The Microbiota of Edam Cheeses Determined by Cultivation and High-Throughput Sequencing of the 16S rRNA Amplicon
Abstract
1. Introduction
2. Materials and Methods
2.1. Cheese Sampling
2.2. Chemical Composition
2.3. Determination of the Counts of Selected Bacterial Groups by the Culture-Dependent Method
2.4. DNA Isolation
2.5. Amplicon Sequencing and Bioinformatics Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition
3.2. Determination of the Counts of Selected Bacterial Groups by the Culture-Dependent Method
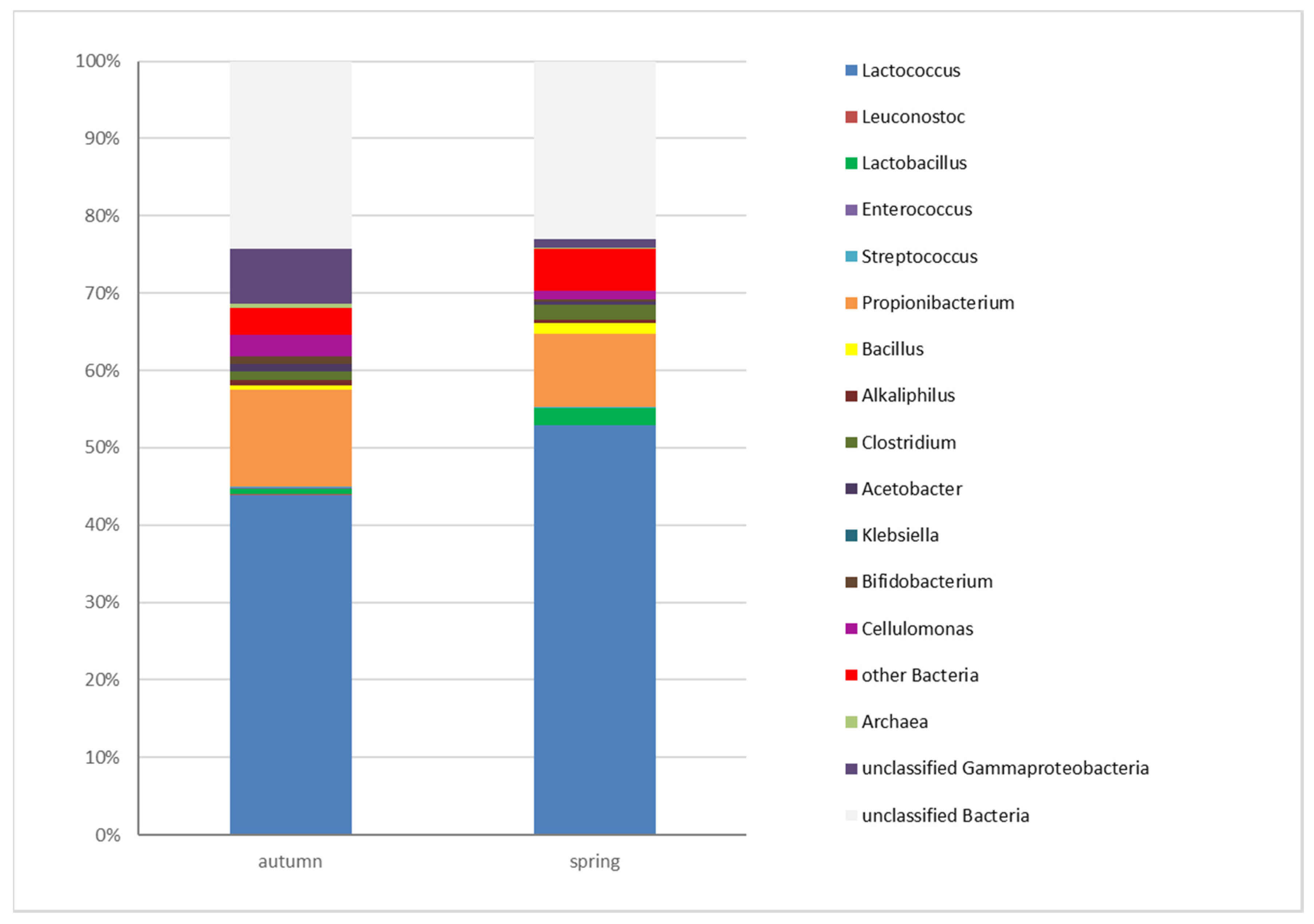
3.3. Determination of the Microbiota of Edam Cheeses by High-Throughput Sequencing (HTS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ogier, J.-C.; Lafarge, V.; Girard, V.; Rault, A.; Maladen, V.; Gruss, A.; Leveau, J.-Y.; Delacroix-Buchet, A. Molecular fingerprinting of dairy microbial ecosystems by use of temporal temperature and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 2004, 70, 5628−5643. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, C.L.; Vaughan, E.E.; Caggia, C. Artisanal and experimental Pecorino Siciliano cheese: Microbial dynamics during manufacture assessed by culturing and PCR-DGGE analyses. Int. J. Food Microbiol. 2006, 109, 1−8. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, A.; Guidone, A.; Ianniello, R.G.; Cioffi, S.; Aponte, M.; Pavlidis, D.; Tsakalidou, E.; Zotta, T.; Parente, E. A survey of non-starter lactic acid bacteria in traditional cheeses: Culture dependent identification and survival to simulated gastrointestinal transit. Int. Dairy J. 2015, 43, 42−50. [Google Scholar] [CrossRef]
- Beresford, T.P.; Fitzsimons, N.A.; Brennan, N.L.; Cogan, T.M. Recent advances in cheese microbiology. Int. Dairy J. 2001, 11, 259–274. [Google Scholar] [CrossRef]
- Czerwińska, E.; Piotrowski, W. Potential sources of milk contamination influencing its quality for consumption. Rocz. Ochrona Środow. 2011, 13, 635–652. (in Polish). [Google Scholar]
- Lawlor, J.; Delahunty, C.M.; Wilkinson, M.G.; Sheehan, J. Swiss-type and Swiss–cheddar hybrid-type cheeses: Effects of manufacture on sensory character and relationships between the sensory attributes and volatile compounds and gross compositional constituents. Int. J. Dairy Technol. 2003, 56, 39–51. [Google Scholar] [CrossRef]
- Joux, F.; Lebaron, P. Use of fluorescent probes to assess physiological functions of bacteria at single-cell level. Microbes Infect. 2000, 2, 1523–1535. [Google Scholar] [CrossRef]
- Dove, A. PCR: Thirty-five years and counting. Science 2018, 360, 670–672. [Google Scholar] [CrossRef][Green Version]
- Cocolin, L.; Mataragas, M.; Bourdichon, F.; Doulgeraki, A.; Pilet, M.-F.; Jagadeesan, B.; Rantsiou, K.; Phister, T. Next generation microbiological risk assessment meta-omics: The next need for integration. Int. J. Food Microbiol. 2018, 287, 3–9. [Google Scholar] [CrossRef]
