melikan, N.; Bowell, G.P.; Pridham, J.; Sampson, J.; Rice-Evans, C. Antioxidant potential of intermediates in phenylopropanoid metabolism in higher plants. FEBS Lett. 1995, 368, 188–192. [Google Scholar] [CrossRef]
- Quideau, S.; Feldman, K.S. Ellagotannin chemistry: The first synthesis of dehydrohexahydroxydiphenoate (DHHDP) esters from oxidative coupling of unetherified methyl gallate. J. Org. Chem. 1997, 62, 8809–8813. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.H.; Törrönen, A. Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: Influence of cultivar, cultivation site and technique. Food Res. Int. 2000, 33, 517–524. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.-S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Gülcü, M.; Uslu, N.; Özcan, M.M.; Gökmen, F.; Özcan, M.M.; Banjanin, T.; Lemiasheuski, V. The investigation of bioactive compounds of wine, grape juice and boiled grape juice wastes. J. Food Process. Preserv. 2019, 43, 1–14. [Google Scholar] [CrossRef]
- Aguilera-Carbó, A.; Augur, C.; Prado-Barragán, L.A.; Aguilar, C.N.; Favela-Torres, E. Extraction and analysis of ellagic acid from novel complex sources. Chem. Pap. 2008, 62, 440–444. [Google Scholar] [CrossRef]
- Narayanan, B.A.; Geoffroy, O.; Willingham, M.C.; Re, G.G.; Nixon, D. p53/p21(WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Cancer Lett. 1999, 136, 215–221. [Google Scholar] [CrossRef]
- Han, D.H.; Lee, M.J.; Kim, J.H. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 2006, 26, 3601–3606. [Google Scholar]
- Festa, F.; Aglitti, T.; Duranti, G.; Ricordy, R.; Perticone, P.; Cozzi, R. Strong antioxidant activity of ellagic acid in mammalian cells in vitro revealed by the comet assay. Anticancer Res. 2001, 21, 3903. [Google Scholar]
- Corbett, S.; Daniel, J.; Drayton, R.; Field, M.; Steinhardt, R.; Garrett, N. Evaluation of the Anti-inflammatory Effects of Ellagic Acid. J. PeriAnesthesia Nurs. 2010, 25, 214–220. [Google Scholar] [CrossRef]
- Lamer-Zarawska, E.; Oszmiański, J. Rola niektórych substancji roślinnych w profilaktyce przeciwnowotworowej. Wiad. Ziel. 1998, 5, 1–4. [Google Scholar]
- Kwiatkowska, E. Kwas elagowy—Zawartość w żywności i rola prozdrowotna. Post. Fitoter. 2010, 4, 211–214. [Google Scholar]
- Sepúlveda, L.; Ascacio, A.; Rodríguez-Herrera, R.; Aguilera-Carbó, A.; Aguilar, C.N. ChemInform Abstract: Ellagic Acid: Biological Properties and Biotechnological Development for Production Processes. Afr. J. Biotechnol. 2012, 43, 4518–4523. [Google Scholar] [CrossRef]
- Lutomski, J.; Alkiewicz, J. Leki Roślinne w Profilaktyce i Terapii; PZWL: Warszawa, Poland, 1993. [Google Scholar]
- Kohlmunzer, S. Farmakognozja; PZWL: Warszawa, Poland, 1993. [Google Scholar]
- Schmid, B.; Kötter, I.; Heide, L. Pharmacokinetics of salicin after oral administration of a standardised willow bark extract. Eur. J. Clin. Pharmacol. 2001, 57, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Schmid, B.; Lüdtke, R.; Selbmann, H.K.; Kötter, I.; Tschirdewahn, B.; Schaffner, W.; Heide, L. Efficacy and tolerability of a standardized willow bark extract in patients with osteoarthritis: Randomized placebo-controlled, double blind clinical trial. Phytother. Res. 2001, 15, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Międzybrodzki, R. Kierunki poszukiwań i zastosowanie niesteroidowych leków przeciwzapalnych. Postęp. Hig. Med. Dośw. 2004, 58, 438–448. [Google Scholar]
- Zejc, A.; Gorczyca, M. Chemia Leków; PZWL: Warszawa, Poland, 2004. [Google Scholar]
- Ożarowski, A.; Jaroniewski, W. Rośliny Lecznicze i ich Praktyczne Zastosowanie; IWZW: Warszawa, Poland, 1987. [Google Scholar]
- Flos Pini Masculinum—Kwiat Męski Sosny w Fitoterapii. Available online: http://rozanski.li/1750/flos-pini-masculinum-kwiat-meski-sosnyw-fitoterapii/ (accessed on 11 November 2019).
