Eco-Physiological Traits and Phenylpropanoid Profiling on Potted Vitis vinifera L. cv Pinot Noir Subjected to Ascophyllum nodosum Treatments under Post-Veraison Low Water Availability
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Location and Plot Settings
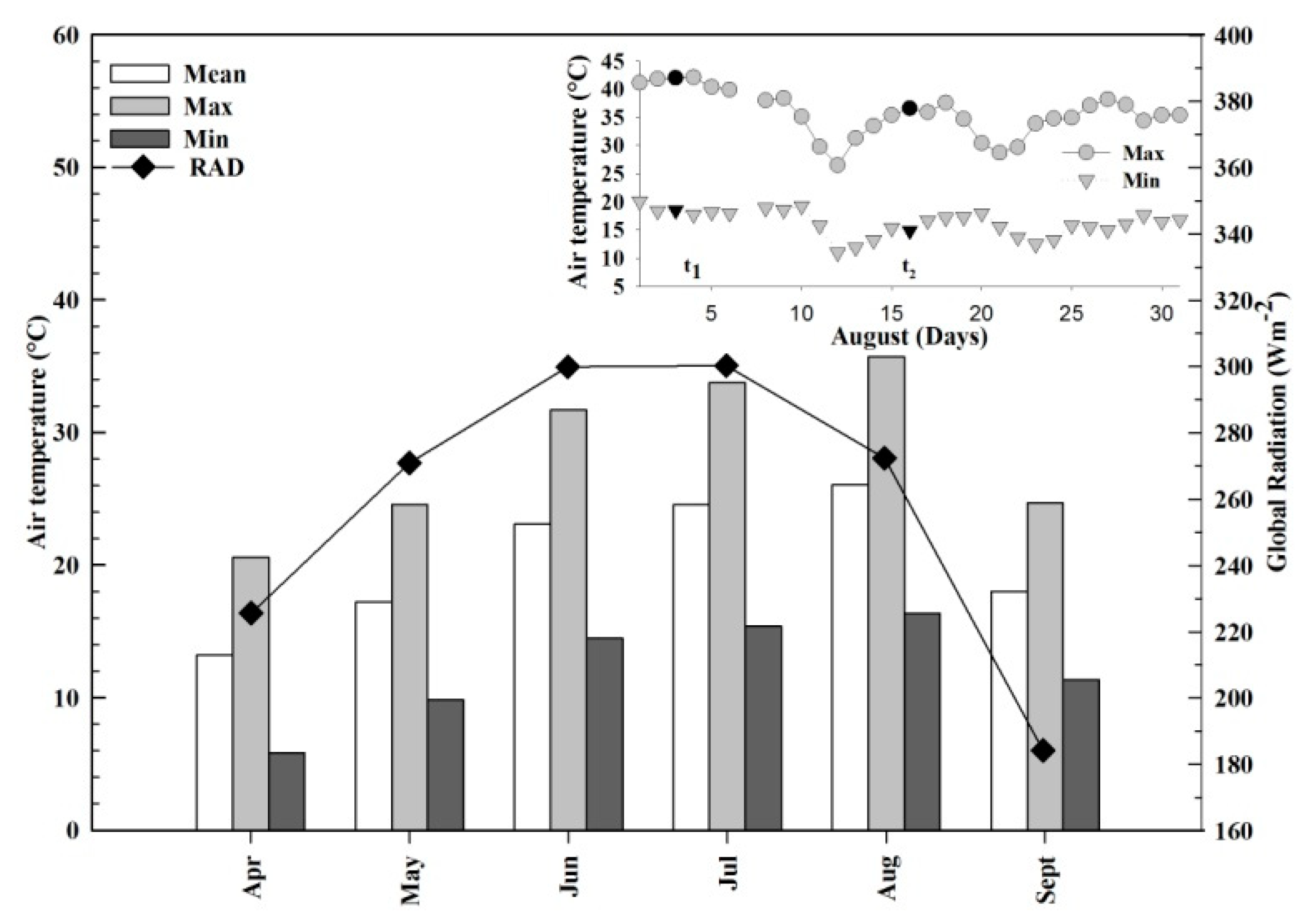
2.2. Climate Parameters
2.3. Leaf Gas Exchange, Chlorophyll Fluorescence, Leaf Water Potential, and Content
2.4. Grape Composition and Yield
2.5. Berry Skin Phenylpropanoids
2.6. Statistical Analysis
3. Results
3.1. Climate Parameters
3.2. Leaf Gas Exchange, Chlorophyll Fluorescence, and Leaf and Stem Water Potential
3.3. Grape Composition and Yield
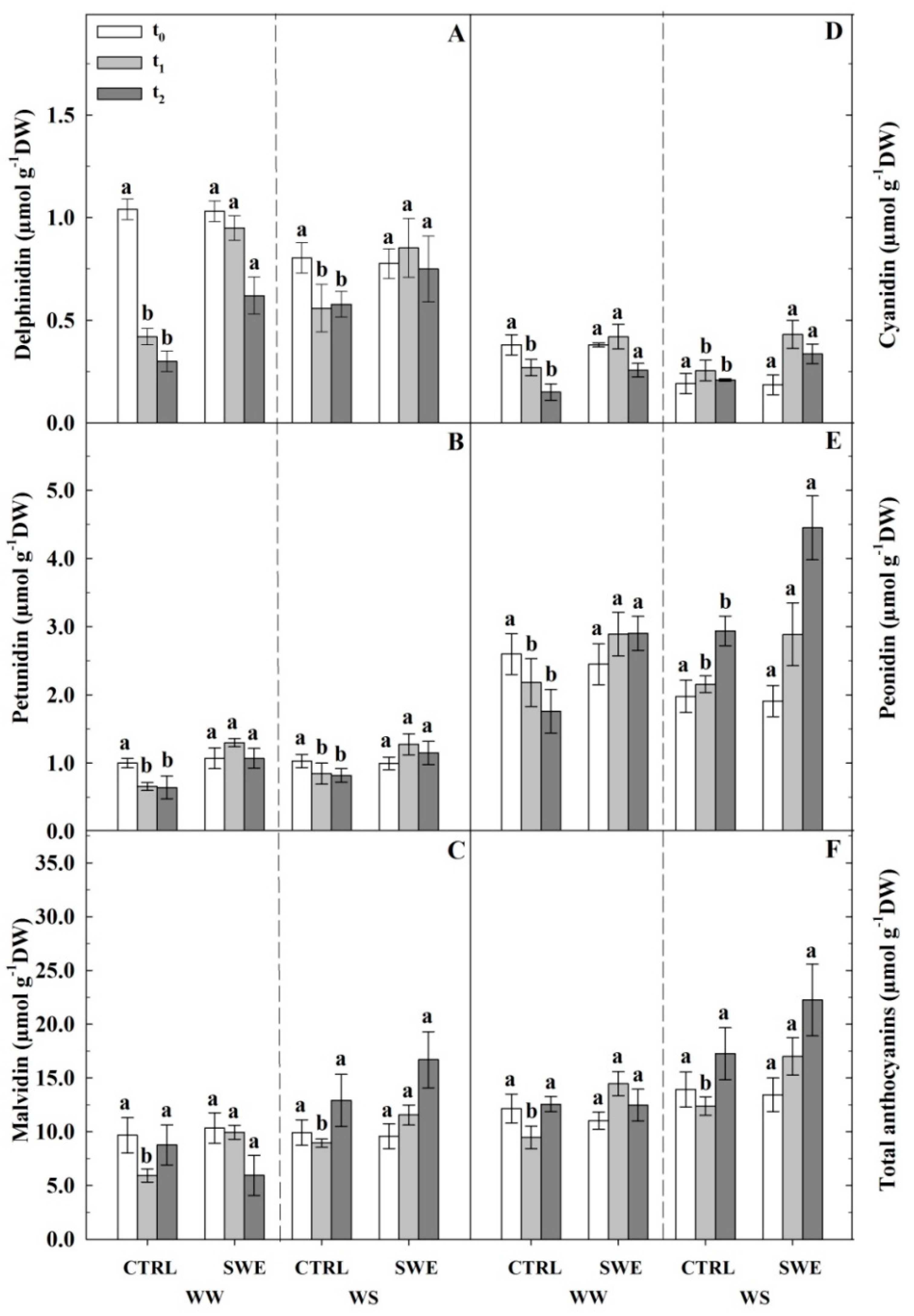
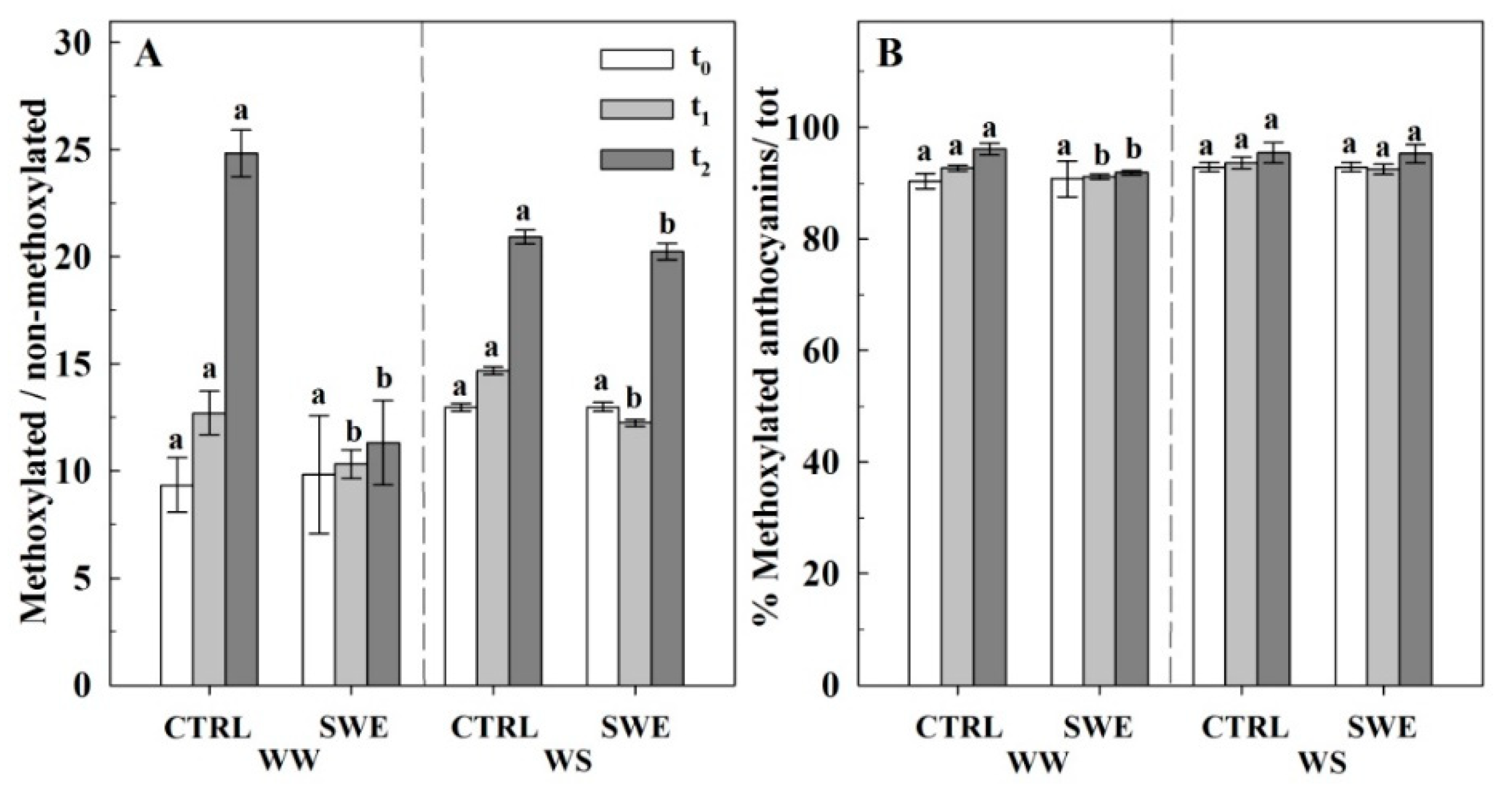
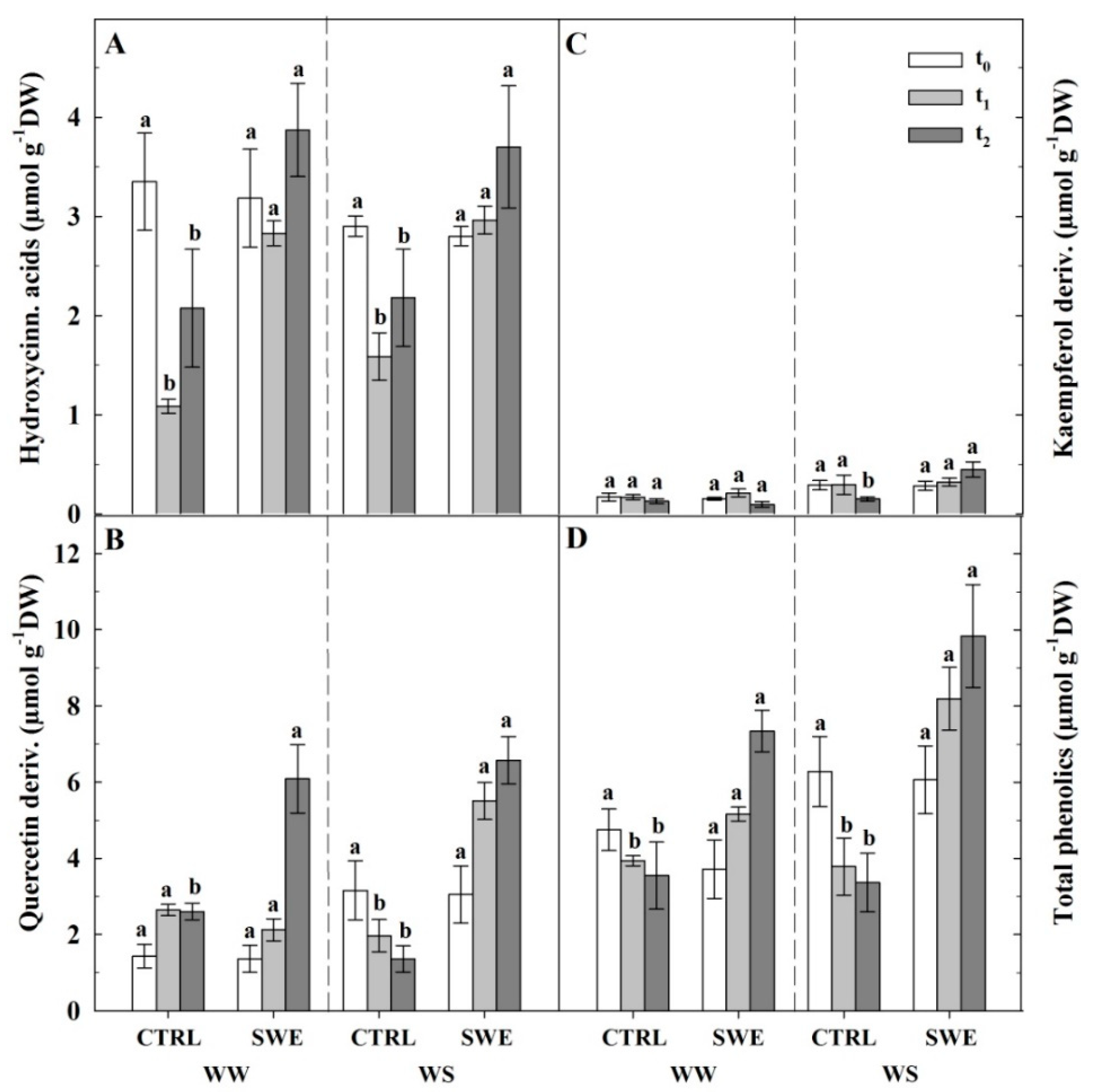
3.4. Berry Skin Phenylpropanoids
4. Discussion
4.1. Consequences of Water Deficit on Pinot Noir Physiology and Berry Skin Anthocyanin Content
4.2. Timing of Action of A. nodosum Extract in Improving Plant Physiological Performance and Water Relations
4.3. A. nodosum Treatments Influenced the Biosynthesis of Berry Skin Anthocyanins Irrespective of Irrigation Regime
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cifre, J.; Bota, J.; Escalona, J.M.; Medrano, H.; Flexas, J. Physiological tools for irrigation scheduling in grapevine (Vitis vinifera L.). An open gate to improve water-use efficiency? Agric. Ecosyst. Environ. 2005, 106, 159–170. [Google Scholar] [CrossRef]
- Chaves, M.M.; Santos, T.P.; Souza, C.D.; Ortuño, M.F.; Rodrigues, M.L.; Lopes, C.M.; Maroco, J.P.; Pereira, J.S. Deficit irrigation in grapevine improves water-use efficiency while controlling vigour and production quality. Ann. Appl. Biol. 2007, 150, 237–252. [Google Scholar] [CrossRef]
- Schultz, H.R.; Jones, G.V. Climate induced historic and future changes in viticulture. J. Wine Res. 2010, 21, 137–145. [Google Scholar] [CrossRef]
- Spinoni, J.; Naumann, G.; Vogt, J.; Barbosa, P. European drought climatologies and trends based on a multi-indicator approach. Glob. Planet. Chang. 2015, 127, 50–57. [Google Scholar] [CrossRef]
- Schultz, H.R. Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant Cell Environ. 2003, 26, 1393–1405. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Greer, D.H.; Hutton, R.J.; Landsberg, J.J. Does night-time transpiration contribute to anisohydric behaviour in a Vitis vinifera cultivar? J. Exp. Bot. 2009, 60, 3751–3763. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.M.; Ortuño, M.F.; Lopes, C.M.; Chaves, M.M. Grapevine varieties exhibiting differences in stomatal response to water deficit. Funct. Plant Biol. 2012, 39, 179–189. [Google Scholar] [CrossRef]
- Tomás, M.; Medrano, H.; Escalona, J.M.; Martorell, S.; Pou, A.; Ribas-Carbó, M.; Flexas, J. Variability of water use efficiency in grapevines. Environ. Exp. Bot. 2014, 103, 148–157. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Flexas, J.; Hernández, E.; Rosselló, J.; Bota, J. From leaf to whole-plant water use efficiency (WUE) in complex canopies: Limitations of leaf WUE as a selection target. Crop J. 2015, 3, 220–228. [Google Scholar] [CrossRef]
