Effect of Co-Ingestion of Collagen Peptides with Yogurt on Blood Absorption of Short Chain Hydroxyproline Peptides
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
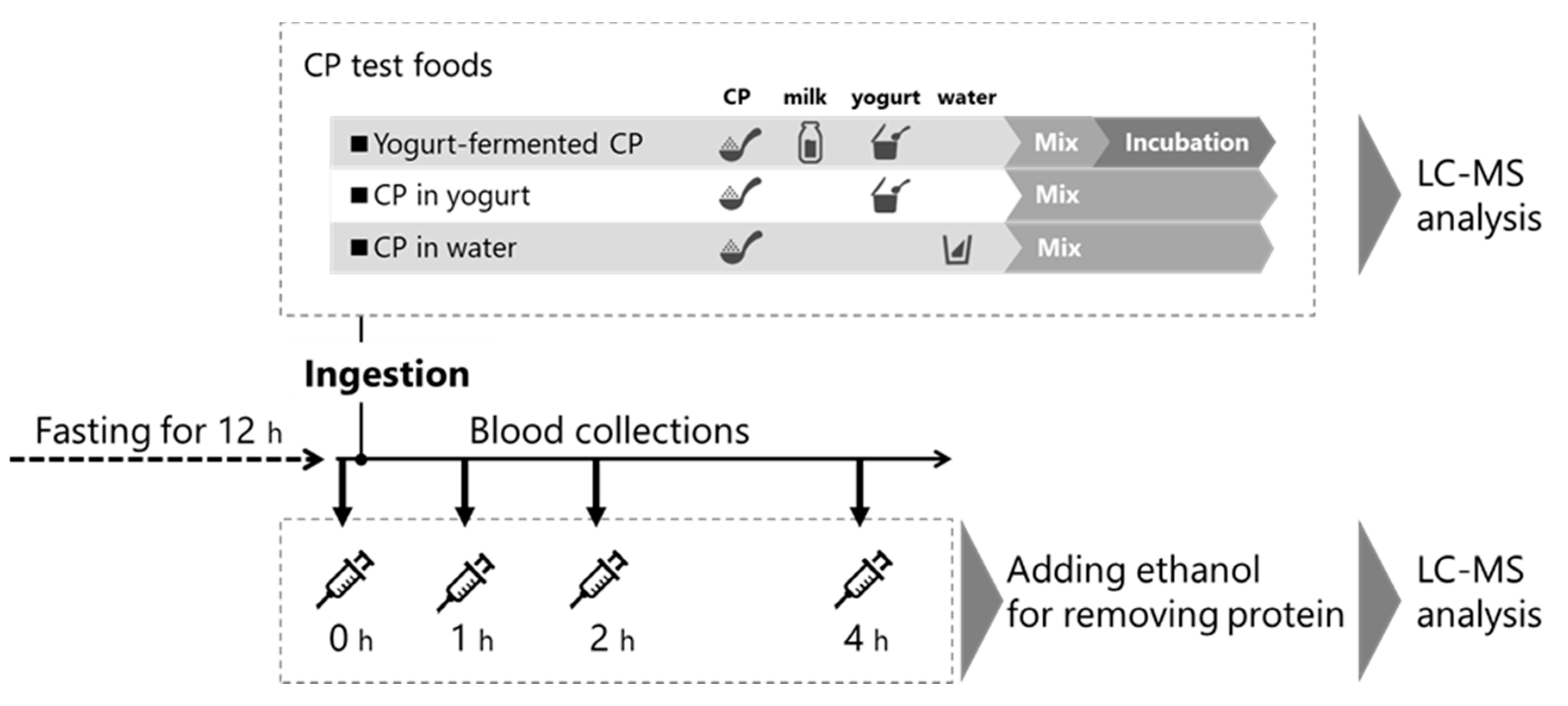
2.2. Preparation of CP Test Foods
2.3. Analysis of Hyp-Containing Di- or Tripeptides in CP Test Foods
2.4. Human Study Design
2.5. Quantitation of Hyp and Hyp-Containing Di- or Tripeptides in Human Plasma
2.6. Statistical Analysis
3. Results
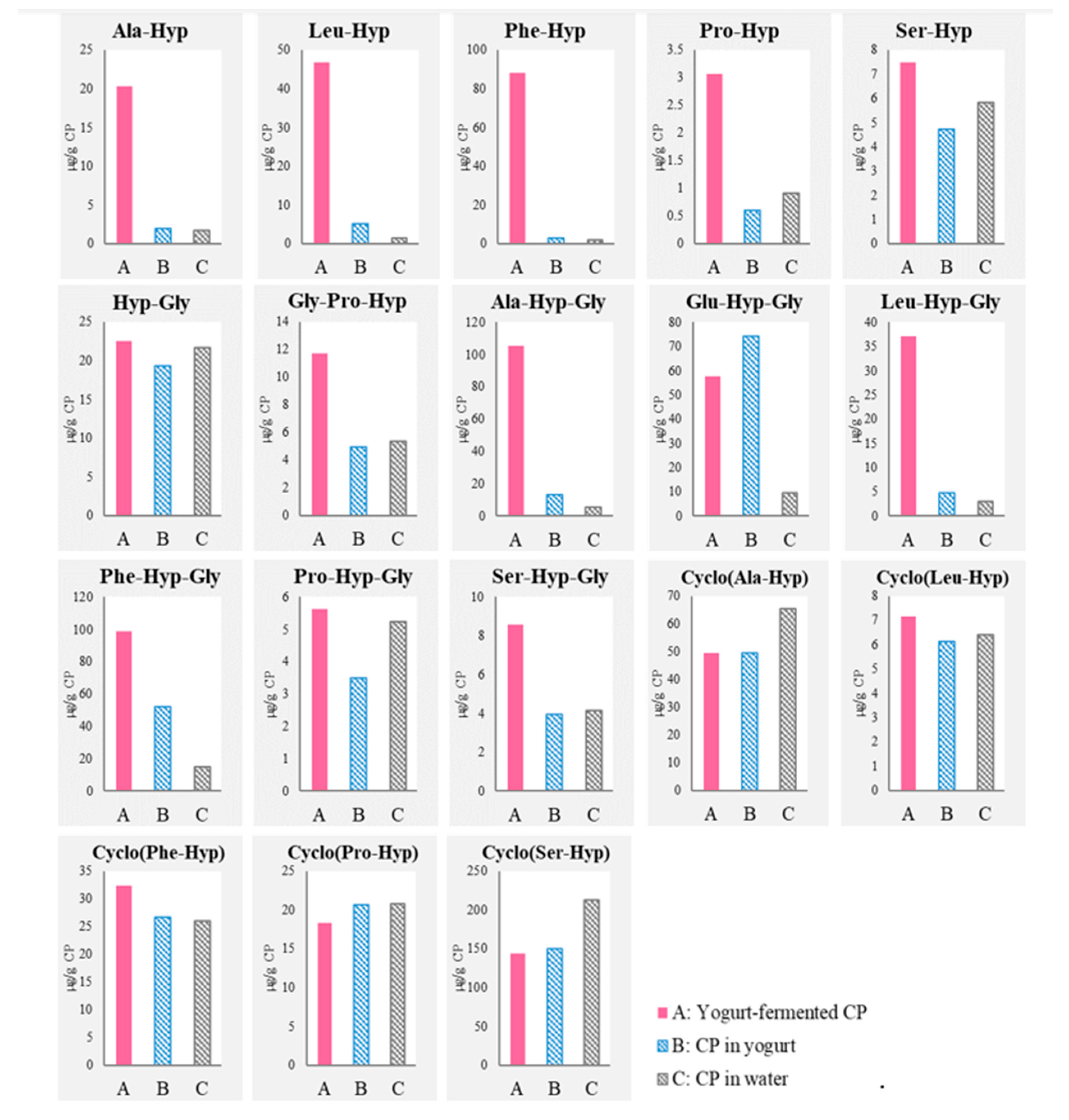
3.1. Detection of Di- and Tripeptides and Cyclic Peptides Derived from CP after Yogurt Fermentation
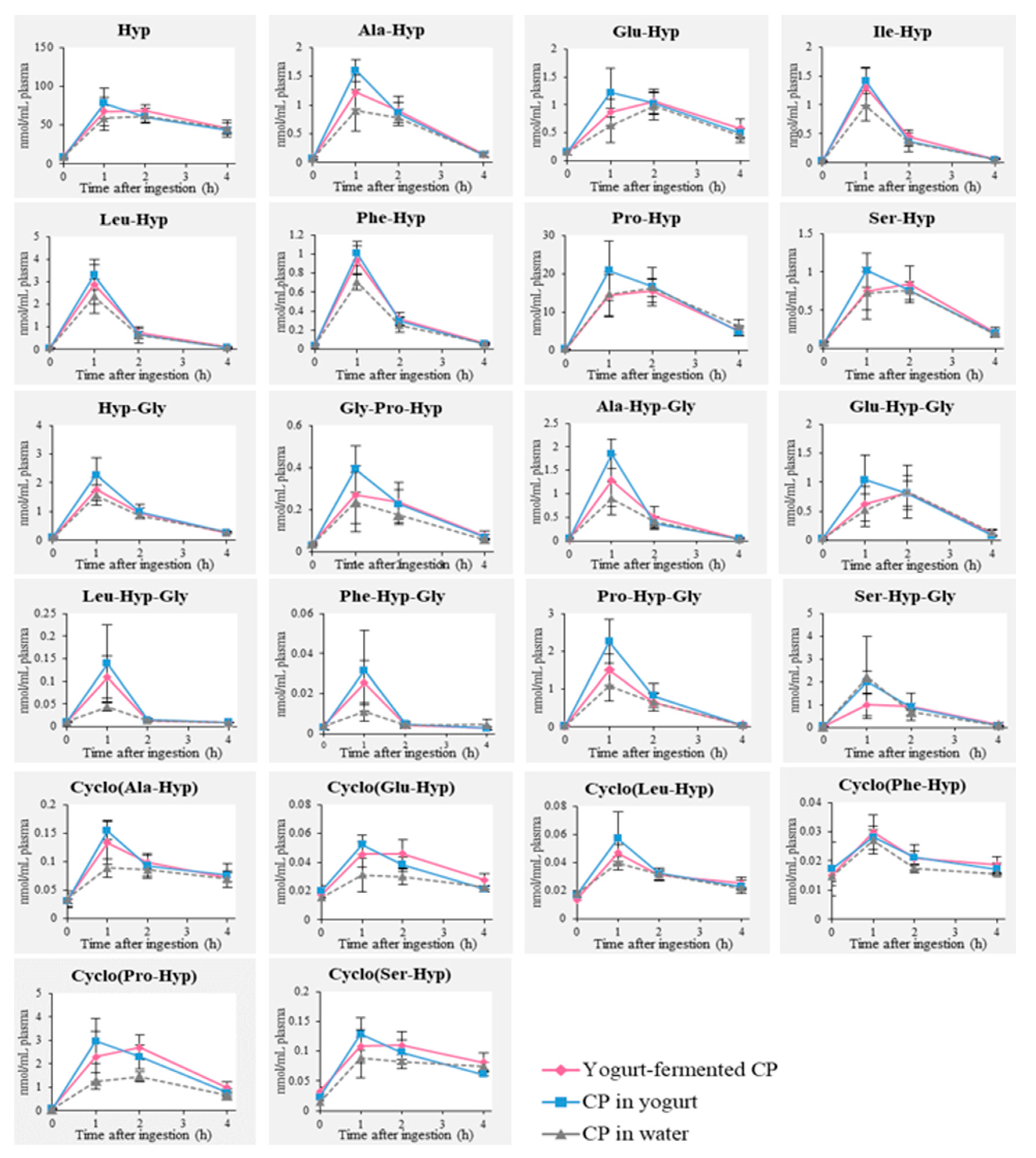
3.2. Detection of Food-Derived Hyp-Containing Peptides in Human Blood after Ingestion of CP with or without Yogurt Fermentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schauss, A.G.; Stenehjem, J.; Park, J.; Endres, J.R.; Clewell, A.E. Effect of the Novel Low Molecular Weight Hydrolyzed Chicken Sternal Cartilage Extract, BioCell Collagen, on Improving Osteoarthritis-Related Symptoms: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Agric. Food Chem. 2012, 60, 4096–4101. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sugihara, F.; Suzuki, K.; Inoue, N.; Venkateswarathirukumara, S. A double-blind, placebo-controlled, randomised, clinical study on the effectiveness of collagen peptide on osteoarthritis. J. Sci. Food Agric. 2014, 95, 702–707. [Google Scholar] [CrossRef]
- Yamanaka, H.; Okada, S.; Sanada, H. A multicenter, randomized, controlled study of the use of nutritional supplements containing collagen peptides to facilitate the healing of pressure ulcers. J. Nutr. Intermed. Metab. 2017, 8, 51–59. [Google Scholar] [CrossRef]