- Pan, H.; Lundgren, L.N. Phenolic extractives from root bark of Picea abies. Phytochemistry 1995, 39, 1423–1428. [Google Scholar] [CrossRef]
- Antonova, G.F.; Chaplygina, I.A.; Varaksina, T.N.; Stasova, V.V. Ascorbic acid and xylem development in trunks of the Siberian larch trees. Russ. J. Plant Physiol. 2005, 52, 83–92. [Google Scholar] [CrossRef]
- Antonova, G.F.; Varaksina, T.N.; Zheleznichenko, T.V.; Stasova, V.V. Changes in phenolic acids during maturation and lignification of Scots pine xylem. Онтoгенез 2012, 43, 199–208. [Google Scholar] [CrossRef]
- Metsämuuronen, S.; Sirén, H. Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem. Rev. 2019, 18, 623–664. [Google Scholar] [CrossRef]
- Strona Archiwalna. Available online: http://www.rozanski.ch/fitoterapia1.htm (accessed on 11 November 2019).
- Strack, D.; Kottke, I.; Oberwinkler, F. Phenolics of mycorrhizas and non-mycorrhizal roots of Norway spruce. Planta 1990, 182, 142–148. [Google Scholar] [CrossRef]
- Cvikrová, M.; Malá, J.; Hrubcová, M.; Eder, J.; Foretová, S. Induced changes in phenolic acids and stilbenes in embryogenic cell cultures of Norway spruce by culture filtrate of Ascocalyx abietina. J. Plant Dis. Prot. 2008, 115, 57–62. [Google Scholar] [CrossRef]
- Malá, J.; Hrubcová, M.; Máchová, P.; Cvrčková, H.; Martincová, O.; Cvikrová, M. Changes in phenolic acids and stilbenes induced in embryogenic cell cultures of Norway spruce by two fractions of Sirococcus strobilinus mycelian. J. For. Sci. 2011, 57, 1–7. [Google Scholar] [CrossRef]
- Koutaniemi, S.; Warinowski, T.; Kärkönen, A.; Alatalo, E.; Fossdal, C.G.; Saranpää, P.; Laakso, T.; Fagerstedt, K.V.; Simola, L.K.; Paulin, L.; et al. Expression profiling of the lignin biosynthetic pathway in Norway spruce using EST sequencing and real-time RT-PCR. Plant Mol. Biol. 2007, 65, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T. Antioxidant Efficiency of Beech (Fagus sylvatica L.) Bark Polyphenols Assessed by Chemometric Methods. Ind. Crops Prod. 2017, 108, 26–35. [Google Scholar] [CrossRef]
- Pirvu, L.; Grigore, A.; Bubueanu, C.; Draghici, E.M. Comparative analytical and antioxidant activity studies on a series of Fagus sylvatica L. leaves extracts. J. Planar Chromatogr. Mod. TLC 2013, 26, 237–242. [Google Scholar] [CrossRef]
- De Simón, B.F.; Cadahía, E.; Conde, E.; García-Vallejo, M.C.; Simón, B.F. Low Molecular Weight Phenolic Compounds in Spanish Oak Woods. J. Agric. Food Chem. 1996, 44, 1507–1511. [Google Scholar] [CrossRef]
- Cadahía, E.; Muñoz, L.; De Simón, B.F.; García-Vallejo, M.C.; Simón, B.F. Changes in Low Molecular Weight Phenolic Compounds in Spanish, French, and American Oak Woods during Natural Seasoning and Toasting. J. Agric. Food Chem. 2001, 49, 1790–1798. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Sanz, M.; Cadahía, E.; Poveda, P.; Broto, M. Chemical characterization of oak heartwood from Spanish forests of Quercus pyrenaica (wild.). Ellagitannins, low molecular weight phenolic, and volatile compounds. J. Agric. Food Chem. 2006, 54, 8314–8321. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Valentão, P.; Andrade, P.B.; Ferreira, I.C.; Ferreres, F.; Bento, A.A.; Seabra, R.; Estevinho, L.M. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295. [Google Scholar] [CrossRef]