- Kergourlay, G.; Taminiau, B.; Daube, G.; Champomier Verges, M.C. Metagenomic insights into the dynamics of microbial communities in food. Int. J. Food Microbiol. 2015, 213, 31–39. [Google Scholar] [CrossRef]
- Ercolini, D.; De Filippis, F.; La Storia, A.; Iacono, M. “Remake” by high-throughput sequencing of the microbiota involved in the production of water buffalo mozzarella cheese. Appl. Environ. Microbiol. 2012, 78, 8142–8145. [Google Scholar] [CrossRef] [PubMed]
- Delcenserie, V.; Taminiau, B.; Delhalle, L.; Nezer, C.; Doyen, P.; Crevecoeur, S.; Roussey, D.; Korsak, N.; Daube, G. Microbiota characterization of a Belgian protected designation of origin cheese, Herve cheese, using metagenomic analysis. J. Dairy Sci. 2014, 97, 6046–6056. [Google Scholar] [CrossRef] [PubMed]
- Dalmasso, A.; de los Dolores Soto del Rio, M.; Civera, T.; Pattono, D.; Cardazzo, B.; Bottero, M.T. Characterization of microbiota in Plaisentif cheese by high-throughput sequencing. LWT-Food Sci. Technol. 2016, 69, 490–496. [Google Scholar] [CrossRef]
- De Filippis, F.; Genovese, A.; Ferranti, P.; Gilbert, J.A.; Ercolini, D. Metatranscriptomics reveals temperature-driven functional changes in microbiome impacting cheese maturation rate. Sci. Rep. 2016, 6, 21871. [Google Scholar] [CrossRef]
- Escobar-Zepeda, A.; Sanchez-Flores, A.; Quirasco Baruch, M. Metagenomic analysis of a Mexican ripened cheese reveals a unique complex microbiota. Food Microbiol. 2016, 57, 116–127. [Google Scholar] [CrossRef]
- Ceugniez, A.; Taminiau, B.; Coucheney, F.; Jacques, P.; Delcenserie, V.; Daube, G.; Drider, D. Use of a metagenetic approach to monitor the bacterial microbiota of “Tomme d’Orchies” cheese during the ripening process. Int. J. Food Microbiol. 2017, 247, 65–69. [Google Scholar] [CrossRef]
- Dugat-Bony, E.; Straub, C.; Teissandier, A.; Onesime, D.; Loux, V.; Monnet, C.; Irlinger, F.; Landaud, S.; Leclercq-Perlat, M.N.; Bento, P.; et al. Overview of a surface-ripened cheese community functioning by meta-omics analyses. PLoS ONE 2015, 10, e0124360. [Google Scholar] [CrossRef]
- Porcellato, D.; Skeie, S.B. Bacterial dynamics and functional analysis of microbial metagenomes during ripening of Dutch-type cheese. Int. Dairy J. 2018, 61, 182–188. [Google Scholar] [CrossRef]
- Duru, I.C.; Laine, P.; Andreevskaya, M.; Paulin, L.; Kananen, S.; Tynkkynen, S.; Auvinen, P.; Smolander, O.-P. Metagenomic and metatranscriptomic analysis of the microbial community in Swiss-type Maasdam cheese during ripening. Int. J. Food Microbiol. 2018, 281, 10–22. [Google Scholar] [CrossRef]
- Albenzio, M.; Corbo, M.R.; Rehman, S.U.; Fox, P.F.; De Angelis, M.; Corsetti, A.; Sevi, A.; Gobbetti, M. Microbiological and biochemical characteristics of Canestrato Pugliese cheese made from raw milk, pasteurized milk or by heating the curd in hot whey. Int. J. Food Microbiol. 2001, 67, 35–48. [Google Scholar] [CrossRef]
