Featured Application
This work suggests that application of both ethephon and root symbioses in fenugreek plants can positively affect the production and accumulation of valuable compounds such as trigonelline and ABA with potentiality for future sustainable management purposes of fenugreek cultivation.
Abstract
Secondary metabolites (SMs) have high economic impact thanks to their exploitability in chemical, pharmaceutical and cosmetic industries. Trigonella foenum-graecum has an importance due to the production of bioactive compounds with pharmaceutical values. Among them, the alkaloid trigonelline is known for its role in the treatment of different human diseases. SM accumulation is influenced by environmental factors but is modulated by the application of exogenous compounds. Ethephon, a precursor of the phytohormone ethylene, was already used to influence SM accumulation. Our work is aimed at evaluating the accumulation of trigonelline and the phytohormone abscisic acid (ABA) when three factors were combined: i) two levels of water regimes (well-watered and water deficit), ii) ethephon treatments and iii) inoculation with an arbuscular mycorrhizal (AM)-based inoculum also leading to nodulation. The content of trigonelline and ABA was significantly affected by symbioses, showing high accumulation in AM-colonized plants irrespective of the water regimes applied. In terms of trigonelline accumulation with respect to ethephon treatments, while symbiotic plants showed a dose-dependent trend, non-symbiotic plants showed a significantly difference only when 550 ppm of ethephon was applied. In conclusion, our work provides new information on the effects of both ethephon and symbioses on plant growth and accumulation of valuable compounds, such as trigonelline, in fenugreek.
1. Introduction
In nature, plants produce a large plethora of organic bioactive compounds. Several of them that do not directly affect life’s fundamental processes are referred as secondary metabolites (SMs) [1]. Plant SMs are widely used as a natural chemical source in pharmaceutics, cosmetics and nutraceuticals, and there is an increased interest in the cultivation of plant species devoted to production of bioactive compounds [2]. However, the optimization of agronomic management strategies for secondary metabolite accumulation in nutraceutical species is still challenging [1,3]. Different studies reported that many environmental factors might affect the production and the accumulation of SMs in plants [1]. Plant SMs are often compounds that have no fundamental function in the maintenance of life processes in the plants, but they are important to interact with the environment for adaptation and defense [4,5]. For example, several SMs are produced and modulated to allow adaptation of plants to the changing environment, thus mitigating the effects of biotic, i.e. herbivores and pathogens, and abiotic stresses [4,5,6] Among these, water deficit is one of the most significant stresses that plants face during their development, dramatically affecting growth and crop yield. It was indicated that, when exposed to drought, plants, in addition to molecules with a role in plant stress response such as the abscisic acid (ABA), accumulate higher concentrations of SMs, suggesting that water stress can be an easy-to-use elicitor of bioactive compounds [4]. On the other hand, bioaccumulation of SMs can be also achieved by application of exogenous plant growth regulators such as phytohormones. As enhancers of SM biosynthesis, synthetic phytohormones have been successfully used as elicitors to stimulate the production of terpenes, phenols and alkaloids [7,8]. In this context, one of the used plant growth regulators is ethephon (2-chloroethylphosphonic acid), a precursor of the phytohormone ethylene, which directly influences different physiological plant processes, such as senescence abscission, floral transition, fruit ripening and maturation [9]. The use of ethephon to influence SM accumulation was so far demonstrated for Coffea arabica and Thalictrum rugosum [10].
In the last decade, it has been often reported that also the establishment of symbiosis with arbuscular mycorrhizal (AM) fungi, which colonize plant roots and help their host plants in nutrient uptake while in return receive essential carbon compounds [11], may also induce changes in SM content in different plant tissues [12,13,14,15,16,17,18]. The real application of root-associated microorganisms, such as AM fungi, is currently considered to have an enormous potential in the frame of innovative and sustainable agriculture, providing benefits to plants not only by enhancing their nutrition and conferring tolerance to abiotic and biotic threats [19,20], but also by increasing the amount of useful SMs produced by host plant [21].
