Abstract
This study evaluates nutrients and health-promoting compounds responsible for antioxidant capacity in eight novel formulations based on lyophilized fruit and vegetable powders. The composition contained lyophilized carrot, pumpkin, lentil sprouts, raspberry, strawberry, and apple. The effect of functional additives on the antioxidant, nutritional, and functional characteristics of powdered beverages was determined in the powders and after rehydration followed by in vitro digestion. The antioxidant activity, phenols, vitamin C, and reducing power were significantly higher in the powders enriched with additives having potential functional properties. Furthermore, the analyses indicated that all the powdered formulations may be potential sources of total starch (100–112 mg/100 mL) and proteins (125–139 mg/100 mL). The designed powdered beverages after reconstitution exhibited high antioxidant content, reasonable consumer acceptance, and good in vitro bioaccessibility. The best results of antioxidant capacity were obtained for beverages enriched with raspberry, i.e., 10.4 mg Trolox equivalent (TE)/100 mL and 12.1 mg TE/100 mL rehydrated at 20 °C and 80 °C, respectively. Additionally, color characteristics were used as indicators of the quality of the powdered beverages. This research promotes the reduction of food waste, since whole plant tissues are used, thus allowing maximum exploitation of food raw materials; moreover, drying provides stable shelf life.
Keywords:
powdered food; fruits; vegetables; beverages; antioxidants; health benefits; freeze-drying; color; rehydration 1. Introduction
It is widely known that fruits and vegetables are primary sources of phytochemicals, which are essential to prevent degenerative diseases [1]. They contain a variety of antioxidants that are useful to maintain oxidative stress [2]. Natural antioxidants in vegetables and fruits, such as polyphenols and vitamins, are considered to be involved in a wide range of health benefits [3,4,5,6]. Therefore, phenolic compounds should be delivered with food every day in an appropriate quantity. Moreover, it is estimated that changes in dietary patterns and lifestyle, such as increasing the intake of fruits and vegetables and more balanced consumption of meat and plant foods, are an essential and efficient strategy for reducing the scope of chronic diseases [7]. There is evidence suggesting that the health benefits of plant foods are attributed to the synergy or interactions between bioactive compounds and other nutrients in whole foods. Consequently, all necessary nutrients, antioxidants, bioactive compounds, and phytochemicals should be delivered from a balanced diet that is rich in a wide variety of fruits, vegetables, whole grains, and other plant foods for optimal nutrition, health, and well-being of consumers, rather than from dietary supplements.
Fresh fruits and vegetables have high moisture content; hence, they may deteriorate within a short time if they are not handled appropriately. Similarly, the high costs of the distribution of these resources worldwide may contribute to the search for alternative novel products. Thus, fruit and vegetable powders could be a more practical form than their raw counterparts. Currently, these powders can be used not only as antioxidant-rich flavor enhancers for bakery [8], confectionery [3], and sweets [9], but also in diverse sauces, puddings, and garnishes, and as food ingredients for infants and children [10]. Moreover, fruit and vegetable powders are applied as intermediate products in the beverage industry, as functional food additives improving the nutritional value of foodstuffs, flavoring agents (in ice creams, yogurts, fruit bars), or natural colorants [11,12].
For example, fruit and vegetable powders likewise serve as ingredients in instant noodles, dried soups, and other food recipes [13,14]. Addition of natural colorants present in blueberry and cranberry fruit powders remarkably increased the attractiveness of breakfast cereal [11]. In turn, Costa, Felipe, Maia, Hernandez, and Brasil [15] emphasized the suitability of guava and cashew-apple powders in the food industry to be used as high-dietary-fiber fortifiers.
Currently, dried products are broadly used by food industries, and dynamic growth is observed in this chain. Consumers place more attention on healthy snacks or meals, which can be prepared quickly. In response to market demand, products that are easy to prepare and easy to eat are being produced. Powdered foods have a number of advantages, most importantly, a longer shelf life, a lower risk of contamination or microbial growth, and no need for refrigerated storage. Airtight packaging keeps the product fresh and unchanged for up to two years, without the need for preservative additives [3]. Of course, the quality of such a product has to be monitored at each step of its production (characteristic of raw material, technological changes, effect of storage). It was previously proven that uncontrolled introduction of new food products and supplements may generate a risk for consumers resulting from a presence of dangerous contamination such as mycotoxins, heavy metals, and pesticides [16,17]. Formulation of new foods using natural products (i.e., plant-based ingredients) has the potential to confer several benefits; such products are lower in calories, fat, salt, phosphates, and other synthetic components but rich in fiber, antioxidants, and other bioactives [8]. In addition, their potential advantages as meal replacement, ready-to-eat, easy-to-prepare, and easy-to-swallow products should be mentioned. As a meal replacement, powdered beverages provide an easy solution to a balanced diet, which must contain carbohydrates, high-quality proteins, and other nutrients [18,19]. Numerous studies analyzed instant powders and instant beverages, although the application of novel powdered beverages containing multiple bioactive ingredients in the same product is limited. Some examples include low-calorie and nutritive powdered sorghum drink mix [8], powdered cocoa beverages [20], or instant ginger beverages [21].