- Sugihara, F.; Inoue, N.; Venkateswarathirukumara, S. Ingestion of bioactive collagen hydrolysates enhanced pressure ulcer healing in a randomized double-blind placebo-controlled clinical study. Sci. Rep. 2018, 8, 11403. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Oesser, S.; Baumstark, M.W.; Gollhofer, A.; Koenig, D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: A randomised controlled trial. Br. J. Nutr. 2015, 114, 1237–1245. [Google Scholar] [CrossRef]
- Iwai, K.; Hasegawa, T.; Taguchi, Y.; Morimatsu, F.; Sato, K.; Nakamura, Y.; Higashi, A.; Kido, Y.; Nakabo, Y.; Ohtsuki, K. Identification of Food-Derived Collagen Peptides in Human Blood after Oral Ingestion of Gelatin Hydrolysates. J. Agric. Food Chem. 2005, 53, 6531–6536. [Google Scholar] [CrossRef]
- Ohara, H.; Matsumoto, H.; Ito, K.; Iwai, K.; Sato, K. Comparison of Quantity and Structures of Hydroxyproline-Containing Peptides in Human Blood after Oral Ingestion of Gelatin Hydrolysates from Different Sources. J. Agric. Food Chem. 2007, 55, 1532–1535. [Google Scholar] [CrossRef]
- Matsui, T.; Tamaya, K.; Seki, E.; Osajima, K.; Matsumoto, K.; Kawasaki, T. Absorption of Val–Tyr with in Vitro Angiotensin I-Converting Enzyme Inhibitory Activity into the Circulating Blood System of Mild Hypertensive Subjects. Boil. Pharm. Bull. 2002, 25, 1228–1230. [Google Scholar] [CrossRef] [PubMed]
- Foltz, M.; Meynen, E.E.; Bianco, V.; Van Platerink, C.; Koning, T.M.M.G.; Kloek, J. Angiotensin converting enzyme inhibitory peptides from a lactotripeptide-enriched milk beverage are absorbed intact into the circulation. J. Nutr. 2007, 137, 953–958. [Google Scholar] [CrossRef]
- Shigemura, Y.; Iwai, K.; Morimatsu, F.; Iwamoto, T.; Mori, T.; Oda, C.; Taira, T.; Park, E.Y.; Nakamura, Y.; Sato, K. Effect of Prolyl-hydroxyproline (Pro-Hyp), a Food-Derived Collagen Peptide in Human Blood, on Growth of Fibroblasts from Mouse Skin. J. Agric. Food Chem. 2009, 57, 444–449. [Google Scholar] [CrossRef]