In this work, attention was focused on the production of one of the main SMs produced by Trigonella foenum-graecum (fenugreek), i.e., trigonelline. Fenugreek belongs to the Fabaceae family and is one of the species that is gaining attention by drug research and industry because of its nutraceutical and pharmaceutical properties. It is indeed one of the oldest medicinal leguminous plants grown and commercialized world-wide, showing resilience to semi-arid conditions [22,23,24]. Its seeds contain a plethora of bioactive compounds with high pharmaceutical value, e.g., the saponin diosgenine and the pyridine alkaloid trigonelline. Particularly, the latter may have a role in the treatment of many different human diseases, for instance diabetes, due to its hypoglycemic, neuroprotective, anti-invasive, estrogenic and antibacterial properties [25]. It is found in many plants, like coffee beans, and in significant quantity in fenugreek seeds [26]. Cell and tissue culture protocols were developed in fenugreek to force trigonelline bioaccumulation [27,28]. It has been also documented that fenugreek plants over-accumulate trigonelline in response to water deficit [29]. In detail, our work aimed to verify the effects on trigonelline production when water stress (as an example of abiotic stress), ethephon treatment (as a plant growth regulator) and an AM-based inoculum (as a biostimulant) were applied alone or in combination. Additionally, abscisic acid (ABA) level was also evaluated to verify the impact of the several treatments on the fenugreek response to a water deficit condition.
2. Materials and Methods
2.1. Experimental Planning
In this experiment, two levels of water regime [non-stress (NS) and water deficit (S)], exogenous ethephon application at different concentrations (0, 350, 550, 850 μg/L) and inoculation with an AM-based inoculum [not-inoculated (NI) and inoculated (I) plants] were evaluated as a factorial experiment based on a randomized complete block design with four replications. At the end of the experiment, leaf samples were collected and stored at −80 °C until biochemical analysis, as below described.
2.2. Fenugreek Sowing and Treatments
Seeds of Trigonella foenum-graecum L., Neyshaboor landrace, were obtained from the Department of Agriculture, Neyshaboor, Iran. These were surface sterilized with 5% (v/v) hypochlorite and then washed with sterilized water 5 times. Sterilized seeds were placed in Petri dishes (5 seeds per each Petri dish) on a wet paper, covered with aluminum foils to avoid light and kept in a plant growth chamber at 28 °C for one week. After that, the seedlings were transferred to pots previously prepared and maintained in greenhouse. Sterilized quartz sand was used as culture medium, and plants were watered with 60 mL of water twice a week and with a Long Ashton solution once a week (3.2 μmol Pi). Mycorrhizal fenugreek plants were prepared using a commercial AM fungal inoculum (Organic Plant Health Company, Hamedan, Iran), containing Rhizophagus irregularis as the AM fungal species (accession numbers MT209826–MT209828). In detail, 100 g of an AM fungal inoculum (consisting of spores, external mycelium and Zea mays mycorrhizal roots) was placed at a depth of 3 cm in the pots. For each pot, a quantity of about 650 g of sand plus inoculum was used, while a quantity of 750 g of sand was used in control plants. The same inoculum also led to the formation of root-nodules formed by Ensifer meliloti (formerly Sinorhizobium meliloti) (accession numbers MT209826–MT209828). To perform treatment with exogenous ethylene, ethephon (Sigma) was dissolved in distilled water and diluted to obtain 350, 550 and 850 μL/L (ppm) solutions (E350, E550, E850, respectively). Water alone was used as control (E0). Two milliliters of each dilution were sprayed on each plant only at the beginning of the experiment, i.e., 30 days after sowing. Plants were then divided in two groups: (i) well-watered (NS) that were regularly watered up to field capacity throughout the entire experimental period and (ii) subjected to water deficit condition (S) that were not watered until the pots reached an averaging weight loss of about 250–280 g. After that, for 20 days, seedlings were watered daily to maintain a relatively stable level of water stress.
2.3. Assessment of Morphometric Parameters and Symbiosis Development
At the end of the experiments, plants were harvested, and stem height, fruit number and root, stem and fruit dry weight were recorded. Due to the scarcity of material, only the root apparatus from one plant for each treatment was used to evaluate the efficiency of the AM-based inoculum according to the Trouvelot system [30] (Supplementary Materials Figure S1). Nodules, with a typical red feature at a stereomicroscope observation, associated with AM-colonized plants, were also found. For this reason, the number of nodules was also considered (n = 4, Supplementary Materials Figure S1), and AM treatment was then called “I” treatment, to include both the AM colonization and nodules present in inoculated Trigonella roots.