As demonstrated by Benincasa, Falcinelli, Lutts, Stagnari, and Galieni [22], among sprouted legumes, lentil sprouts contain many functional health-promoting components such as starch, proteins, and fiber. Additionally, they have a low glycemic index, slowly release glucose into the blood stream, and ensure a more stable insulin response [23]. For these reasons, lentils are suited to diabetic and overweight people. Similarly, there are many reports proving that pumpkin is a great source of natural complexes of soluble and insoluble fiber, fatty acids, vitamins, minerals, antioxidants, and other bioregulators [24]. It was demonstrated that dietary fiber from pumpkin pulp exhibits strong antioxidant, cholesterol-lowering, hypoglycemic, hepatoprotective, and antidiabetic activities [25,26,27]. Carrot is rich in β-carotene, thiamine, vitamin B-complex, riboflavin, and minerals. Therefore, the use of powdered lentil sprouts, pumpkin, and carrot as a base for creating functional drinks seems to be the right choice. Furthermore, functional additives such as lyophilized strawberry (rich in phenolics 27.3 mg gallic acid equivalent (GAE)/g, vitamin C 12.1 mg/g), raspberry (total phenolic content (TPC), 21.7 mg GAE/g; vitamin C, 2.5 mg/g), and apple (TPC, 9.5 mg GAE/g) were well tested [10,28,29]. Berries are also reported to have antioxidant and anti-inflammatory biological properties [30,31]. Apples contain pectins, dietary fibers, vitamins, and oligosaccharides. They are also a good source of different classes of phenolic compounds. Moreover, various studies identified chlorogenic, coumaric, coumaroylquinic, caffeic, ferulic, cinnamic, vanillic, gentisic, protochatehuic, and gallic acids in apples [32]. Such compounds play a remarkable role in antioxidant activity and represent many various benefits for human health. Consequently, the addition of fruits provides acceptable color and masks the undesirable taste of legumes [3].
An important step of the evaluation of food quality is assessment of the potential bioaccessibility of nutrients and bioactives. In general, in vitro gastrointestinal digestion is useful in assessing the bioaccessibility of food compounds with biological activity [23]. It was proven that the total concentration of individual elements in food does not reflect the real nutritional value of a diet, as the entire nutrient is very seldom digested and absorbed completely. Generally, the bioaccessible fraction refers to the amount of compounds or elements that are released from the matrix and are soluble in the gastrointestinal tract [33].
The aim of this study was to evaluate nutrients and health-promoting compounds and their antioxidant capacity in novel powdered beverages based on lyophilized fruit and vegetable powders. The effect of these additives on the functional properties and consumer quality of the beverages was determined in the powders and after rehydration followed by in vitro digestion.
2. Materials and Methods
2.1. Chemicals
All chemicals, ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)), ammonium thiocyanate, α-amylase (EC 3.2.1.1), pancreatin, and pepsin (EC 3.4.23.1), were purchased from Sigma–Aldrich (Poznan, Poland) and BTL Ltd. (Łodz, Poland). All other chemicals were of analytical grade.
2.2. Plant Material and Composition of Beverages
All plant materials (carrot, pumpkin, lentil sprouts, raspberry, strawberry, and apple) were obtained from a local market. Before freeze-drying, the raw materials were peeled and grated to obtain ca. 2.5-mm fragments. Afterward, the samples were lyophilized (LABCONCO, Kansas City, MO, USA), milled using a laboratory grinder (MRC GRINDING MACHINE, SM-450, Israel), sieved (0.45 mm), and stored at −20 °C. The output drink (MO1) contained 30% carrot, 30% pumpkin, and 40% lentil sprouts. To diversify the quality of the beverage, lyophilized lentil sprouts were partially replaced with 10% raspberry (MO2), 10% strawberry (MO3), 10% apple (MO4), 5% raspberry and 5% strawberry (MO5), 5% raspberry and 5% apple (MO6), 5% strawberry and 5% apple (MO7), and 4% apple, 3% raspberry, and 3% strawberry (MO8). The mixtures were prepared in three replications.
2.3. Production of Beverages
Powdered samples (1 g) were rehydrated in 30 mL of distilled water and shaken (three intervals of 30 s). This ratio and time were selected based on previous studies of consumer acceptability, where a proper consistency of the ready-to-drink beverage was the most desirable factor. The samples were rehydrated with water at two temperatures (20 °C and 80 °C) to reflect cold and hot beverages, respectively.