- Bota, J.; Tomás, M.; Flexas, J.; Medrano, H.; Escalona, J.M. Differences among grapevine cultivars in their stomatal behavior and water use efficiency under progressive water stress. Agric. Water Manag. 2016, 164, 91–99. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Cifre, J.; Mariano Escalona, J.; Galmés, J.; Gulías, J.; Riera, D. Understanding down-regulation of photosynthesis under water stress: Future prospects and searching for physiological tools for irrigation management. Ann. Appl. Biol. 2004, 144, 273–283. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Sadras, V.O.; Petrie, P.R. Climate shifts in south-eastern Australia: Early maturity of Chardonnay, Shiraz and Cabernet Sauvignon is associated with early onset rather than faster ripening. Aust. J. Grape Wine Res. 2011, 17, 199–205. [Google Scholar] [CrossRef]
- Petrie, P.R.; Sadras, V.O. Advancement of grapevine maturity in Australia between 1993 and 2006: Putative causes, magnitude of trends and viticultural consequences. Aust. J. Grape Wine Res. 2008, 14, 33–45. [Google Scholar] [CrossRef]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.D.; Gerós, H. Berry phenolics of grapevine under challenging environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef]
- Sadras, V.O.; Moran, M.A. Elevated temperature decouples anthocyanins and sugars in berries of Shiraz and Cabernet Franc. Aust. J. Grape Wine Res. 2012, 18, 115–122. [Google Scholar] [CrossRef]
- Adams, D.O. Phenolics and ripening in grape berries. Am. J. Enol. Vitic. 2006, 57, 246–256. [Google Scholar]
- Waterhouse, A.L. Wine phenolics. Ann. N. Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef]
- Silva, L.R.; Queiroz, M. Bioactive compounds of red grapes from Dão region (Portugal): Evaluation of phenolic and organic profile. Asian Pac. J. Trop. Biomed. 2016, 6, 315–321. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Romero-Cascales, I.; López-Roca, J.M.; Ros-García, J.M.; Gómez-Plaza, E. Anthocyanin fingerprint of grapes: Environmental and genetic variations. J. Sci. Food Agric. 2006, 86, 1460–1467. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Di Gaspero, G. Transcriptional control of anthocyanin biosynthetic genes in extreme phenotypes for berry pigmentation of naturally occurring grapevines. BMC Plant Biol. 2007, 7, 46. [Google Scholar] [CrossRef]
- Zarrouk, O.; Brunetti, C.; Egipto, R.; Pinheiro, C.; Genebra, T.; Gori, A.; Lopes, C.M.; Tattini, M.; Chaves, M.M. Grape ripening is regulated by deficit irrigation/elevated temperatures according to cluster position in the canopy. Front. Plant Sci. 2016, 7, 1640. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Nardi, S.; Altissimo, A. Long-term research activity on the biostimulant properties of natural origin compounds. In Proceedings of the I World Congress on the Use of Biostimulants in Agriculture, Strasbourg, France, 26–29 November 2012; Volume 1009, pp. 181–187. [Google Scholar]