2.4. Trigonelline and ABA Quantification
Collected leaves were freeze dried and homogenized and then transferred in a 2 mL centrifuge tube (20–40 mg) and extracted with 1 mL of methanol: water (8:2 v/v) acidified with 0.1% of acetic acid in an ultrasonic bath for 1 h. Samples were centrifuged at 15,000 rpm and 4 °C for 10 min, and the supernatant was analyzed by HPLC–DAD technique. Original standard of Trigonelline hydrochloride (Trig) (purity ≥ 99%) and ABA (purity ≥ 98.5%), purchased from Sigma-Aldrich, were used for the identification of the studied metabolites by comparing retention times and UV spectra. The quantification was made by the external calibration method. The HPLC apparatus was an Agilent 1220 Infinity LC system model G4290B (Agilent®, Waldbronn, Germany), equipped with gradient pump, autosampler and column oven set at 30 °C. A 170 Diode Array Detector (Gilson, Middleton, USA) set at 265 nm (for ABA and Trig) was employed. A Nucleodur C18 analytical column (250 × 4.6 mm i.d., 5 μm, Macherey Nagel) was used. The mobile phases consisted of (A) water acidified with formic acid 0.1% and (B) acetonitrile at a flow rate of 0.600 mL min–1 in gradient mode, 0–6 min: 30% of B, 6–16 min: from 30% to 100% of B, 16–21 min: 100% of B. Twenty microliters was injected for each sample, and three biological replicates were run for each analysis.
2.5. Statistical Analysis
Analysis of variance (ANOVA) and graphs of the experimental data were performed using R. When ANOVA indicated that either stress (NS, S), symbiosis colonization (NI, I) or ethephon application (E0-850) or their interaction was statistically significant, mean separation was performed using the Tukey HSD test, adopting a probability level of P ≤ 0.05.
3. Results and Discussion
Our study aimed to evaluate the water deficit effects on target metabolites (trigonelline and abscisic acid—ABA) in fenugreek, colonized by both AM fungi and nodule-forming symbionts in combination with exogenous ethephon application. Leguminous plants can in fact host within their roots both symbionts concomitantly, forming the so-called tripartite symbioses largely studied to elucidate the complex dialogue among the partners [31]. Over the last decade, several studies have been performed on the impact of a single symbiont in plant physiology features considering several environmental factors [32,33,34]. Conversely, poor studies are available on tripartite symbiosis effects in response to abiotic stressful events.
In detail, after about six weeks from inoculation, control not-inoculated (NI) and inoculated (I) plants were subjected to several combined treatments: well irrigated (NS) and not-watered (S), both treated with different ethephon concentrations (E0, E350, E550, E850 ppm). Results (Table 1) showed that biometric parameters were not affected by the considered factors (Stress, I, E), with the exception of inoculation (I) factor for the fruit number where not -inoculated (NI) plants showed higher number of fruits with respect to the inoculated (I) ones.

Table 1.
Results of morphometric measurements. Average and standard deviation (±) values of four plants for treatment/condition are reported (with the exception of root dry weight from inoculated plants, n = 3). Results of three-way ANOVA for the three considered effects are also included (stress, inoculation and ethephon application). NS, no stress; S, water stress; NI, no inoculation; I, inoculation; E0, E350, E550, E850, ethephon treatments. Codes for p-value: *** 0 ≤ 0.001, ** 0.001 ≤ 0.01, * 0.01 ≤ 0.05, ns not significant. Different letters within each column indicate significant differences according to Tukey HSD test (P ≤ 0.05). DW: dry weight, h: height.