2.4. Sensory Evaluation
A descriptive test was used to evaluate the sensory profile of the samples. An untrained panel composed of 86 members aged from 20 to 44 years (44 women, 42 men) collaborated on the sensory evaluation. The panel evaluated the overall quality of the beverages by scoring the following attributes: color (0.2), taste (0.25), aroma (0.25), consistency (0.1), and acceptability (0.2) using a nine-point scale (1: dislike extremely, 5: neither like nor dislike, 9: like extremely). Samples were prepared according to the procedure described in Section 2.3. The beverages tested were numerically coded and served in transparent glasses. Tap water was provided to the panelists for cleansing their palate between sampling. The data were expressed as the medians of all the scores [34].
2.5. Physical Properties of the Beverages
The color of the samples was measured in the reflectance mode using a Chroma Meter NH310 portable colorimeter (China) according to the CIE L*a* b* system. The measurement was performed on the sample surface using CIE Standard Illuminate D65, di:8 (diffuse illumination/8 C viewing angle), and CIE: 2 Standard Observer. Before analysis, the Chroma Meter was calibrated with white and black ceramic standard plates. The analysis was conducted in 10 repetitions for each powder. L* (lightness), a* (redness), and b* (yellowness) were assessed to determine the rate of color changes after drying. pH was measured using a pH-meter (pH-meter Symphony SB20, VWR, West Chester, PA, USA). WSI (water solubility index) and WAI (water absorption index) were assayed with the centrifuge method as described previously [35].
2.6. In Vitro Digestion
In vitro digestion was performed as described previously [36]. Then, 2-mL beverage samples were subjected to the digestion process. After digestion, the samples were centrifuged (15 min, 6900× g) and the supernatants were mixed with an equal volume of methanol to stop enzyme activity.
2.7. Analysis of Low-Molecular-Weight Antioxidants
2.7.1. Phenolic Content
Powdered samples (500 mg) were subsequently extracted using a three-step extraction procedure. In the first step, the sample was extracted with 5 mL of 50% methanol for 30 min at room temperature using a multi-rotator (RS-60, Biosan) (300 rpm). The samples were centrifuged (15 min, 6000× g), and the pellets were subsequently re-extracted with 5 mL of 1% HCl in 50% methanol and finally with 5 mL of 80% acetone. The supernatants from all steps were combined and stored for further analysis [37].
The amount of total phenolics was determined using Folin–Ciocalteau reagent [38] and expressed as gallic acid equivalent (GAE) in mg per g of powder.
2.7.2. Vitamin C Content
The vitamin C content was determined with methods described earlier by Campos et al. [39]. Ascorbic acid was extracted from the powders using 5% meta-phosphoric acid (MPA). Then, 50 mg of powders were extracted with 2 mL of 5% (w/v) m-phosphoric acid (MPA). The mixtures were centrifuged (15 min, 6000× g, 4 °C) and the supernatant was used for further determination. Dehydroascorbic acid was converted to ascorbic acid using 5 mmol/L phosphine [39]. The vitamin C content was expressed in mg per g of powder.
2.7.3. Carotenoid Content
The powdered sample was extracted with 5 mL of 80% acetone for 30 min at room temperature using a multi-rotator (RS-60, Biosan) (300 rpm). The samples were centrifuged (15 min, 6000× g) and the pellets were subsequently re-extracted five times with 80% acetone until the powders became colorless. The extracts were combined and used for the assay. The carotenoid content was calculated using equations proposed by Sumanta, Haque, Nishika, and Suprakash [40]. Carotenoids were expressed in mg per g of powder.
2.8. Antioxidant Properties
2.8.1. Reducing Power (RP)
Reducing power was determined with the method used by Pulido, Bravo, and Saura-Calixto [41]. The samples for the assay were prepared according to the procedure described in Section 2.7.1. Reducing power was expressed as Trolox equivalent (TE) in mg per g of powder or 100 mL of beverage.
2.8.2. Ability to Quench ABTS Radicals
The experiments were carried out using the ABTS decolorization assay [42]. The samples for the assay were prepared according to the procedure described in Section 2.7.1. The free radical scavenging ability was expressed as Trolox equivalent in mg per g of powder or 100 mL of beverage.
2.9. Nutrients in Functional Powders
2.9.1. Starch Analysis
Starch Content
Total starch (TS) and resistant starch contents in powdered beverages were determined after hydrolysis of starch solubilized with thermostable α-amylase and amyloglucosidase according to the manufacturer’s procedure (Total Starch Kit; Resistant Starch Kit, Megazyme).