- Pobłocka-Olech, L. Zastosowanie Metod Chromatograficznych w Badaniach Składu Chemicznego Kory Niektórych Gatunków Klonów Wierzby. Ph.D. Thesis, Farmecautical Department, Medicial Academy, Gdańsk, Poland, 2006. [Google Scholar]
- Bisset, N.; Wichtl, M. Herbal Drugs and Phytopharmaceuticals; CRC: London, UK, 2001. [Google Scholar]
- Duke, J.A. Handbook of Phytochemical Constituents of GRAS Herbs and Other Economic Plants; CRC: London, UK, 1992. [Google Scholar]
- Pohjamo, S.P.; Hemming, J.E.; Willför, S.; Reunanen, M.H.; Holmbom, B.R. Phenolic extractives in Salix caprea wood and knots. Phytochemistry 2003, 63, 165–169. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Moreirinha, C.; Silva, S.; Costa, E.M.; Veiga, M.; Coscueta, E.R.; Santos, S.A.O.; Almeida, A.; Pintado, M.M.E.; Freire, C.S.; et al. The Health-Promoting Potential of Salix spp. Bark Polar Extracts: Key Insights on Phenolic Composition and In Vitro Bioactivity and Biocompatibility. Antioxidants 2019, 8, 609. [Google Scholar] [CrossRef] [PubMed]
- Najda, A.; Bekier, J.; Guleac, E.; Filiks, A. Profil Kwasów Fenolowych Liści Brzozy Brodawkowatej (Betula pendula roth) [Phenolic Acid Profile of Silver Birch (Betula pendula Roth) Leaves]. Episteme 2014, 25, 235–243. [Google Scholar]
- Bobinaitė, R.; Grootaert, C.; Van Camp, J.; Šarkinas, A.; Liaudanskas, M.; Žvikas, V.; Viškelis, P.; Rimantas Venskutonis, P. Chemical composition, antioxidant, antimicrobial and antiproliferative activities of the extracts isolated from the pomace of rowanberry (Sorbus aucuparia L.). Food Res. Int. 2020, 136, 109310. [Google Scholar] [CrossRef] [PubMed]
- Kuchukhidze, J.; Jokhadze, M.; Murtazashvili, T.; Mshvildadze, V. Antioxidant polyphenols from Populus alba growing in Georgia. Georgian Med. News 2011, 199, 94–97. [Google Scholar]
- Dimkić, I.; Ristivojević, P.; Janakiev, T.; Berić, T.; Trifković, J.; Milojković-Opsenica, D.; Stanković, S. Phenolic profiles and antimicrobial activity of various plant resins as potential botanical sources of Serbian propolis. Ind. Crop. Prod. 2016, 94, 856–871. [Google Scholar] [CrossRef]
- Telichowska, A.; Kobus-Cisowska, J.; Ligaj, M.; Stuper-Szablewska, K.; Szymanowska, D.; Tichoniuk, M.; Szulc, P. Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties. Open Chem. 2020, 18, 1125–1135. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; De Biaggi, M.; Riondato, I.; Rakotoniaina, E.N.; Beccaro, G.L. New Findings in Prunus padus L. Fruits as a Source of Natural Compounds: Characterization of Metabolite Profiles and Preliminary Evaluation of Antioxidant Activity. Molecules 2018, 23, 725. [Google Scholar] [CrossRef]
- Slavin, J.; Marquart, L.; Jakobs, D. Consumption of whole-grain food and decreased risk of cancer: Proposed mechanisms. Cereal Foods World 2000, 45, 54–58. [Google Scholar]
- Colette, N.; Bernier, J.L.; Catteau, J.P.; Pommery, J.; Wallet, J.C.; Gaydou, E.M. Antioxidant properties of hydroxyflavones. Free Radic. Biol. Med. 1996, 20, 35–43. [Google Scholar]
- Ligor, M. Polifenole. In Badanie Substancji Biologicznie Aktywnych w Surowcach Roślinnych i Produktach Naturalnych z Zastosowaniem Łączonych technik Chromatograficznych; Ligor, M., Ed.; UMK: Toruń, Poland, 2013; pp. 55–63. [Google Scholar]
- Lamer-Zarawska, E. Flawonoidy, Fitoterapia i Leki Roślinne; PZWL: Warszawa, Poland, 2007; pp. 64–67. [Google Scholar]