- Posmyk, M.M.; Szafrańska, K. Biostimulators: A new trend towards solving an old problem. Front. Plant Sci. 2016, 7, 748. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Sangha, J.S.; Kelloway, S.; Critchley, A.T.; Prithiviraj, B. Seaweeds (macroalgae) and their extracts as contributors of plant productivity and quality: The current status of our understanding. Adv. Bot. Res. 2014, 71, 189–219. [Google Scholar]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef]
- Richardson, A.D.; Aikens, M.; Berlyn, G.P.; Marshall, P. Drought stress and paper birch (Betula papyrifera) seedlings: Effects of an organic biostimulant on plant health and stress tolerance, and detection of stress effects with instrument-based, noninvasive methods. J. Arboric. 2004, 30, 52–61. [Google Scholar]
- Spann, T.M.; Little, H.A. Application of commercial extract of the brown seaweed Ascophyllum nodosum increases drought tolerance in container-grown ‘Hamlin’ Sweet Orange nursery trees. HortScience 2011, 46, 577–582. [Google Scholar] [CrossRef]
- Elansary, H.O.; Skalicka-Woźniak, K.; King, I.W. Enhancing stress growth traits as well as phytochemical and antioxidant contents of Spiraea and Pittosporum under seaweed extract treatments. Plant Physiol. Bioch. 2016, 105, 310–320. [Google Scholar] [CrossRef]
- Martynenko, A.; Shotton, K.; Astatkie, T.; Petrash, G.; Fowler, C.; Neily, W.; Critchley, A.T. Thermal imaging of soybean response to drought stress: The effect of Ascophyllum nodosum seaweed extract. Springerplus 2016, 5, 1393. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K.; Abdel-Hamid, A.M.; El-Esawi, M.A.; Ali, H.M.; Elshikh, M.S. Seaweed extracts enhance Salam turfgrass performance during prolonged irrigation intervals and saline shock. Front. Plant Sci. 2017, 8, 830. [Google Scholar] [CrossRef]
- Santaniello, A.; Scartazza, A.; Gresta, F.; Loreti, E.; Biasone, A.; Di Tommaso, D.; Piaggesi, A.; Perata, P. Ascophyllum nodosum seaweed extract alleviates drought stress in Arabidopsis by affecting photosynthetic performance and related gene expression. Front. Plant Sci. 2017, 8, 1362. [Google Scholar] [CrossRef]
- Khan, W.; Rajirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; dell’Aversana, E.; Carillo, P.; Maggio, A. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 2018. [Google Scholar] [CrossRef]
- Berlyn, G.P.; Russo, R.O. The use of organic biostimulants in nitrogen fixing trees. Nitrogen Fix. Tree Res. Rep. 1990, 8, 78–80. [Google Scholar]
- Blunden, G.; Gordon, S.M.; Smith, B.E.; Fletcher, R.L. Quaternary ammonium compounds in species of the Fucaceae (Phaeophyceae) from Britain. Br. Phycol. J. 1985, 20, 105–108. [Google Scholar] [CrossRef]
- Laetitia, A.; Fauchon, M.; Blanc, N.; Hauchard, D.; ArGall, E. Phenolic compounds in the brown seaweed Ascophyllum nodosum: Distribution and radical-scavenging activities. Phytochem. Anal. 2010, 21, 399–405. [Google Scholar]