Reducing Sugar Content
For determination of free reducing sugars, 200 mg of a powdered sample or 1 mL of a beverage was extracted with 5 mL of 50% methanol. The samples were centrifuged at 12,000× g at 4 °C for 20 min, and the supernatants were collected. The content of free reducing sugars was determined using the standard dinitrosalicylic acid (DNSA) method [43].
2.9.2. Protein Analysis
Protein Content
Total protein content in powdered beverages was determined as a sum of protein fractions (albumins, globulins, prolamins, and glutelins) isolated based on the solubility criterion according to the method proposed by Ribeiro, Teixeira, and Ferreira (2004). The protein content in the extracts was determined with the Bradford method using bovine serum albumin as the standard protein [44]. The protein content was expressed in mg/g of powder or 100 mL of beverage.
Content of Free Amino Acids and Peptides
Non-protein nitrogen was determined with 2,4,6-trinitrobenzene sulfonic acid (TNBS) according to the methods described by Adler-Nissen (2002) using l-leucine as the standard. The content of free amino acids and peptides was expressed in mg per 100 mL of beverage [45].
2.10. Statistical Analysis
The distribution of the data was estimated using Shapiro–Wilk tests. Except for the consumer quality test, statistical significance was estimated by Tukey’s test for data obtained from three independent samples of each extract in three parallel experiments (n = 9). The experimental data were shown as means ± SD. For estimation of the effect on consumer quality, the results from 86 panelists were subjected to statistical analyses using the nonparametric Mann–Whitney test. Experimental data were shown as medians with a range referring to minimum and maximum values. Unless stated otherwise, the statistical tests were carried out at a significance level of α = 0.05. The statistical tests were performed using Statistica 13.1 software (StatSoft, Inc., Tulsa, USA).
3. Results and Discussion
The beverages were produced based on integral exploitation of several fruits and vegetables: lentil sprouts, carrot, pumpkin, raspberry, strawberry, and apple. The whole raw materials were lyophilized and processed into powders. They were combined in eight variants, and their functional properties were evaluated.
The composition and antioxidant properties of functional powders dedicated for production of beverages are shown in Table 1. It was found that functional additives accounting for 10% of dry mass of powdered beverage significantly diversified low-molecular-weight antioxidants and antioxidant properties.

Table 1.
Characteristic of powders mixtures—low-molecular-weight antioxidants and antioxidant properties.
The total phenolic content in the basal (control) composition was estimated at 3.6 mg GAE/g. The functional additives significantly increased the amount of phenolics in a range of 4.9–6.9 mg GAE/g (an increase by 36–92%, respectively). The highest phenolic content was recorded for MO2 enriched with the raspberry powder (6.9 mg GAE/g). The content of carotenoids was similar in all samples, and there were no significant differences between the control and enriched powders. The highest increase in the vitamin C content was determined in the samples enriched with the strawberry powder (MO3, 2.0 mg/g; an increase by 186% compared to the control). A slight increase compared to the initial composition was also recorded in the MO4 and MO6 samples enriched with apples, as well as raspberry and apple powder. The increase in phenolics and vitamin C observed after partial replacement of sprouted lentil flour with lyophilized fruit was reflected in elevation of antioxidant capacity. The highest antiradical activity was recorded for the MO4 sample (6.2 mg TE/g). Surprisingly, compared to the control sample, the MO2, MO5, and MO8 samples had a lower ability to quench free radicals by 8%, 28%, and 36%, respectively. The reducing power ranged from 2.2 to 4.2 mg TE/g for the basal powders (MO1) and raspberry-enriched powders (MO2), respectively. With respect to the control, the increases in reducing abilities were between 23% and 91%.
The content of bioactive compounds in each mixture is a sum of individual components, which indirectly depends on their variety, harvest season, method of extraction, or solvent used. Ogunjobi and Ogunwolu [46] showed that the quality of cassava flour biscuit was improved by supplementation with apple powder. Similarly, addition of mango peel powder into biscuits resulted in higher polyphenol and carotenoid contents and improved antioxidant activity [47]. It was previously indicated that the activity of phenolics depends on conditions such as pH and interactions of phenolics with other dietary constituents released during digestion, e.g., iron, other minerals, dietary fiber, or proteins. All these factors are known to influence polyphenol solubility and availability [48]. The decrease in the antioxidant ability (MO2, MO5, MO8) observed in the present study may be a result of antagonistic interactions between the ingredients of various food matrices. This was previously reported by Gawlik-Dziki [49] in a study of a commonly prepared culinary mixture of vegetables, where inhibitors of lipoxygenases from tomato/garlic or tomato/lettuce acted antagonistically. Similarly, Wang, Meckling, Marcone, Kakuda, and Tsao [50] found an antagonistic effect in a combination of raspberry/legume or raspberry/blackberry shown by the TRAP assay.