- Dahl, S.; Tavaria, F.K.; Malcata, F.X. Relationships between flavour and microbiological profiles in Serra da Estrela cheese throughout ripening. Int. Dairy J. 2000, 10, 255–262. [Google Scholar] [CrossRef]
- Marini, M.; Maifreni, M.; Rondinini, G. Microbiological characterization of artisanal Montasio cheese: Analysis of its indigenous lactic acid bacteria. FEMS Microbiol. Lett. 2003, 229, 133–140. [Google Scholar] [CrossRef]
- ISO 707:2008 (IDF 50:2008). Milk and Milk Products—Guidance on Sampling; International Standard Organization: London, UK, 2008. [Google Scholar]
- ISO 3433:2008 (IDF 222: 2008). Cheese-Determination of Fat Content-Van Gulik Method; International Standard Organization: London, UK, 2008. [Google Scholar]
- ISO 5943:2006 (IDF 88:2006). Cheese and Processed Cheese Products-Determination of Chloride Content--Potentiometric Titration Method; International Standard Organization: London, UK, 2006. [Google Scholar]
- AOAC International 2005. Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Paarmann, D.; D’Souza, M.; Olson, R.; Glass, E.M.; Kubal, M.; Paczian, T.; Rodriguez, A.; Stevens, R.; Wilke, A.; et al. The metagenomics RAST server–a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics 2008, 9, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Aljewicz, M.; Cichosz, G. Influence of probiotic (Lactobacillus acidophilus NCFM, L. paracasei LPC37, and L. rhamnosus HN001) strains on starter cultures and secondary microbiota in Swiss-and Dutch-type cheeses. J. Food Process. Preserv. 2017, 41, e13253. [Google Scholar] [CrossRef]
- de Souza, C.F.V.; Dalla Rosa, T.; Ayub, M.A.Z. Changes in the microbiological and physicochemical characteristics of Serrano cheese during manufacture and ripening. Braz. J. Microbiol. 2003, 34, 260–266. [Google Scholar] [CrossRef]
- Park, W.; Yoo, J.; Oh, S.; Ham, J.S.; Jeong, S.G.; Kim, Y. Microbiological characteristics of Gouda cheese manufactured with pasteurized and raw milk during ripening using next generation sequencing. Food Sci. Animal Resource 2019, 39, 585–600. [Google Scholar] [CrossRef]
- Van Hoorde, K.; Heyndrickx, M.; Vandamme, P.; Huys, G. Influence of pasteurization, brining conditions and production environment on the microbiota of artisan Gouda-type cheeses. Food Microbiol. 2010, 27, 425–433. [Google Scholar] [CrossRef]
- Martín-Platero, A.M.; Maqueda, M.; Valdivia, E.; Purswani, J.; Martínez-Bueno, M. Polyphasic study of microbial communities of two Spanish farmhouse goats’ milk cheeses from Sierra de Aracena. Food Microbiology 2009, 6, 294–304. [Google Scholar] [CrossRef]
- Sánchez-Gamboa, C.; Hicks-Pérez, L.; Gutiérrez-Méndez, N.; Heredia, N.; García, S.; Nevárez-Moorillón, G. Microbiological changes during ripening of Chihuahua cheese manufactured with raw milk and its seasonal variations. Foods 2018, 7, 153. [Google Scholar] [CrossRef]