- Benhamou, N.; Nicole, M. Cell biology of plant immunization against microbial infection: The potential of induced resistance in controlling plant diseases. Plant Physiol. Biochem. 1999, 37, 703–719. [Google Scholar] [CrossRef]
- Skipp, R.A.; Bailey, J.A. The fungitoxicity of isoflavonoid phytoalexins measured using different types of bioassay. Physiol. Plant Pathol. 1977, 11, 101–112. [Google Scholar]
- Phillips, D.; Kapulnik, Y. Plant isoflavonoids, pathogens and symbionts. Trends Microbiol. 1995, 3, 58–64. [Google Scholar] [CrossRef]
- Dakora, F.D.; Phillips, D. Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins. Physiol. Mol. Plant Pathol. 1996, 49, 1–20. [Google Scholar] [CrossRef]
- Laracine-Pittet, C.; Lebreton, P. Flavonoid variability within Pinus sylvestris. Phytochemistry 1988, 27, 2663–2666. [Google Scholar] [CrossRef]
- Oleszek, W.; Stochmal, A.; Karolewski, P.; Simonet, A.M.; Macias, F.A.; Tava, A. Flavonoids from Pinus sylvestris needles and their variation in trees of different origin grown for nearly a century at the same area. Biochem. Syst. Ecol. 2002, 30, 1011–1022. [Google Scholar] [CrossRef]
- Viriot, C.; Scalbert, A.; Lapierre, C.; Moutounet, M. Ellagitannins and lignins in aging of spirits in oak barrels. J. Agric. Food Chem. 1993, 41, 1872–1879. [Google Scholar] [CrossRef]
- Vovk, I.; Simonovska, B.; Andrenšek, S.; Vuorela, H. Rotation planar extraction and rotation planar of oak Quercus robus L bark. J. Chromatogr. A 2003, 991, 267–274. [Google Scholar] [CrossRef]
- Benković, E.T.; Grohar, T.; Žigon, D.; Švajger, U.; Janeš, D.; Kreft, S.; Strukelj, B. Chemical composition of the silver fir (Abies alba) bark extract Abigenol® and its antioxidant activity. Ind. Crop. Prod. 2014, 52, 23–28. [Google Scholar] [CrossRef]
- Petrakis, P.V.; Spanos, K.; Feest, A.; Daskalakou, E.N. Phenols in Leaves and Bark of Fagus sylvatica as Determinants of Insect Occurrences. Int. J. Mol. Sci. 2011, 12, 2769–2782. [Google Scholar] [CrossRef]
- Dübeler, A.; Voltmer, G.; Gora, V.; Lunderstädt, J.; Zeeck, A. Phenols from Fagus sylvatica and their role in defence against Cryptococcus fagisuga. Phytochemistry 1997, 45, 51–57. [Google Scholar] [CrossRef]
- Zeneli, G.; Krokene, P.; Christiansen, E.; Krekling, T.; Gershenzon, J. Methyl jasmonate treatment of large Norway spruce (Picea abies) trees increases the accumulation of terpenoid resin components and protects against infection by Ceratocystis polonica, a bark beetle-associated fungus. Tree Physiol. 2006, 26, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Krajnc, A.U.; Novak, M.; Felicijan, M.; Kraševec, N.; Lešnik, M.; Zupanec, N.; Komel, R. Antioxidative response patterns of Norway spruce bark to low-density Ceratocystis polonica inoculation. Trees 2014, 28, 1145–1160. [Google Scholar] [CrossRef]
- Broda, B.; Mowszowicz, J. Przewodnik do Oznaczania Roślin Leczniczych, Trujących i Użytkowych. [Guide for the Determination of Medicinal, Poisonous and Usable Plants]; PZWL: Warszawa, Poland, 2000. [Google Scholar]
- Sulima, P.; Krauze-Baranowska, M.; Przyborowski, J.A. Variations in the chemical composition and content of salicylic glycosides in the bark of Salix purpurea from natural locations and their significance for breeding. Fitoterapia 2017, 118, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Salix in Practical Phytotherapy. Available online: http://rozanski.li/438/wierzba-salix-w-praktycznej-fitoterapii/ (accessed on 13 June 2019).