Consumer perception of a new product should be regarded as a key strategy in the development of novel food products. The powders were rehydrated with water at two temperatures (20 °C and 80 °C) to reflect cold and hot beverages, respectively. Next, the beverages were subjected to consumer analysis, which covered quality-related properties including optical properties (color, appearance, consistency), sensory properties (aroma, taste, flavor), and overall acceptability. The results of the sensory panel are presented in Figure 1. The functional additives increased the value of color acceptance significantly. These values ranged from 3–7. The highest values for color were recorded for MO3 (enriched with strawberry) and MO5 (with the addition of raspberry and strawberry) in the case of “cold” rehydration and for MO2 (enriched with raspberry) and MO3 after “warm” hydration. Lentil sprouts, i.e., the basal source in our beverages, showed that the “beany” and “insipid” flavor negatively influenced the acceptance of the drinks. Probably, the lentil taste was still perceived; however, these undesirable organoleptic properties were slightly improved by enrichment with 10% of the functional additives. The best results were observed after the addition of raspberry (MO2). The supplemented beverages were well accepted by the panelists; however, higher overall acceptability was noted for beverages obtained at 20 °C.

Figure 1.
Consumer quality of powdered beverages: A—rehydration at 20 °C; B—rehydration at 80 °C; MO1—control beverage; MO2—beverage enriched with 10% raspberry; MO3—beverage enriched with 10% strawberry; MO4—beverage enriched with 10% apple; MO5—beverage enriched with 5% raspberry and 5% strawberry; MO6—beverage enriched with 5% raspberry and 5% apple; MO7—beverage enriched with 5% strawberry and 5% apple; MO8—beverage enriched with 4% apple, 3% raspberry, and 3% strawberry;.
Sun-Waterhouse [3] described challenges in developing drinks; high insoluble fiber content in a drink causes sensory issues like grittiness, which leads to lower consumer acceptability. On the other hand, consumer acceptance and interest are focused on naturally colored foods rich in naturally present colorants such as carotenoids or anthocyanins [51].
The wetting and rehydration stage is the key step in the process of powdered beverage manufacture. After dehydration, powdered beverages should retain the color, flavor, and nutritional value of the raw materials [12]. Knowledge of the rehydration properties of lyophilized ingredients can contribute to improvement of the quality of ready-to-drink products.
The color parameters of rehydrated samples are presented in Table 2. In general, the color of each beverage was very similar, and only slight differences in individual parameters were noted. All drinks had the same base (90%), the content of which dominated in the final color of the individual samples. The color coordinates of MO1 indicated that the additives had a slight influence on the lightness (L*) of the beverages. In the case of beverages rehydrated at the lower temperature, the minimum value for L* (31.50) was obtained for MO4, whereas the maximum value of this parameter (33.03) was found for the sample with the 10% addition of apple and strawberry. The color component a* (redness) changed across a rather narrow range from 6.59 (control sample) to 9.61 (sample with the 10% addition of apple and strawberry). The redness of MO1 (control beverage) was significantly lower compared to that of the drinks with the addition of fruits. The values for yellowness (b*) ranged from 12.53 (MO7) to 14.68 (MO4). Except MO4, the yellowness of the control beverage (MO1) obtained at 20 °C was significantly higher compared to the other beverages. The loss in the b* value indicates that the yellowness of the sample decreased when the functional lyophilizates were added. It may also be related to partial decomposition of carotenoids and generation of brown pigments. An opposite result was found for samples rehydrated at 80 °C, where the functional additives significantly increased the yellowness of the beverages.

Table 2.
Differences in physical properties after different rehydration temperatures application.
The rehydration at the higher temperature resulted in a slight decrease in the pH value. The pH of the cold drinks ranged between 5.16 and 6.22, while those rehydrated at 80 °C had a pH value from 4.99 to 5.92. It may be suggested that organic acids were better soluble at the higher temperature, which was previously confirmed in the study of Priecina and Karklina [52] on steamed carrot and pumpkin. There were slight differences in the WAI and WSI parameters in the powdered beverages; however, they did not exceed 10%. The loose porous structure of dried materials makes water penetration easy; hence, it can be expected that the rehydration in hot water may help to release bound compounds and cause decomposition of the main compounds responsible for the color parameters. On the other hand, tissue structure disruption and technological procedures were found to cause degradation of natural colorants. Roongruangsri and Bronlund [51] reported a high correlation between color changes and carotenoid content in pumpkin powders. The degradation of carotenoids was shown to be mainly induced by temperature and oxygen. Jirasatid, Chaikham, and Nopharatana [53] found that, after treatment at 80 °C, the content of carotenoids in banana–pumpkin puree decreased by ca. 20%. Additionally, enzymes present in tissue may be involved in degradation of pigments. The most visible losses of green color following chlorophyll degradation were related to non-enzymatic processes (changes in pH) and the action of specific enzymes such as chlorophyllase. Moreover, PPO and POD responsible for oxidation of phenolics may generate dark-pigmented quinones. The effect of these enzymes on color development was previously reported in shredded iceberg lettuce [54], broccoli [55], or apple and pear purées [56]. On the one hand, a higher temperature of rehydration may destroy native enzymes responsible for the undesirable changes; however, on the other hand, it accelerates thermal degradation of chlorophylls and carotenoids.