- Norrie, J.; Branson, T.; Keathley, P.E. Marine plant extracts impact on grape yield and quality. Acta Hortic. 2002, 594, 315–319. [Google Scholar] [CrossRef]
- Colapietra, M.; Alexander, A. Effect of foliar fertilization on yield and quality of table grapes. Acta Hortic. 2006, 721, 213–218. [Google Scholar] [CrossRef]
- Norrie, J.; Keathley, J.P. Benefits of Ascophyllum nodosum marine-plant extract applications to ‘Thompson Seedless’ grape production. Acta Hortic. 2006, 727, 243–247. [Google Scholar] [CrossRef]
- Kok, D.; Bal, E.; Celik, S.; Ozer, C.; Karauz, A. The influences of different seaweed doses on table quality characteristics of cv. trakya ilkeren (Vitis vinifera). Bulg. J. Agric. Sci. 2010, 16, 429–435. [Google Scholar]
- Mancuso, S.; Briand, X.; Mugnai, S.; Azzarello, E. Marine bioactive substances (IPA Extract) improve foliar ion uptake and water stress tolerance in potted “Vitis vinifera” plants. Adv. Hortic. Sci. 2006, 20, 156–161. [Google Scholar]
- Salvi, L.; Brunetti, C.; Cataldo, E.; Niccolai, A.; Centritto, M.; Ferrini, F.; Mattii, G.B. Effects of Ascophyllum nodosum extract on Vitis vinifera: Consequences on plant physiology, grape quality and secondary metabolism. Plant Physiol. Biochem. 2019, 139, 21–32. [Google Scholar] [CrossRef]
- Khan, A.S.; Ahmad, B.; Jaskani, M.J.; Ahmad, R.; Malik, A.U. Foliar application of mixture of amino acids and seaweed (Ascophylum nodosum) extract improve growth and physicochemical properties of grapes. Int. J. Agric. Biol. 2012, 14, 383–388. [Google Scholar]
- Sabir, A.; Yazar, K.; Sabir, F.; Kara, Z.; Yazici, M.A.; Goksu, N. Vine growth, yield, berry quality attributes and leaf nutrient content of grapevines as influenced by seaweed extract (Ascophyllum nodosum) and nanosize fertilizer pulverization. Sci. Hortic. 2014, 175, 1–8. [Google Scholar] [CrossRef]
- Frioni, T.; Sabbatini, P.; Tombesi, S.; Norrie, J.; Poni, S.; Gatti, M.; Palliotti, A. Effects of a biostimulant derived from the brown seaweed Ascophyllum nodosum on ripening dynamics and fruit quality of grapevines. Sci. Hortic. 2018, 232, 97–106. [Google Scholar] [CrossRef]
- Frioni, T.; Tombesi, S.; Quaglia, M.; Calderini, O.; Moretti, C.; Poni, S.; Palliotti, A. Metabolic and transcriptional changes associated with the use of Ascophyllum nodosum extracts as tools to improve the quality of wine grapes (Vitis vinifera cv. Sangiovese) and their tolerance to biotic stress. J. Sci. Food Agric. 2019, 99, 6350–6363. [Google Scholar] [CrossRef]
- Priori, S.; Barbetti, R.; L’Abate, G.; Bucelli, P.; Storchi, P.; Costantini, E.A.C. Natural terroir unit, Siena Province, Tuscany. J. Maps 2014, 10, 466–477. [Google Scholar] [CrossRef]