- Ohara, H.; Ichikawa, S.; Matsumoto, H.; Akiyama, M.; Fujimoto, N.; Kobayashi, T.; Tajima, S. Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J. Dermatol. 2010, 37, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, S.; Mano, H.; Sampei, C.; Shimizu, J.; Wada, M. Chondroprotective effect of the bioactive peptide prolyl-hydroxyproline in mouse articular cartilage in vitro and in vivo. Osteoarthr. Cartil. 2009, 17, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, Y.; Iwasaki, Y.; Tateno, M.; Suzuki, A.; Kurokawa, M.; Sato, Y.; Sato, K. A Pilot Study for the Detection of Cyclic Prolyl-Hydroxyproline (Pro-Hyp) in Human Blood after Ingestion of Collagen Hydrolysate. Nutrients 2018, 10, 1356. [Google Scholar] [CrossRef]
- Taga, Y.; Iwasaki, Y.; Shigemura, Y.; Mizuno, K. Improved in Vivo Tracking of Orally Administered Collagen Hydrolysate Using Stable Isotope Labeling and LC–MS Techniques. J. Agric. Food Chem. 2019, 67, 4671–4678. [Google Scholar] [CrossRef]
- Adibi, S.A.; Soleimanpour, M.R. Functional Characterization of Dipeptide Transport System in Human Jejunum. J. Clin. Investig. 1974, 53, 1368–1374. [Google Scholar] [CrossRef]
- Adibi, S.A.; Morse, E.L.; Masilamani, S.S.; Amin, P.M. Evidence for two different modes of tripeptide disappearance in human intestine. Uptake by peptide carrier systems and hydrolysis by peptide hydrolases. J. Clin. Investig. 1975, 56, 1355–1363. [Google Scholar] [CrossRef]
- Walrand, S.; Chiotelli, E.; Noirt, F.; Mwewa, S.; Lassel, T. Consumption of a Functional Fermented Milk Containing Collagen Hydrolysate Improves the Concentration of Collagen-Specific Amino Acids in Plasma. J. Agric. Food Chem. 2008, 56, 7790–7795. [Google Scholar] [CrossRef]
- Griffiths, M.W.; Tellez, A.M. Lactobacillus helveticus: The proteolytic system. Front. Microbiol. 2013, 4, 30. [Google Scholar] [CrossRef]