The content of total starch, resistant starch, and free reducing sugars in the beverages, as well as the effect of the functional additives and temperature of reconstitution, are presented in Figure 2. The highest content of total starch was recorded for the basal powder (MO1), and the lowest value was found for the MO5 sample enriched with the raspberry and strawberry lyophilizate. As a rich source of starch, lentil sprouts determined the high content of total starch in the beverages. In turn, in the enriched beverages, it was replaced with lyophilized fruits, which caused a significant decrease in the content of this compound. There were no significant differences in the level of resistant starch between the tested samples; however, the addition of raspberries caused a ca. 12% increase, compared to MO1. The functional additives significantly increased the content of free reducing sugars in the beverages. The observed increase ranged between 3% and 30% for the raspberry-enriched drink and the beverage supplemented with the combination of three additives (apple, raspberry, and strawberry), respectively. There were some differences in the starch digestibility parameter.
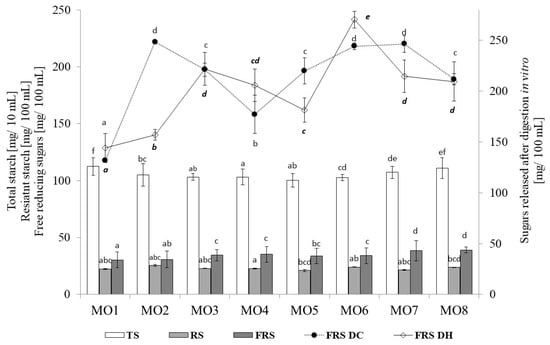
Figure 2.
Total starch, resistant starch, and free reducing sugars, in the powders and after digestion in vitro. MO1—control beverage; MO2—beverage enriched with 10% raspberry; MO3—beverage enriched with 10% strawberry; MO4—beverage enriched with 10% apple; MO5—beverage enriched with 5% raspberry and 5% strawberry; MO6—beverage enriched with 5% raspberry and 5% apple; MO7- beverage enriched with 5% strawberry and 5% apple; MO8- beverage enriched with 4% apple, 3% raspberry, and 3% strawberry; TS—total starch, RS—resistant starch, FRS—free reducing sugars, FRS DC—free reducing sugars in digested beverages rehydrated at 20 °C, FRS DH—free reducing sugars in digested beverages rehydrated at 80 °C.
Surprisingly, the addition of the phenolic-rich powders to the beverages increased starch digestibility (regardless of the temperature of rehydration). The highest amounts of sugar were released after digestion of MO6 rehydrated at 80 °C, i.e., there was a ca. 88% increase compared to the control beverage. There was no effect of the temperature in the MO1, MO3, and MO8 samples; however, reconstitution of MO2, MO5, and MO7 at 20 °C improved starch digestibility. High temperatures usually loosen the structure of food ingredients, but this aspect seems to be marginal in the case of powdered beverages.
Figure 3 shows the results of the analysis of the protein, free amino acid, and peptide content. The protein content differed statistically significantly between the beverages and ranged between 245 and 294 mg/100 mL. The functional additives reduced the protein content; the lowest value was determined for the MO6 beverages with the addition of raspberry and apple (245 mg/100 mL). The lower protein content may have been caused by the reduction of the amount of lentil sprouts, which are the main source of protein in the formulas of the enriched beverages. The content of FRAA ranged from 125 mg/100 mL to 139 mg/100 mL. As indicated by the in vitro digestion results, the reconstitution at the higher temperature clearly promoted the release of amino acids and enhanced their potential bioaccessibility. There is no information in the literature about changes in amino acid content in various fruit-based formulation of beverages; however, there are some reports on the increase in the protein and amino acid content induced by addition of legume flour [57,58].

Figure 3.
Total starch, resistant starch, and free reducing sugars, in the powders and after digestion in vitro. MO1—control beverage; MO2—beverage enriched with 10% raspberry; MO3—beverage enriched with 10% strawberry; MO4—beverage enriched with 10% apple; MO5—beverage enriched with 5% raspberry and 5% strawberry; MO6- beverage enriched with 5% raspberry and 5% apple; MO7—beverage enriched with 5% strawberry and 5% apple; MO8- beverage enriched with 4% apple, 3% raspberry, and 3% strawberry; P—proteins, FAA—free amino acids, FAA DC—free amino acids in digested beverages rehydrated at 20 °C, FAA DH—free amino acids in digested beverages rehydrated at 80 °C.