- Bermúdez, J.; González, M.J.; Olivera, J.A.; Burgueño, J.A.; Juliano, P.; Fox, E.M.; Reginensi, S.M. Seasonal occurrence and molecular diversity of clostridia species spores along cheesemaking streams of 5 commercial dairy plants. J. Dairy Sci. 2016, 99, 3358–3366. [Google Scholar] [CrossRef] [PubMed]
- Matijasic, B.B.; Rajsp, M.K.; Perko, B.; Rogelj, I. Inhibition of Clostridium tyrobutyricum in cheese by Lactobacillus gasseri. Int. Dairy J. 2007, 17, 157–166. [Google Scholar] [CrossRef]
- Alegria, A.; Szczęsny, P.; Mayo, B.; Bardowski, J.; Kowalczyk, M. Biodiversity in Oscypek, a traditional Polish cheese, determined by culture-dependent and -independent approaches. Appl. Environ. Microbiol. 2012, 78, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.J.R.; Oliveira, M.B.P.P.; Mafra, I. Tracing transgenic maize as affected by breadmaking process and raw material for the production of a traditional maize bread, broa. Food Chem. 2013, 138, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Cichosz, G.; Szpendowski, J.; Cichosz, A.J.; Kornacki, M. Paracasein degradation in gouda cheeses produced with Lactobacillus culture. ŻYWNOŚĆ. Nauka. Technologia. Jakość 2006, 1, 58–65, (in Polish, abstract in English). [Google Scholar]
- Czaran, T.; Rattray, F.P.; Møller, C.O.A.; Christensen, B.B. Modelling the influence of metabolite diffusion on non-starter lactic acid bacteria growth in ripening Cheddar cheese. Int. Dairy J. 2018, 80, 35–45. [Google Scholar] [CrossRef]
- Alessandria, V.; Ferrocino, I.; De Filippis, F.; Fontana, M.; Rantsiou, K.; Ercolini, D.; Cocolin, L. Microbiota of an Italian Grana-like cheese during manufacture and ripening, unraveled by 16S rRNA-based approaches. Appl. Environ. Microbiol. 2016, 82, 3988–3995. [Google Scholar] [CrossRef]
- Almeida, M.; Hébert, A.; Abraham, A.-L.; Rasmussen, S.; Monnet, C.; Pons, N.; Delbès, C.; Loux, V.; Batto, J.-M.; Leonard, P.; et al. Construction of a dairy microbial genome catalog opens new perspectives for the metagenomic analysis of dairy fermented products. BMC Genomics 2014, 15, 1101–1117. [Google Scholar] [CrossRef]
- Klijn, N.; Nieuwenhof, F.F.J.; Hoolwerf, J.D.; van der Waals, C.B.; Weerkamp, A.H. Identification of Clostridium tyrobutyricum as the causative agent of late blowing in cheese by species-specific PCR amplification. Appl. Environ. Microbiol. 1995, 61, 2919–2924. [Google Scholar] [CrossRef]
- Bassi, D.; Puglisi, E.; Cocconcelli, P.S. Understanding the bacterial communities of hard cheese with blowing defect. Food Microbiol. 2015, 52, 106–118. [Google Scholar] [CrossRef]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Global Rumen Census Collaborators; Janssen, P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; Mizrahi, I. Similarity of the ruminal bacteria across individual lactating cows. Anaerobes 2012, 18, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, M.; Jones, A.L. Laceyella. Bergey’s Man. Syst. Arch. Bact. 2015. [Google Scholar] [CrossRef]