The only statistically significant difference was found in the nodules that were significantly higher in inoculated stressed (IS) plants than in inoculated non-stressed (INS) ones (Supplementary Materials Figure S1). Results suggest a similar trend for AM fungal colonization (Supplementary Materials Figure S2) in response to ethephon, although it will need further observations to confirm it due to the absence of a sufficient number of replicates to perform a statistical analysis. Ethylene was reported as an inhibitor of nodule and AM development at early stages of symbiosis formation [31]. In addition, exogenous ethylene application induced much lower heights in maize-treated plants with respect to their untreated controls [35]. In this study, exogenous ethephon application and water deficit conditions were applied when symbioses were already established. This could unfold the absence of significant differences in the morphometric measurements recorded. It is worth noting that nodule development was higher under S conditions irrespective of the ethephon concentration used, which opens new questions on symbioses development mechanisms that deserve further investigations, at least in the fenugreek model system. In terms of the SMs production, our results provide evidence that the interaction between the application of exogenous ethylene and the use of an AM-based inoculum significantly increases trigonelline content in fenugreek plants independently of the water deficit treatment (Figure 1 and Supplementary Materials Table S1). The content of trigonelline was significantly affected by symbioses, both in NS and S conditions. Looking at ethephon application, while inoculated plants (I) showed a dose-dependent trend, independent from the water regimes, not-inoculated (NI) plants showed a significant difference only in NISE550. The fact that trigonelline levels were significantly higher in NISE550 with respect to NINSE550 suggests a combined effect of the E550 treatment and water deficit at least in the absence of root colonization (Figure 1 and Supplementary Materials Table S1). Differently from not-inoculated (NI) plants, inoculated (I) ones responded proportionally to the different concentrations of ethephon, and the highest amount of trigonelline was determined in inoculated non-stressed (INS) plants treated with the highest concentration of ethephon (850 ppm) (P ≤ 0.05; Figure 1). An average increase of approximately 3000 μg g–1 was measured in inoculated compared to not-inoculated plants (Figure 1 and Supplementary Materials Table S1). Low amounts of this SM were determined in not-inoculated plants treated with all concentration of ethephon (Figure 1 and Supplementary Materials Table S1).
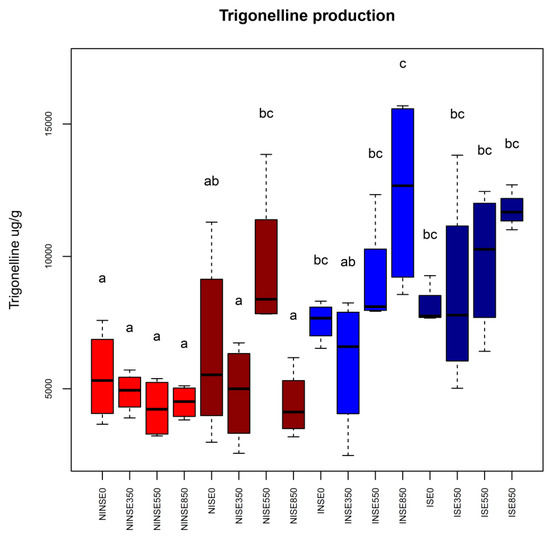
Figure 1.
Boxplots representing trigonelline production in the different conditions (NI, I: not-inoculated and inoculated plants, respectively; S, NS: water stressed and non-stressed plants; E0, E350, E550, E850: different ethephon concentrations in ppm). Different conditions were color coded as follows: red and brown boxes represent not-inoculated plants (NI) non-stressed (NS) and water stressed (S), respectively; blue and dark-blue boxes represent inoculated plants (I) non-stressed (NS) and water stressed (S), respectively. Letters are plotted according to outcomes of Tukey’s test.
Valuable secondary metabolites often can be accumulated by application of synthetic elicitors acting as molecular signals such as ethephon. Ethephon treatment increased the accumulation of proline and antioxidant enzymes activity in maize subjected to water stress by maintaining water status, membrane stability and modulate cuticular wax biosynthesis [35]. In addition, foliar-applied ethephon markedly improve anthocyanin and total phenolic content in black carrots [36]. Several lines of evidence indicated the stimulating effect of ethylene on diosgenin content and enzyme activities also in fenugreek [37,38]. During tripartite interaction in fenugreek, water stress did not significantly influence trigonelline accumulation. However, both inoculated (I) and not-inoculated (NI) plants seemed to accumulate higher amount of trigonelline during water deficit compared to non-stressed plants. It has been recently reported that fenugreek tolerated water deficit by increasing the endogenous melatonin and trigonelline molecules [39]. Interestingly, moderate drought conditions can lead to positively modulate secondary metabolites useful for food, cosmetic and pharmaceutical industries as previously reported in the model medicinal and aromatic sage plant [40].