- Krauze-Baranowska, M.; Pobłocka-Olech, L.; Głód, D.; Wiwart, M.; Zieliński, J.; Migas, P. HPLC of flavanones and chalcones in different species and clones of Salix. Acta Pol. Pharm. 2013, 70, 27–34. [Google Scholar] [PubMed]
- Harborne, J.B.; Baxter, H. The Handbook of Natural Flavonoids; Willey: Chichester, UK, 1999. [Google Scholar]
- Thapliyal, R.P.; Bahugana, R.P. Fatty acids and flavonoids of Salix Lindlevana. Int. J. Pharmacog. 1993, 31, 165–166. [Google Scholar] [CrossRef]
- Gorobets, A.B.; Bandyukova, V.A.; Shapirom, D.K. Flavonoid composition of pollen of Salix caprea and S. alba. Khim. Prir. Soedin. 1982, 6, 781–782. [Google Scholar]
- Fong, H.H.S.; Bauman, J.L. Hawthorn. J. Cardiovasc. Nurs. 2002, 16, 1–8. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Christensen, L.P.; Kaack, K.; Fretté, X. Selection of elderberry (Sambucus nigra L.) genotypes best suited for the preparation of elderflower extracts rich in flavonoids and phenolic acids. Eur. Food Res. Technol. 2007, 227, 293–305. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Presler, A.; Michel, P. Profiling of Phenolic Compounds and Antioxidant Activity of Dry Extracts from the Selected Sorbus Species. Molecules 2012, 17, 3093–3113. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M. Flavonoids from the Genus Taxus. Z. Nat. C 2004, 59, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž.P. proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Čukanović, J.; Tešević, V.V.; Jadranin, M.B.; Ljubojević, M.; MladenoviĆ, E.; Kostic, S. Horse chestnut (Aesculus hippocastanum L.) seed fatty acids, flavonoids and heavy metals plasticity to different urban environments. Biochem. Syst. Ecol. 2020, 89, 103980. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Czeczot, H. Biological activities of flavonoids—A review. Pol. J. Food Nutr. Sci. 2000, 50, 3–13. [Google Scholar]
- Martínez-Flórez, S.; González-Gallego, J.; Culebras, J.M.; Tuñón, M.J. Flavonoids: Properties and anti-oxidizing action. Nutr. Hosp. 2003, 17, 271–278. [Google Scholar]
- Olszewska, M. Flawonoidy i ich zastosowanie w lecznictwie. Farm. Pol. 2003, 59, 391–401. [Google Scholar]
- Yao, L.H.; Jiang, Y.; Shi, J.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in Food and Their Health Benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Zaprometov, M.N. Tannins, Lignans, and Lignins. In Phytochemicals in Plant Cell Cultures; Academic Press: Cambridge, MA, USA, 1988; pp. 89–97. [Google Scholar]
- Nonaka, G. Isolation and structure elucidation of tannins. Pure Appl. Chem. 1989, 61, 357–360. [Google Scholar] [CrossRef]
- Porter, L.J. Flavans and Proanthocyanidins; Harbone, J.B., Ed.; The Flavonoids 23–53; Chapman & Hall: London, UK, 1994. [Google Scholar]
- Bahorun, T.; Aumjaud, E.; Ramphul, H.; Rycha, M.; Luximon-Ramma, A.; Trotin, F.; Aruoma, O.I. Phenolic constituents and antioxidant capacities of Crataegus monogyna (Hawthorn) callus extracts. Nahrung 2003, 47, 191–198. [Google Scholar] [CrossRef]
- Rohdewald, P. Pycnogenol®, a Plant Extract for Women’s Health. Int. J. Women’s Health Care 2017, 2, 10–33140. [Google Scholar]
- Zhang, Z.; Chang, Q.; Zhu, M.; Huang, Y.; Ho, W.K.; Chen, Z. Characterization of antioxidants present in hawthorn fruits. J. Nutr. Biochem. 2001, 12, 144–152. [Google Scholar] [CrossRef]
- Cisár, P.; Jány, R.; Waczulíková, I.; Sumegová, K.; Muchová, J.; Vojtassák, J.; Duraćková, Z.; Lisý, M.; Rohdewald, P. Effect of pine bark extract (Pycnogenol) on symptoms of knee osteoarthritis. Phytother. Res. 2008, 22, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Grimm, T.; Schäfer, A.; Högger, P. Antioxidant activity and inhibition of matrix metalloproteinases by metabolites of maritime pine bark extract (pycnogenol). Free Radic. Biol. Med. 2004, 36, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Oliff, H. Scientific and Clinical Monograph for Pycnogenol, 2019 Update. The American Botanical Council. Available online: http://abc.herbalgram.org/site/PageServer?pagename=Pycnogenol (accessed on 14 January 2020).