- Taga, Y.; Kusubata, M.; Ogawa-Goto, K.; Hattori, S. Efficient Absorption of X-Hydroxyproline (Hyp)-Gly after Oral Administration of a Novel Gelatin Hydrolysate Prepared Using Ginger Protease. J. Agric. Food Chem. 2016, 64, 2962–2970. [Google Scholar] [CrossRef]
- Ohara, H.; Iida, H.; Ito, K.; Takeuchi, Y.; Nomura, Y. Effects of Pro-Hyp, a Collagen Hydrolysate-Derived Peptide, on Hyaluronic Acid Synthesis Using in Vitro Cultured Synovium Cells and Oral Ingestion of Collagen Hydrolysates in a Guinea Pig Model of Osteoarthritis. Biosci. Biotechnol. Biochem. 2010, 74, 2096–2099. [Google Scholar] [CrossRef]
- Trč, T.; Bohmova, J. Efficacy and tolerance of enzymatic hydrolysed collagen (EHC) vs. glucosamine sulphate (GS) in the treatment of knee osteoarthritis (KOA). Int. Orthop. 2010, 35, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fujioka, M.; Sugimoto, K.; Mu, G.; Ishimi, Y. Assessment of effectiveness of oral administration of collagen peptide on bone metabolism in growing and mature rats. J. Bone Miner. Metab. 2004, 22, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Taga, Y.; Kusubata, M.; Ogawa-Goto, K.; Hattori, S.; Funato, N. Collegen-derived X-Hyp-Gly type tripeptides promote differentiation of MC3T3-E1 pre osteoblasts. J. Funct. Foods 2018, 46, 456–462. [Google Scholar] [CrossRef]
- Aguirre, L.; Hebert, E.M.; Garro, M.S.; De Giori, G.S. Proteolytic activity of Lactobacillus strains on soybean proteins. LWT 2014, 59, 780–785. [Google Scholar] [CrossRef]
- Hafeez, Z.; Cakir-Kiefer, C.; Girardet, J.M.; Jardin, J.; Perrin, C.; Dary, A.; Miclo, L. Hydrolysis of milk-derived bioactive peptides by cell-associated extracellular peptidases of Streptococcus thermophiles. Appl. Microbiol. Biotechnol. 2013, 97, 9787–9799. [Google Scholar] [CrossRef]