In our experimental context, with respect to not-inoculated (NI) plants, trigonelline was generally higher in inoculated (I) ones with a peak recorded when ethephon was delivered at 550 and 850 ppm in both NS and S conditions. Here, an AM-based inoculum also leading to nodulation was used, rendering it impossible to verify the effect of the single microorganism. Although further experiments might be also needed to verify the efficiency of the rhizobia-forming nodules, we preferred to take in account their presence in the root apparatus of the inoculated plants. Our findings could be explained by the biochemical, hormonal and physiological crosstalk taking place as a consequence of the tripartite association between AM fungi, rhizobia and legumes. It was already reported that these complex interactions lead to a functional diversity of the root-associated symbionts, which can complement each other in acquiring different limiting nutrients and in driving important ecosystem functions [41].
Previously, it has been proposed that trigonelline may be a regulatory factor during early signal events in the establishment of the AM symbiosis in Prosopis laevigata [42]. In this context, we can hypothesize that the over production of trigonelline in fenugreek plants used in our study can be explained by the combinations of both ethephon application and presence of the symbionts. The use of AM-based inoculants in fenugreek cultivation has started to be considered as an effective and useful agronomic practice, showing an efficacy in improving tolerance of fenugreek to salinity [43] and to oxidative stresses [44].
The plant hormone ABA is a chemical signal involved in the plant response to various environmental stresses. Here, the content of ABA in the inoculated (I) plants was in general significantly higher than in not-inoculated ones, although no differences were found in the non-stress condition (NS) and in the absence of ethephon treatment (E0). On the contrary, it was significantly affected by interaction between different ethephon concentration and irrigation regimes. The highest accumulation of ABA was measured when inoculated non-stressed plants (INS) were subjected to high concentrations of ethephon (550 and 850 ppm, Figure 2 and Supplementary Materials Table S1). Interestingly, the lowest ABA content was recorded in not-inoculated (NI) and in inoculated stressed (IS) plants at 550 and 850 ppm of ethephon, further confirming the fenugreek tolerance to water deficit conditions [39] and the positive interactions of symbiosis and the exogenously applied ethylene (at least at some concentrations) in trigonelline and ABA accumulation, particularly in non-stressed conditions (Figure 1, Figure 2 and Supplementary Materials Table S1). Behind the well-known ABA-mediated stomata closure during water deficit, ABA and ethylene quickly accumulate in plant tissues when subjected to water stress triggering plant defense responses (e.g., defense-related SMs), modulating also growth processes to cope with stress events [45].
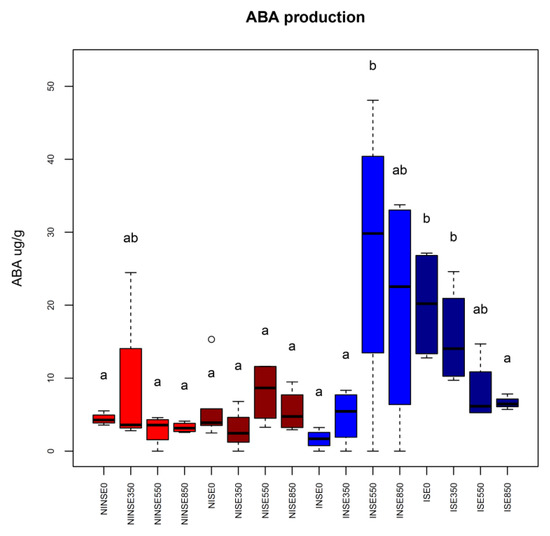
Figure 2.