The results shown in Table 3 demonstrate that the addition of functional powders was a successful attempt at increasing the antioxidant potential and reducing power of rehydrated powdered beverages. Two different mechanisms of antioxidant activity were examined in all the samples: free radical-scavenging ability (ABTS) and reducing power (RP). The highest antioxidant activities were obtained for the beverages supplemented with 10% of raspberry, at both lower and higher temperatures of rehydration. The increase in the ability to quench ABTS radicals for MO2 rehydrated at 20 °C and 80 °C was estimated at 38% and 31%, respectively. In comparison to the control, the antioxidant potential of the beverages rehydrated at both temperatures was significantly higher in the supplemented beverages. It was in the range of 8.5–10.4 mg TE/100 mL and 9.7–12.1 mg TE/100 mL in the beverages rehydrated at 20 °C and 80 °C, respectively. Generally, the beverages reconstituted at the higher temperature showed a greater ability to quench radicals and corresponding reducing power. Furthermore, similar trends in the ability of the rehydrated beverages to reduce Fe3+ ions (RP) were observed.

Table 3.
Antioxidant properties of beverages rehydrated in 20 °C and 80 °C.
Most importantly, the beverages obtained after the simulated digestion processes (both with the control and with the addition of functional powders) were characterized by significantly higher antioxidant potentials than the other ones. These results suggest higher bioavailability of these compounds. Between the two temperatures of rehydration, the bioaccessibility of antioxidants was surprisingly higher at 20 °C. The in vitro digestion significantly influenced reducing power and antioxidant capacity, while optimal results of overall bioaccessibility were obtained for MO4 and MO7. Generally, it was observed in our study that the bioaccessible fractions of antioxidants in the gastric medium were almost similar to or higher than those natively found in the reconstituted samples.
The in vitro digestion released compounds with strong abilities to quench radicals. Such behavior was previously observed after in vitro digestion of bread enriched with onion skin [59], fruit and vegetable juices [60], and powdered seasonings [61]. The bioactivity of food ingredients depends primarily on their bioaccessibility in the gastrointestinal tract, and secondly on the food matrix. Previous in vitro studies confirm that the digestion process may promote the release of bioactive compounds from a food matrix responsible for antioxidant abilities and support reliable prediction of bioaccessibility [7,62]
4. Conclusions
Powdered beverages can be an excellent source of fruit and vegetables in the diet. It appears from the present study that functional components diversify the sensory quality, thus improving the overall acceptability. However, an equally important parameter is the rehydration temperature. In this case, the beverages rehydrated at the higher temperature were not positively assessed by the evaluation panel. Unfortunately, it seems that the addition of fruits cannot totally cover the unpleasant “beany” odor of lentil sprouts. Powdered fruits and vegetables have great potential as valuable ingredients for functional food production such as powdered beverages due to their high content of biologically active compounds and contribution to the organoleptic features of food. Differences in the antioxidant content related to the different rehydration temperatures were observed. As such, antioxidants also play an important role in the maintenance of the overall quality of products. The designed powdered beverages exhibited high antioxidant content, reasonable consumer acceptance, and good in vitro bioaccessibility. This research promotes reduction of food waste, since whole plant tissues were used, thus allowing maximum exploitation of food raw materials; however, the study is only a preliminary step for possible commercial production in the future.