- Palliotti, A.; Tombesi, S.; Frioni, T.; Famiani, F.; Silvestroni, O.; Zamboni, M.; Poni, S. Morpho-structural and physiological response of container-grown Sangiovese and Montepulciano cvv. (Vitis vinifera) to re-watering after a pre-veraison limiting water deficit. Funct. Plant Biol. 2014, 41, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under deficit irrigation: Hints from physiological and molecular data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence: A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Lovisolo, C.; Perrone, I.; Carra, A.; Ferrandino, A.; Flexas, J.; Medrano, H.; Schubert, A. Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: A physiological and molecular update. Funct. Plant Biol. 2010, 37, 98–116. [Google Scholar] [CrossRef]
- Bravdo, B.; Hepner, Y.; Loinger, C.; Cohen, S.; Tabacman, H. Effect of irrigation and crop level on growth, yield and wine quality of Cabernet Sauvignon. Am. J. Enol. Vitic. 1985, 36, 132–139. [Google Scholar]
- Dokoozlian, N.K.; Kliewer, W.M. Influence of light on grape berry growth and composition varies during fruit development. J. Am. Soc. Hortic. Sci. 1996, 121, 869–874. [Google Scholar] [CrossRef]
- Greer, D.H.; Weston, C. Heat stress affects flowering, berry growth, sugar accumulation and photosynthesis of Vitis vinifera cv. Semillon grapevines grown in a controlled environment. Funct. Plant Biol. 2010, 37, 206–214. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Tregoat, O.; Choné, X.; Bois, B.; Pernet, D.; Gaudillère, J.P. Vine water status is a key factor in grape ripening and vintage quality for red Bordeaux wine. How can it be assessed for vineyard management purposes. J. Int. Sci. Vigne Vin 2009, 43, 121–134. [Google Scholar] [CrossRef]
- Dry, P.R.; Loveys, B.R. Grapevine shoot growth and stomatal conductance are reduced when part of the root system is dried. Vitis 1999, 38, 151–156. [Google Scholar]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulias, J.; Flexas, J. Regulation of photosynthesis of C3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef]
- Poni, S.; Lakso, A.N.; Turner, J.R.; Melious, R.E. The effects of pre-and post-veraison water stress on growth and physiology of potted Pinot Noir grapevines at varying crop levels. Vitis 1993, 32, 207–214. [Google Scholar]
- Zufferey, V.; Spring, J.L.; Verdenal, T.; Dienes, A.; Belcher, S.; Lorenzini, F.; Viret, O. The influence of water stress on plant hydraulics, gas exchange, berry composition and quality of Pinot Noir wines in Switzerland. OENO One 2017, 51. [Google Scholar] [CrossRef]
- Rossouw, G.C.; Smith, J.P.; Barril, C.; Deloire, A.; Holzapfel, B.P. Carbohydrate distribution during berry ripening of potted grapevines: Impact of water availability and leaf-to-fruit ratio. Sci. Hortic. 2017, 216, 215–225. [Google Scholar] [CrossRef]
- Davies, C.; Robinson, S.P. Sugar accumulation in grape berries: Cloning of two putative vacuolar invertase cDNAs and their expression in grapevine tissues. Plant Physiol. 1996, 111, 275–283. [Google Scholar] [CrossRef]