Boxplots representing abscisic acid (ABA) production in the different conditions (NI, I: not-inoculated and inoculated plants, respectively; S, NS: water stressed and non-stressed plants; E0, E350, E550, E850: different ethephon concentrations in ppm). Different conditions were color coded as follows: red and brown boxes represent not-inoculated plants (NI) non-stressed (NS) and water stressed (S), respectively, while blue and dark-blue boxes represent inoculated plants (I) non-stressed (NS) and water stressed (S), respectively. Letters are plotted according to outcomes of Tukey’s test.
4. Conclusions
In conclusion, tripartite interaction studies represent an intriguing model that mimics the natural conditions useful to deepen physiological responses to abiotic stresses (e.g., water limitation), biostimulants (e.g., AM fungi) and bioregulators application (e.g., exogenous ethylene application). From an applicative point of view, although field experiments will be pivotal to further support our results, this work suggests that application of ethephon and use of an AM-based inoculum can improve the production and accumulation of a phytohormone with an important role in the response to stress, such as ABA, as well as valuable secondary metabolites such as trigonelline.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/10/7/2338/s1: Figure S1: Boxplot showing the number of observed nodules in inoculated plants; Figure S2: Results of AM colonization assessment; Table S1: Results on trigonelline and ABA content with statistical analysis.
Author Contributions
Conceptualization, S.I., A.G., W.C. and R.B.; formal analysis, L.N., W.C. and F.S.; investigation, S.I., L.N., W.C. and R.B.; resources, S.I. and A.G.; writing—original draft preparation, S.I. and F.S.; writing—review and editing, F.S., L.N., W.C. and R.B.; supervision, R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors thanks Maria Teresa della Beffa for the help in plant preparation and maintenance.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Oksman-Caldentey, K.M.; Inzé, D. Plant cell factories in the post-genomic era: New ways to produce designer secondary metabolites. Trends Plant Sci. 2004, 9, 433–440. [Google Scholar] [CrossRef]
- Jimenez-Garcia, S.N.; Vazquez-Cruz, M.A.; Guevara-Gonzalez, R.G.; Torres-Pacheco, I.; Cruz-Hernandez, A.; Feregrino-Perez, A.A. Current approaches for enhanced expression of secondary metabolites as bioactive compounds in plants for agronomic and human health purposes–a review. Pol. J. Food Nutr. Sci. 2013, 63, 67–78. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, L.; Tonelli, M.; Pellegrini, E.; Cotrozzi, L.; Pucciariello, C.; Trivellini, A.; Lorenzini, G.; Nali, C. Accumulation of rosmarinic acid and behaviour of ROS processing systems in Melissa officinalis L. under heat stress. Ind. Crop Prod. 2019, 138, 111469. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Puig, D.G.; Perez, M.L.; Fuster, M.D.; Ortuno, A.; Sabater, F.; Porras, I.; Lidón, A.G.; Del Rio, J.A. Effect of ethylene on naringin, narirutin and nootkatone accumulation in grapefruit. Planta Med. 1995, 61, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-García, D.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as biofactories: Postharvest stress-induced accumulation of phenolic compounds and glucosinolates in broccoli subjected to wounding stress and exogenous phytohormones. Front. Plant Sci. 2016, 7, 45. [Google Scholar] [CrossRef]
- Kieber, J.J. The ethylene response pathway in Arabidopsis. Ann. Rev. Plant Biol. 1997, 48, 277–296. [Google Scholar] [CrossRef]
- Cho, G.H.; Kim, D.I.; Pedersen, H.; Chin, C.K. Ethephon enhancement of secondary metabolite synthesis in plant cell cultures. Biotechnol. Prog. 1988, 4, 184–188. [Google Scholar] [CrossRef]