- Atlan, D.; Laloi, P.; Portalier, R. X-Prolyl-Dipeptidyl Aminopeptidase of Lactobacillus delbrueckii subsp. bulgaricus: Characterization of the Enzyme and Isolation of Deficient Mutants. Appl. Environ. Microbiol. 1990, 56, 2174–2179. [Google Scholar] [CrossRef]
- Daniel, H.; Vohwinkel, M.; Rehner, G. Effect of Casein and β-Casomorphins on Gastrointestinal Motility in Rats. J. Nutr. 1990, 120, 252–257. [Google Scholar] [CrossRef]
- Tomé, D.A.; Dumontier, A.M.; Hautefeuille, M.A.; Desjeux, J.F. Opiate activity and transepithelial passage of intact β-casomorphins in rabbit ileum. Am. J. Physiol. 1987, 253, 737–744. [Google Scholar] [CrossRef]
- Meisel, H.; Fitzgerald, R.J. Opioid peptides encrypted in intact milk protein sequences. Br. J. Nutr. 2000, 84, 27–31. [Google Scholar] [CrossRef]
- Lacroix, I.M.E.; Chen, X.-M.; Kitts, D.D.; Li-Chan, E.C. Investigation into the bioavailability of milk protein-derived peptides with dipeptidyl-peptidase IV inhibitory activity using Caco-2 cell monolayers. Food Funct. 2017, 8, 701–709. [Google Scholar] [CrossRef]
- Takeda, J.; Park, H.-Y.; Kunitake, Y.; Yoshiura, K.; Matsui, T. Theaflavins, dimeric catechins, inhibit peptide transport across Caco-2 cell monolayers via down-regulation of AMP-activated protein kinase-mediated peptide transporter PEPT1. Food Chem. 2013, 138, 2140–2145. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.; Kunitake, Y.; Hirasaki, N.; Tanaka, M.; Matsui, T. Theaflavins enhance intestinal barrier of Caco-2 Cell monolayers through the expression of AMP-activated protein kinase-mediated Occludin, Claudin-1, and ZO-1. Biosci. Biotechnol. Biochem. 2014, 79, 130–137. [Google Scholar] [CrossRef] [PubMed]
 ), “CP in yogurt” (
), “CP in yogurt” ( ), and “CP in water” (
), and “CP in water” ( ).