- Gupta, R.K.; Hasla, E. Plant proanthocyanidins. Part 7. Prodelphinidins from Pinus sylvestris. Chem. Soc. Perkin Trans. 1981, 1, 1148–1150. [Google Scholar] [CrossRef]
- Karapandzova, M.; Stefkov, G.; Cvetkovikj, I.; Stanoeva, J.P.; Stefova, M.; Kulevanova, S. Flavonoids and Other Phenolic Compounds in Needles of Pinus peuce and Other Pine Species from the Macedonian Flora. Nat. Prod. Commun. 2015, 10, 987–990. [Google Scholar] [CrossRef]
- Kim, N.-Y.; Jang, M.-K.; Lee, D.-G.; Yu, K.H.; Jang, H.; Kim, M.; Kim, S.G.; Yoo, B.H.; Lee, S.-H. Comparison of methods for proanthocyanidin extraction from pine (Pinus densiflora) needles and biological activities of the extracts. Nutr. Res. Pract. 2010, 4, 16–22. [Google Scholar] [CrossRef]
- Ku, C.S.; Mun, S.P.; Jang, J.P. Effects of Water Extraction Temperatures on the Yield, Molecular Weight, and Antioxidant Activity of Proanthocyanidins Extracted from Pinus radiata Bark. For. Prod. J. 2011, 61, 321–325. [Google Scholar] [CrossRef]
- Vivas, N.; Nonier, M.-F.; Pianet, I.; De Gaulejac, N.V.; Fouquet, E. Proanthocyanidins from Quercus petraea and Q. robur heartwood: Quantification and structures. Comptes Rendus Chim. 2006, 9, 120–126. [Google Scholar] [CrossRef]
- Pallenbach, E.; Scholz, E.; König, M.; Rimpler, H. Proanthocyanidins from Quercus petraea Bark. Planta Med. 1993, 59, 264–268. [Google Scholar] [CrossRef]
- Oak in Phytotherapy. Available online: http://rozanski.li/1193/quercus-dab-w-fitoterapii/ (accessed on 13 June 2019).
- Yang, B.; Liu, P. Composition and health effects of phenolic compounds in hawthorn (Crataegus spp.) of different origins. J. Sci. Food Agric. 2012, 92, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Arya, V.; Bhat, Z.A.; Khan, N.A.; Prasad, D.N. The genus Crataegus: Chemical and pharmacological perspectives. Rev. Bras. Farm. 2012, 22, 1187–1200. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Oszmiański, J. Anthocyanins in fruits of Prunus padus (bird cherry). J. Sci. Food Agric. 2002, 82, 1483–1486. [Google Scholar] [CrossRef]
- Bridle, P.; Stott, K.; Timberlake, C. Anthocyanins in Salix species: A new anthocyanin in Salix purpurea bark. Phytochemistry 1973, 12, 1103–1106. [Google Scholar] [CrossRef]
- Wilska-Jeszka, J. Polifenole, Glukozynolany i inne Związki Prozdrowotne i Antyżywieniowe. Chemia Żywności. T. I. Składniki Żywności; Sikorski, Z.E., Ed.; WN-T: Warszawa, Poland, 2007; pp. 206–226. [Google Scholar]
- Lila, M.A. Anthocyanins and Human Health: An In Vitro Investigative Approach. J. Biomed. Biotechnol. 2004, 2004, 306–313. [Google Scholar] [CrossRef]
- Cos, P.; Bruyne, T.D.; Hermans, N.; Apers, S.; Berghe, D.V.; Vlietinck, A. Proanthocyanidins in health care: Current and new trends Curr. Med. Chem. 2000, 11, 1345–1359. [Google Scholar] [CrossRef]
- Karonen, M.; Loponen, J.; Ossipov, V.; Pihlaja, K. Analysis of procyanidins in pine bark with reversed-phase and normal-phase high-performance liquid chromatography–electrospray ionization mass spectrometry. Anal. Chim. Acta 2004, 522, 105–112. [Google Scholar] [CrossRef]
- Muszyński, S.; Guzewski, W. Antocyjany Roślin Wyższych; PTB: Kraków, Poland, 1976; Volume 20, p. 4. [Google Scholar]