- Azuma, A.; Yakushiji, H.; Koshita, Y.; Kobayashi, S. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 2012, 236, 1067–1080. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Rai, A.C.; Singh, M.; Shah, K. Environmental stresses and transgenics: Role of ZFP (ZAT) gene in multiple stress tolerance in plants. Adv. Plant Physiol. 2012, 13, 197–232. [Google Scholar]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Flexas, J.; Scoffoni, C.; Gago, J.; Sack, L. Leaf mesophyll conductance and leaf hydraulic conductance: An introduction to their measurement and coordination. J. Exp. Bot. 2013, 64, 3965–3981. [Google Scholar] [CrossRef]
- Hubbard, R.M.; Bond, B.J.; Ryan, M.G. Evidence that hydraulic conductance limits photosynthesis in old Pinus ponderosa trees. Tree Physiol. 1999, 19, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.M.; Woodruff, D.R.; McCulloh, K.A.; Meinzer, F.C. Leaf hydraulic conductance, measured in situ, declines and recovers daily: Leaf hydraulics, water potential and stomatal conductance in four temperate and three tropical tree species. Tree Physiol. 2009, 29, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vilalta, J.; Garcia-Forner, N. Water potential regulation, stomatal behaviour and hydraulic transport under drought: Deconstructing the iso/anisohydric concept. Plant Cell Environ. 2017, 40, 962–976. [Google Scholar] [CrossRef] [PubMed]
- Roman, D.T.; Novick, K.A.; Brzostek, E.R.; Dragoni, D.; Rahman, F.; Phillips, R.P. The role of isohydric and anisohydric species in determining ecosystem-scale response to severe drought. Oecologia 2015, 179, 641–654. [Google Scholar] [CrossRef]
- Villalobos-González, L.; Muñoz-Araya, M.; Franck, N.; Pastenes, C. Controversies in the midday water potential regulation and stomatal behauvior might result by the environment, genotype and/or roostock: Evidence from Carménère and Syrah grapevine varieties. Front. Plant Sci. 2019, 10, 1522. [Google Scholar] [CrossRef]
- Düring, H. Evidence for osmotic adjustment to drought in grapevines (Vitis vinifera L.). Vitis 2016, 23, 1–10. [Google Scholar]
- Dokoozlian, N.K. Grape berry growth and development. In Raisin Production Manual; Agricultural and Natural Resources Publication: Oakland, CA, USA, 2000; Volume 3393, p. 30. [Google Scholar]
- Kumari, R.; Kaur, I.; Bhatnagar, A.K. Effect of aqueous extract of Sargassum johnstonii Setchell & Gardner on growth, yield and quality of Lycopersicon esculentum Mill. J. Appl. Phycol. 2011, 23, 623–633. [Google Scholar]
- Fan, D.; Hodges, D.M.; Zhang, J.; Kirby, C.W.; Ji, X.; Locke, S.J.; Critchley, A.T.; Prithiviraj, B. Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem. 2011, 124, 195–202. [Google Scholar] [CrossRef]
- Lola-Luz, T.; Hennequart, F.; Gaffney, M. Effect on health promoting phytochemicals following seaweed application, in potato and onion crops grown under a low input agricultural system. Sci. Hortic. 2014, 170, 224–227. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficit accelerate ripening and induce change in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef]