- Balestrini, R.; Lumini, E. Focus on mycorrhizal symbioses. Appl. Soil Ecol. 2019, 123, 299–304. [Google Scholar] [CrossRef]
- Fester, T.; Hause, G. Accumulation of reactive oxygen species in arbuscular mycorrhizal roots. Mycorrhiza 2005, 15, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Lohse, S.; Schliemann, W.; Ammer, C.; Kopka, J.; Strack, D.; Fester, T. Organization and metabolism of plastids and mitochondria in arbuscular mycorrhizal roots of Medicago truncatula. Plant Physiol. 2005, 139, 329–340. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schliemann, W.; Ammer, C.; Strack, D. Metabolite profiling of mycorrhizal roots of Medicago truncatula. Phytochemistry 2008, 69, 112–146. [Google Scholar] [CrossRef] [PubMed]
- Sbrana, C.; Avio, L.; Giovannetti, M. Beneficial mycorrhizal symbionts affecting the production of health promoting phytochemicals. Electrophoresis 2014, 35, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Wipf, D.; Mongelard, G.; van Tuinen, D.; Gutierrez, L.; Casieri, L. Transcriptional responses of Medicago truncatula upon sulfur deficiency stress and arbuscular mycorrhizal symbiosis. Front. Plant Sci. 2014, 5, 680. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, R.; Müller, C. Leaf metabolome in arbuscular mycorrhizal symbiosis. Curr. Opin. Plant Biol. 2015, 26, 120–126. [Google Scholar] [CrossRef]
- Cervantes-Gámez, R.G.; Bueno-Ibarra, M.A.; Cruz-Mendívil, A.; Calderón-Vázquez, C.L.; Ramírez-Douriet, C.M.; Maldonado-Mendoza, I.E.; Villalobos-López, M.A.; Valdez-Ortíz, A.; López-Meyer, M. Arbuscular mycorrhizal symbiosis-induced expression changes in Solanum lycopersicum leaves revealed by RNA-seq analysis. Plant Mol. Biol. Rep. 2016, 34, 89–102. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: let’s benefit from past successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef]
- Balestrini, R.; Chitarra, W.; Antoniou, C.; Ruocco, M.; Fotopoulos, V. Improvement of plant performance under water deficit with the employment of biological and chemical priming agents. J. Agric. Sci. 2018, 156, 680–688. [Google Scholar] [CrossRef]
- Pedone-Bonfim, M.V.L.; da Silva, F.S.B.; Maia, L.C. Production of secondary metabolites by mycorrhizal plants with medicinal or nutritional potential. Acta Physiol. Plant. 2015, 37, 27. [Google Scholar] [CrossRef]
- Flammang, A.M.; Cifone, M.A.; Erexson, G.L.; Stankowski Jr, L.F. Genotoxicity testing of a fenugreek extract. Food Chem. Toxicol. 2004, 42, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Altuntaş, E.; Özgöz, E.; Taşer, Ö.F. Some physical properties of fenugreek (Trigonella foenum-graecum L.) seeds. J. Food Eng. 2005, 71, 37–43. [Google Scholar] [CrossRef]
- Acharya, S.N.; Thomas, J.E.; Basu, S.K. Fenugreek: An “old world” crop for the “new world”. Biodiversity 2006, 7, 27–30. [Google Scholar] [CrossRef]
- Ashihara, H.; Ludwig, I.A.; Katahira, R.; Yokota, T.; Fujimura, T.; Crozier, A. Trigonelline and related nicotinic acid metabolites: Occurrence, biosynthesis, taxonomic considerations, and their roles in planta and in human health. Phytochem. Rev. 2015, 14, 765–798. [Google Scholar] [CrossRef]
- Minorsky, P.V. Trigonelline: A diverse regulator in plants. Plant Physiol. 2002, 128, 7–8. [Google Scholar] [CrossRef]
- Raheleh, A.; Hasanloo, T.; Khosroshahli, M. Evaluation of trigonelline production in Trigonella foenum-graecum hairy root cultures of two Iranian masses. POJ 2011, 4, 408–412. [Google Scholar]
- Qaderi, A.; Akbari, Z.; Kalateh-jari, S.; Fatehi, F.; Tolyat, M.; Jalali Moghadam, M.; Naghdi Badi, H. Improving trigonelline production in hairy root culture of fenugreek (Trigonella foenum-graecum). J. Med. Plant 2016, 3, 73–80. [Google Scholar]