- Morales, M.; Ros Barcelo, A.; Pedreno, M.A. Plant stilbenes: Recent advances in their chemistry and biology. Adv. Plant Physiol. 2000, 3, 39–70. [Google Scholar]
- Bavaresco, L.; Fregoni, C.; Cantù, E.; Trevisan, M. Stilbene compounds: From the grapevine to wine. Drugs Under Exp. Clin. Res. 1999, 25, 57–63. [Google Scholar]
- Roupe, K.; Remsberg, C.M.; Yáñez, J.A.; Davies, N. Pharmacometrics of Stilbenes: Seguing Towards the Clinic. Curr. Clin. Pharmacol. 2006, 1, 81–101. [Google Scholar] [CrossRef]
- Gu, X.; Chu, Q.; O’Dwyer, M.; Zeece, M. Analysis of resveratrol in wine by capillary electrophoresis. J. Chromatogr. A 2000, 881, 471–481. [Google Scholar] [CrossRef]
- Shakibaei, M.; Harikumar, K.B.; Aggarwal, B.B. Resveratrol addiction: To die or not to die. Mol. Nutr. Food Res. 2009, 53, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Arichi, H.; Kimura, Y.; Okuda, H.; Baba, K.; Kozawa, M.; Arichi, S. Effects of stilbene components of the roots of Polygonum cuspidatum Sieb. et Zucc. on lipid metabolism. Chem. Pharm. Bull. 1982, 30, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Lange, B.M.; Trost, M.; Heller, W.; Langebartels, C.; Sandermann, H. Elicitor-induced formation of free and cell-wall-bound stilbenes in cell-suspension cultures of Scots pine (Pinus sylvestris L.). Planta 1994, 194, 143–148. [Google Scholar] [CrossRef]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.-M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317. [Google Scholar] [CrossRef] [PubMed]
- Fliegmann, J.; Schanz, S.; Britsch, L. Molecular analysis of chalcone and dihydropinosylvin synthase from Scots pine (Pinus sylvestris), and differential regulation of these and related enzyme activities in stressed plants. Plant Mol. Biol. 1992, 18, 489–503. [Google Scholar] [CrossRef]
- Erdtman, H.; Misiorny, A. Constituents of pine heartwood. XXXI. The content of pinosylvin phenols in Swedish pines. Sven. Pap. 1952, 55, 605–608. [Google Scholar]
- Kiselev, K.V.; Grigorchuk, V.P.; Ogneva, Z.V.; Suprun, A.R.; Dubrovina, A.S. Stilbene biosynthesis in the needles of spruce. Picea Jezoensis Phytochem. 2016, 131, 57–67. [Google Scholar] [CrossRef]
- Sheng-Hong, L.; Xue-Mei, N.; Zahn, S.; Gershenzon, J.; Weston, J.; Schneider, B. Diastereomeric stilbene glucoside dimers from the bark of Norway spruce (Picea abies L.). Phytochemistry 2008, 69, 772–782. [Google Scholar]
- Ganthaler, A.; Stöggl, W.; Mayr, S.; Kranner, I.; Schüler, S.; Wischnitzki, E.; Sehr, E.M.; Fluch, S.; Trujillo-Moya, C. Association genetics of phenolic needle compounds in Norway spruce with variable susceptibility to needle bladder rust. Plant Mol. Biol. 2017, 94, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Sergent, T.; Kohnen, S.; Jourez, B.; Beauve, C.; Schneider, Y.-J.; Vincke, C. Characterization of black locust (Robinia pseudoacacia L.) heartwood extractives: Identification of resveratrol and piceatannol. Wood Sci. Technol. 2014, 48, 1005–1017. [Google Scholar] [CrossRef]
- Song, W.; Wang, H.-J.; Bucheli, P.; Zhang, P.-F.; Wei, N.-Z.; Lu, Y.-H. Phytochemical Profiles of Different Mulberry (Morussp.) Species from China. J. Agric. Food Chem. 2009, 57, 9133–9140. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).