- Jayaraman, J.; Norrie, J.; Punja, Z.K. Commercial extract from the brown seaweed A. nodosum reduces fungal diseases in greenhouse cucumber. J. Appl. Phycol. 2011, 23, 353–361. [Google Scholar] [CrossRef]
- Jackman, R.L.; Smith, J.L. Anthocyanins and betalains. In Natural Food Colorants, 2nd ed.; Hendry, G.A.F., Houghton, J.D., Eds.; Chapman & Hall: London, UK, 1996; pp. 244–309. [Google Scholar]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules 2010, 15, 9057–9191. [Google Scholar] [CrossRef] [PubMed]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Romboli, Y.; Mangani, S.; Buscioni, G.; Granchi, L.; Vincenzini, M. Effect of Saccharomyces cerevisiae and Candida zemplinina on quercetin, vitisin A and hydroxytyrosol contents in Sangiovese wines. World J. Microbiol. Biotechnol. 2015, 31, 1137–1145. [Google Scholar] [CrossRef]
- Jeong, S.T.; Goto-Yamamoto, N.; Hashizume, K.; Esaka, M. Expression of the flavonoid 3′-hydroxylase and flavonoid 3,5′-hydroxylase genes and flavonoid composition in grape (Vitis Vinifera). Plant Sci. 2006, 170, 61–69. [Google Scholar] [CrossRef]
- Tarara, J.M.; Lee, J.; Spayd, S.E.; Scagel, C.F. Berry temperature and solar radiation alter acylation, proportion, and concentration of anthocyanin in Merlot grapes. Am. J. Enol. Vitic. 2008, 59, 235–247. [Google Scholar]
- Griesser, M.; Weingart, G.; Schoedl-Hummel, K.; Neumann, N.; Becker, M.; Varmuza, K.; Forneck, A. Severe drought stress is affecting selected primary metabolites, polyphenols, and volatile metabolites in grapevine leaves (Vitis vinifera cv. Pinot noir). Plant Physiol. Biochem. 2015, 88, 17–26. [Google Scholar] [CrossRef]

| Pn (µmol/m2 s) | gs (mmol/m2 s) | Fv/Fm | WUE (mmol/m2 s) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Irrig. Regime | Samp. Time | CTRL | SWE | CTRL | SWE | CTRL | SWE | CTRL | SWE |
| t0 | 13.9 ± 0.7 a | 15.0 ± 1.5 a | 162.2 ± 19.6 a | 146.1 ± 9.3 a | 0.77 ± 0.01 a | 0.77 ± 0.01 a | 3.4 ± 0.4 a | 3.5 ± 0.4 a | |
| WW | t1 | 6.6 ± 0.6 a | 8.6 ± 0.5 a | 94.4 ± 3.4 a | 92.8 ± 5.4 a | 0.76 ± 0.02 a | 0.78 ± 0.01 a | 4.9 ± 0.2 a | 3.7 ± 0.2 b |
| t2 | 6.6 ± 1.9 b | 11.6 ± 0.7 a | 86.0 ± 14.7 b | 161.5 ± 12.6 a | 0.80 ± 0.01 a | 0.77 ± 0.03 a | 2.6 ± 0.7 a | 2.6 ± 0.1 a | |
| t0 | 4.8 ± 1.1 a | 5.3 ± 2.3 a | 92.4 ± 14.8 a | 82.2 ± 12.4 a | 0.77 ± 0.01 a | 0.77 ± 0.01 a | 1.8 ± 0.5 a | 1.0 ± 0.5 a | |
| WS | t1 | 8.1 ± 0.4 a | 7.0 ± 0.6 a | 62.3 ± 3.4 a | 62.2 ± 6.4 a | 0.73 ± 0.03 a | 0.76 ± 0.02 a | 4.6 ± 0.4 a | 3.4 ± 0.1 b |
| t2 | 1.0 ± 0.2 b | 4.3 ± 1.1 a | 18.2 ± 5.0 b | 49.5 ± 11.4 a | 0.80 ± 0.01 a | 0.80 ± 0.01 a | 1.1 ± 0.4 b | 2.1 ± 0.3 a | |
| Parameter | Pn | gs | E | WUE | Ψpd | Ψstem | RWC | Kplant | TA | Berry Weight | Cluster Weight | Yield/Vine |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | mol m−2 s−1 | mmol m−2 s−1 | mmol m−2 s−2 | Mmol m−2 s−2 | MPa | MPa | % | mmol MPa−1 s−1 m−2 | gL−1 | g | g | g |
| Treatments | ||||||||||||
| Treated | 8.9152 | 111.0000 | 3.2707 | 2.7552 | −0.6176 | −1.2872 | 73.5383 | 3.24 | 10.4737 | 0.6873 | 52.4 | 312.1 |