- Dadrasan, M.; Chaichi, M.R.; Pourbabaee, A.A.; Yazdani, D.; Keshavarz-Afshar, R. Deficit irrigation and biological fertilizer influence on yield and trigonelline production of fenugreek. Ind. Crop Prod. 2015, 77, 156–162. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthode d’estimation ayant une signification fonctionnelle. In Physiological and genetical aspects of mycorrhizae. In Proceedings of the 1st European Symposium on Mycorrhizae, Dijon, France, 1–5 July 1985; pp. 217–221. [Google Scholar]
- Foo, E. Plant hormones play common and divergent roles in nodulation and arbuscular mycorrhizal symbioses. In The Model Legume Medicago truncatula; Jhon Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 753–765. [Google Scholar]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef]
- Volpe, V.; Chitarra, W.; Cascone, P.; Volpe, M.G.; Bartolini, P.; Moneti, G.; Pieraccini, G.; Di Serio, C.; Maserti, B.; Guerrieri, E.; et al. The association with two different arbuscular mycorrhizal fungi differently affects water stress tolerance in tomato. Front. Plant Sci. 2018, 9, 1480. [Google Scholar] [CrossRef] [PubMed]
- Alagna, F.; Balestrini, R.; Chitarra, W.; Marsico, A.D.; Nerva, L. Getting ready with the priming: Innovative weapons against biotic and abiotic crop enemies in a global changing scenario. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Academic Press: Cambridge, MA, USA, 2020; pp. 35–56. [Google Scholar]
- Yu, H.; Zhang, Y.; Xie, Y.; Wang, Y.; Duan, L.; Zhang, M.; Li, Z. Ethephon improved drought tolerance in maize seedlings by modulating cuticular wax biosynthesis and membrane stability. J. Plant Physiol. 2017, 214, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Barba-Espín, G.; Glied, S.; Crocoll, C.; Dzhanfezova, T.; Joernsgaard, B.; Okkels, F.; Lutken, H.; Muller, R. Foliar-applied ethephon enhances the content of anthocyanin of black carrot roots (Daucus carota ssp. Sativus var. atrorubens Alef.). BMC Plant Biol. 2017, 17, 70. [Google Scholar]
- Oncina, R.; Del Río, J.A.; Gomez, P.; Ortuno, A. Effect of ethylene on diosgenin accumulation in callus cultures of Trigonella foenum-graecum L. Food Chem. 2002, 76, 475–479. [Google Scholar] [CrossRef]
- Gomez, S.; Roy, S.K.; Pal, R.K. Primary processing of fenugreek (Trigonella foenum graecum L.)–An eco-friendly approach for convenience and quality. Plant Food Hum. Nutr. 2003, 58, 1–10. [Google Scholar] [CrossRef]
- Zamani, Z.; Amiri, H.; Ismaili, A. Improving drought stress tolerance in fenugreek (Trigonella foenum-graecum) by exogenous melatonin. Plant Biosyst. 2019. [Google Scholar] [CrossRef]
- Caser, M.; Chitarra, W.; D’Angiolillo, F.; Perrone, I.; Demasi, S.; Lovisolo, C.; Pistelli, L.; Pistelli, L.; Scariot, V. Drought stress adaptation modulates plant secondary metabolite in Salvia dolomitica Codd. Ind. Crop Prod. 2019, 129, 85–96. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; De Bruin, S.; Luckerhoff, L.; Van Logtestijn, R.S.; Schlaeppi, K. A widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. ISME J. 2016, 10, 389–399. [Google Scholar] [CrossRef]
- Rojas-Andrade, R.; Cerda-García-Rojas, C.; Frías-Hernández, J.; Dendooven, L.; Olalde-Portugal, V.; Ramos-Valdivia, A. Changes in the concentration of trigonelline in a semi-arid leguminous plant (Prosopis laevigata) induced by an arbuscular mycorrhizal fungus during the presymbiotic phase. Mycorrhiza 2003, 13, 49–52. [Google Scholar] [CrossRef]
- Metwally, R.A.; Abdelhameed, R.E. Synergistic effect of arbuscular mycorrhizal fungi on growth and physiology of salt-stressed Trigonella foenum-graecum plants. Biocatal. Agric. Biotechnol. 2018, 16, 538–544. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R. Arbuscular mycorrhizal symbiosis modulates antioxidant response in salt-stressed Trigonella foenum-graecum plants. Mycorrhiza 2014, 24, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).