- Brooke, C.J.; Riley, T.V. Erysipelothrix rhusiopathiae: Bacteriology, epidemiology and clinical manifestations of an occupational pathogen. J. Medical Microbiol. 1999, 48, 789–799. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Mills, D.A. Facility-specific “house” microbiome drives microbial landscapes of artisan cheesemaking plants. Appl. Environ. Microbiol. 2013, 79, 5214–5223. [Google Scholar] [CrossRef] [PubMed]

| Microorganism | Medium | Incubation Conditions |
|---|---|---|
| Lactobacillus spp. | MRS agar (Merck) | 37 °C, 48 h, anaerobic incubation |
| Lactococcus spp. | M17 agar (Merck) | 30 °C, 48 h |
| Leuconostoc spp. | Sucrose agar: (sucrose—50 g/L; yeast extract—10 g/L; agar—15 g/L; pH 7.2–7.4) | 30 °C, 72 h |
| Propionibacterium spp. | Lactate agar: (peptone—10 g/L; yeast extract—5 g/L; calcium lactate—10 g/L; agar—15 g/L; pH 7.0–7.2) | 30 °C, 72–96 h, anaerobic incubation |
| Enterobacteriaceae | VRBG (Merck) | 37 °C, 24–48 h |
| Enterococcus spp. | Slanetz–Bartley agar (Merck) | 37 °C, 48 h |
| Staphylococcus spp. | RPF agar (Merck) | 37 °C, 48 h |
| Clostridium spp. | RCM agar (Oxoid) | 37 °C, 48 h, anaerobic incubation |
| Bacillus spp. | Nutrient agar (Merck) | 30 °C, 48 h |
| Yeasts and molds | YGC agar (Merck) | 25 °C, 72–96 h |
| Composition [%] | Season | |
|---|---|---|
| Autumn | Spring | |
| water | 42.50 ± 0.87 | 42.20 ± 0.77 |
| fat | 27.51 ± 0.38 | 27.19 ± 1.26 |
| sodium chloride | 1.49 ± 0.07 | 1.51 ± 0.06 |
| Bacteria | Season | |
|---|---|---|
| Autumn | Spring | |
| Lactococcus | 6.55 ± 0.25 b,* | 7.16 ± 0.33 a |
| Leuconostoc | 4.60 ± 0.99 | 4.25 ± 0.86 |
| Propionibacterium | 5.64 ± 0.53 | 5.85 ± 0.12 |
| Lactobacillus | 5.97 ± 0.55 | 6.64 ± 0.51 |
| Enterobacteriaceae | 2.50 ± 0.55 a | 2.00 ± 0.01 b |
| Enterococcus | 3.63 ± 0.36 a | 2.77 ± 0.85 b |
| Staphylococcus | 3.00 ± 0.24 | 2.70 ± 0.79 |
| Clostridium | 4.32 ± 0.40 | 3.87 ± 1.15 |
| Bacillus | 3.35 ± 0.66 b | 4.53 ± 0.47 a |
| Yeasts | 2.83 ± 0.26 a | 2.00 ± 0.01 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nalepa, B.; Ciesielski, S.; Aljewicz, M. The Microbiota of Edam Cheeses Determined by Cultivation and High-Throughput Sequencing of the 16S rRNA Amplicon. Appl. Sci. 2020, 10, 4063. https://doi.org/10.3390/app10124063
Nalepa B, Ciesielski S, Aljewicz M. The Microbiota of Edam Cheeses Determined by Cultivation and High-Throughput Sequencing of the 16S rRNA Amplicon. Applied Sciences. 2020; 10(12):4063. https://doi.org/10.3390/app10124063
Chicago/Turabian StyleNalepa, Beata, Sławomir Ciesielski, and Marek Aljewicz. 2020. "The Microbiota of Edam Cheeses Determined by Cultivation and High-Throughput Sequencing of the 16S rRNA Amplicon" Applied Sciences 10, no. 12: 4063. https://doi.org/10.3390/app10124063
APA StyleNalepa, B., Ciesielski, S., & Aljewicz, M. (2020). The Microbiota of Edam Cheeses Determined by Cultivation and High-Throughput Sequencing of the 16S rRNA Amplicon. Applied Sciences, 10(12), 4063. https://doi.org/10.3390/app10124063