| Nontreated | 8.1578 | 95.2000 | 2.8696 | 2.8891 | −0.7547 | −1.4238 | 68.6305 | 2.50 | 10.6412 | 0.6852 | 46.1 | 276.7 |
| Irrig. Regime | ||||||||||||
| WW | 11.73 | 133.32 | 3.73 | 3.33 | −0.43 | −1.10 | 70.43 | 3.52 | 12.46 | 0.75 | 51.72 | 312.00 |
| WS | 5.13 | 71.00 | 2.37 | 2.28 | −0.95 | −1.63 | 71.68 | 2.22 | 9.34 | 0.58 | 46.81 | 289.79 |
| Significance | ||||||||||||
| Treatments | 0.512 | 0.039 | 0.019 | 0.387 | 0.017 | 0.035 | 0.020 | 0.042 | 0.874 | 0.001 | 0.606 | 0.459 |
| Irrig. Regime | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.506 | 0.025 | 0.005 | 0.000 | 0.031 | 0.018 |
| Treat. × Irrig. Regime | 0.500 | 0.634 | 0.646 | 0.002 | 0.005 | 0.003 | 0.967 | 0.011 | 0.571 | 0.041 | 0.694 | 0.412 |
| F 3′5′OH Activity | 3′-OMT Activity | 5′-OMT Activity | |||||
|---|---|---|---|---|---|---|---|
| Irrig. Regime | Samp. Time | CTRL | SWE | CTRL | SWE | CTRL | SWE |
| t0 | 3.93 ± 0.05 a | 4.40 ± 0.05 a | 6.84 ± 0.7 a | 6.45 ± 0.7 a | 9.30 ± 0.6 a | 10.04 ± 0.7 a | |
| WW | t1 | 2.86 ± 0.40 a | 3.69 ± 0.56 a | 8.08 ± 0.6 a | 6.88 ± 1.4 b | 14.12 ± 1.4 a | 10.48 ± 0.4 b |
| t2 | 5.09 ± 0.15 a | 2.42 ± 0.09 b | 11.73 ± 0.9 a | 11.31 ± 2.2 a | 29.23 ± 3.9 a | 9.60 ± 3.2 b | |
| t0 | 5.42 ± 0.07 a | 5.42 ± 0.07 a | 10.30 ± 1.9 a | 10.30 ± 1.9 a | 12.34 ± 0.4 a | 12.20 ± 0.4 a | |
| WS | t1 | 4.29 ± 0.15 a | 4.13 ± 0.14 a | 8.47 ± 0.3 a | 6.69 ± 1.6 b | 16.00 ± 1.4 a | 13.57 ± 1.1 b |
| t2 | 4.55 ± 0.06 a | 3.89 ± 0.31 b | 14.02 ± 0.3 a | 13.25 ± 0.9 a | 22.36 ± 2.0 a | 22.27 ± 4.9 a | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvi, L.; Brunetti, C.; Cataldo, E.; Storchi, P.; Mattii, G.B. Eco-Physiological Traits and Phenylpropanoid Profiling on Potted Vitis vinifera L. cv Pinot Noir Subjected to Ascophyllum nodosum Treatments under Post-Veraison Low Water Availability. Appl. Sci. 2020, 10, 4473. https://doi.org/10.3390/app10134473
Salvi L, Brunetti C, Cataldo E, Storchi P, Mattii GB. Eco-Physiological Traits and Phenylpropanoid Profiling on Potted Vitis vinifera L. cv Pinot Noir Subjected to Ascophyllum nodosum Treatments under Post-Veraison Low Water Availability. Applied Sciences. 2020; 10(13):4473. https://doi.org/10.3390/app10134473
Chicago/Turabian StyleSalvi, Linda, Cecilia Brunetti, Eleonora Cataldo, Paolo Storchi, and Giovan Battista Mattii. 2020. "Eco-Physiological Traits and Phenylpropanoid Profiling on Potted Vitis vinifera L. cv Pinot Noir Subjected to Ascophyllum nodosum Treatments under Post-Veraison Low Water Availability" Applied Sciences 10, no. 13: 4473. https://doi.org/10.3390/app10134473
APA StyleSalvi, L., Brunetti, C., Cataldo, E., Storchi, P., & Mattii, G. B. (2020). Eco-Physiological Traits and Phenylpropanoid Profiling on Potted Vitis vinifera L. cv Pinot Noir Subjected to Ascophyllum nodosum Treatments under Post-Veraison Low Water Availability. Applied Sciences, 10(13), 4473. https://doi.org/10.3390/app10134473