).
 ), “CP in yogurt” (
), “CP in yogurt” ( ), and “CP in water” (
), and “CP in water” ( ).
).
| Food | Milk (g) | Yogurt (g) | Water (g) | Collagen Peptide (CP, g) | Incubation |
|---|---|---|---|---|---|
| Yogurt-fermented CP | 45 | 5 | 0 | 10 | ◯ |
| CP in yogurt | 0 | 50 | 0 | 10 | × |
| CP in water | 0 | 0 | 200 | 10 | × |
| Free and Peptide Form of Hyp | Cmax (nmol/mL) | AUC (nmol/h·mL) | ||||
|---|---|---|---|---|---|---|
| Yogurt-Fermented CP | CP in Yogurt | CP in Water | Yogurt-Fermented CP | CP in Yogurt | CP in Water | |
| Hyp | 68.15 ± 8.37 | 78.13 ± 19.39 | 61.80 ± 9.31 | 55.00 ± 6.82 | 51.54 ± 12.41 | 48.68 ± 8.95 |
| Ala-Hyp | 1.12 ± 0.29 | 1.60 ± 0.19 ** | 0.90 ± 0.36 | 0.69 ± 0.12 | 0.75 ± 0.12 * | 0.56 ± 0.08 |
| Glu-Hyp | 1.06 ± 0.21 | 1.22 ± 0.44 | 0.98 ± 0.25 | 0.78 ± 0.10 | 0.81 ± 0.22 | 0.63 ± 0.21 |
| Ile-Hyp | 1.31 ± 0.32 | 1.42 ± 0.23 | 0.98 ± 0.26 | 0.52 ± 0.10 | 0.51 ± 0.85 | 0.42 ± 0.07 |
| Leu-Hyp | 2.88 ± 0.87 | 3.30 ± 0.67 | 2.35 ± 0.76 | 1.03 ± 0.27 | 1.09 ± 0.23 | 0.92 ± 0.24 |
| Phe-Hyp | 0.93 ± 0.15 * | 1.01 ± 0.13 ** | 0.71 ± 0.08 | 0.37 ± 0.06 | 0.38 ± 0.04 | 0.32 ± 0.07 |
| Pro-Hyp | 15.53 ± 2.96 | 20.86 ± 7.70 | 16.36 ± 2.43 | 10.75 ± 1.45 | 12.47 ± 4.31 | 11.06 ± 3.10 |
| Ser-Hyp | 0.84 ± 0.23 | 1.02 ± 0.22 | 0.76 ± 0.02 | 0.57 ± 0.08 | 0.58 ± 0.11 | 0.51 ± 0.12 |
| Hyp-Gly | 1.77 ± 0.40 | 2.28 ± 0.59 | 1.56 ± 0.35 | 0.88 ± 0.16 | 1.01 ± 0.25 | 0.83 ± 0.09 |
| Gly-Pro-Hyp | 0.27 ± 0.14 | 0.39 ± 0.11 | 0.24 ± 0.14 | 0.18 ± 0.07 | 0.20 ± 0.06 | 0.14 ± 0.06 |
| Ala-Hyp-Gly | 1.29 ± 0.55 | 1.84 ± 0.31 * | 0.90 ± 0.35 | 0.52 ± 0.18 | 0.62 ± 0.11 * | 0.40 ± 0.04 |
| Glu-Hyp-Gly | 0.82 ± 0.30 | 1.14 ± 0.32 | 0.84 ± 0.45 | 0.50 ± 0.12 | 0.58 ± 0.13 | 0.47 ± 0.24 |
| Leu-Hyp-Gly | 0.11 ± 0.05 | 0.14 ± 0.09 * | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.02 ± 0.01 |
| Phe-Hyp-Gly | 0.025 ± 0.011 | 0.032 ± 0.020 | 0.011 ± 0.005 | 0.01 ± 0.003 | 0.01 ± 0.01 | 0.01 ± 0.002 |
| Pro-Hyp-Gly | 1.51 ± 0.41† | 2.26 ± 0.57 ** | 1.10 ± 0.40 | 0.64 ± 0.05† | 0.89 ± 0.18 ** | 0.54 ± 0.14 |
| Ser-Hyp-Gly | 1.00 ± 0.48 | 1.99 ± 0.47 | 2.19 ± 1.79 | 0.62 ± 0.32 | 0.85 ± 0.12 | 0.81 ± 0.45 |
| Cyclo(Ala-Hyp) | 0.13 ± 0.04 * | 0.15 ± 0.02 ** | 0.09 ± 0.02 | 0.09 ± 0.01 | 0.09 ± 0.02 | 0.08 ± 0.01 |
| Cyclo(Glu-Hyp) | 0.046 ± 0.006 | 0.052 ± 0.007 * | 0.031 ± 0.012 | 0.04 ± 0.004 ** | 0.03 ± 0.004 * | 0.03 ± 0.01 |
| Cyclo(Leu-Hyp) | 0.05 ± 0.52 | 0.06 ± 0.02 | 0.04 ± 0.00 | 0.03 ± 0.003 | 0.03 ± 0.01 | 0.03 ± 0.004 |
| Cyclo(Phe-Hyp) | 0.030 ± 0.02 | 0.028 ± 0.002 | 0.027 ± 0.05 | 0.02 ± 0.002 | 0.02 ± 0.004 | 0.02 ± 0.002 |
| Cyclo(Pro-Hyp) | 2.69 ± 0.69 * | 2.97 ± 0.97 ** | 0.09 ± 0.03 | 1.84 ± 0.28 ** | 1.76 ± 0.52 * | 1.00 ± 0.21 |
| Cyclo(Ser-Hyp) | 0.11 ± 0.02 | 0.13 ± 0.03 | 0.11 ± 0.01 | 0.09 ± 0.01 * | 0.08 ± 0.01 | 0.07 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasaki, Y.; Taga, Y.; Suzuki, A.; Kurokawa, M.; Sato, Y.; Shigemura, Y. Effect of Co-Ingestion of Collagen Peptides with Yogurt on Blood Absorption of Short Chain Hydroxyproline Peptides. Appl. Sci. 2020, 10, 4066. https://doi.org/10.3390/app10124066
Iwasaki Y, Taga Y, Suzuki A, Kurokawa M, Sato Y, Shigemura Y. Effect of Co-Ingestion of Collagen Peptides with Yogurt on Blood Absorption of Short Chain Hydroxyproline Peptides. Applied Sciences. 2020; 10(12):4066. https://doi.org/10.3390/app10124066
Chicago/Turabian StyleIwasaki, Yu, Yuki Taga, Asahi Suzuki, Mihoko Kurokawa, Yoshio Sato, and Yasutaka Shigemura. 2020. "Effect of Co-Ingestion of Collagen Peptides with Yogurt on Blood Absorption of Short Chain Hydroxyproline Peptides" Applied Sciences 10, no. 12: 4066. https://doi.org/10.3390/app10124066
APA StyleIwasaki, Y., Taga, Y., Suzuki, A., Kurokawa, M., Sato, Y., & Shigemura, Y. (2020). Effect of Co-Ingestion of Collagen Peptides with Yogurt on Blood Absorption of Short Chain Hydroxyproline Peptides. Applied Sciences, 10(12), 4066. https://doi.org/10.3390/app10124066