Author Contributions
Conceptualization, J.B.-N. and M.Ś.; methodology, J.B.-N. and M.Ś.; software, J.B.-N.; validation, J.B.-N.; formal analysis, J.B.-N.; investigation, J.B.-N.; resources, J.B.-N.; data curation, J.B.-N.; writing—original draft preparation, J.B.-N.; writing—review and editing, J.B.-N. and M.Ś.; visualization, J.B.-N.; supervision, M.Ś. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pang, G.; Xie, J.; Chen, Q.; Hu, Z. How functional foods play critical roles in human health. Food Sci. Hum. Wellness 2012, 1, 26–60. [Google Scholar] [CrossRef]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Review of dried fruits: Phytochemicals, antioxidant efficacies, and health benefits. J. Funct. Foods 2016, 21, 113–132. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D. The development of fruit-based functional foods targeting the health and wellness market: A review. Int. J. Food Sci. Technol. 2011, 46, 899–920. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Ryan, L. Improving public health? The role of antioxidant-rich fruit and vegetable beverages. FRIN 2011, 44, 3135–3148. [Google Scholar] [CrossRef]
- Neacsu, M.; Vaughan, N.; Raikos, V.; Multari, S.; Duncan, G.J.; Duthie, G.G.; Russell, W.R. Phytochemical profile of commercially available food plant powders: Their potential role in healthier food reformulations. Food Chem. 2015, 179, 159–169. [Google Scholar] [CrossRef]
- Ganesan, K. Polyphenol-rich lentils and their health promoting effects. Int. J. Mol. Sci. 2017, 18, 2390. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. Changes in vitamin C, phenolic, and carotenoid profiles throughout in vitro gastrointestinal digestion of a blended fruit juice. J. Agric. Food Chem. 2013, 61, 1859–1867. [Google Scholar] [CrossRef]
- Queiroz, V.A.; da Silva Aguiar, A.; de Menezes, C.B.; de Carvalho, C.W.; Paiva, C.L.; Fonseca, P.C.; da Conceição, R.R. A low calorie and nutritive sorghum powdered drink mix: Influence of tannin on the sensorial and functional properties. J. Cereal Sci. 2018, 79, 43–49. [Google Scholar] [CrossRef]
- Hasan, M.U.; Ullah, A.; Sajid, M.; Imtiaz, A. Modern drying techniques in fruits and vegetables to overcome postharvest losses: A review. J. Food Process. Preserv. 2019, 43, 1–15. [Google Scholar] [CrossRef]
- Müller, L.; Gnoyke, S.; Popken, A.M.; Böhm, V. Antioxidant capacity and related parameters of different fruit formulations. LWT Food Sci. Technol. 2010, 43, 992–999. [Google Scholar] [CrossRef]
- Camire, M.E.; Dougherty, M.P.; Briggs, J.L. Functionality of fruit powders in extruded corn breakfast cereals. Food Chem. 2007, 101, 765–770. [Google Scholar] [CrossRef]
- Karam, M.C.; Petit, J.; Zimmer, D.; Baudelaire, E.; Marie, C. Effects of drying and grinding in production of fruit and vegetable powders: A review. J. Food Eng. 2016, 188, 32–49. [Google Scholar] [CrossRef]
- Argyropoulos, D.; Heindl, A.; Muller, J. Assessment of convection, hot-air combined with microwave- vacuum and freeze-drying methods for mushrooms with regard to product quality. Int. J. Food Sci. Technol. 2011, 46, 333–342. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Mujumdar, A.S.; Tang, J.; Miao, S.; Wang, Y. Recent developments in high-quality drying of vegetables, fruits, and aquatic products. Crit. Rev. Food Sci. Nutr. 2017, 57, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Costa-Font, M.; Gil, M.; Traill, W.B. Consumer acceptance, valuation of and attitudes towards genetically modified food: Review and implications for food policy. Food Policy 2008, 33, 99–111. [Google Scholar] [CrossRef]
- Piemontese, L. Plant Food Supplements with Antioxidant Properties for the Treatment of Chronic and Neurodegenerative Diseases: Benefits or Risks? J. Diet. Suppl. 2017, 14, 478–484. [Google Scholar] [CrossRef]
- Moncalvo, A.; Marinoni, L.; Dordoni, R.; Garrido, G.D.; Lavelli, V.; Spigno, G. Waste grape skins: Evaluation of safety aspects for the production of functional powders and extracts for the food sector. Food Addit. Contam. Part A 2016, 33, 1116–1126. [Google Scholar] [CrossRef]
- Lim, H.; Lee, H.J.; Choue, R.; Wang, Y. Trends in fast-food and sugar-sweetened beverage consumption and their association with social environmental status in south korea. J. Acad. Nutr. Diet. 2018, 118, 1228–1236. [Google Scholar] [CrossRef]
- Nazir, M.; Arif, S.; Sanaullah, R.; Nazir, W.; Khalid, N. Opportunities and challenges for functional and medicinal beverages: Current and future trends. Trends Food Sci. Technol. 2019, 88, 513–526. [Google Scholar] [CrossRef]
- Shittu, T.A.; Lawal, M.O. Factors affecting instant properties of powdered cocoa beverages. Food Chem. 2007, 100, 91–98. [Google Scholar] [CrossRef]
- Diah, R.; Budiwati, T.A.; Kosasih, W.; Pudjiraharti, S. Sensory and physicochemical evaluation od instant ginger drinks fortified with DFA III. Procedia Chem. 2015, 16, 177–183. [Google Scholar]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted grains: A comprehensive review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Świeca, M.; Gawlik-Dziki, U. Effects of sprouting and postharvest storage under cool temperature conditions on starch content and antioxidant capacity of green pea, lentil and young mung bean sprouts. Food Chem. 2015, 185, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Nawirska-Olszańska, A.; Biesiada, A.; Sokół-Łętowska, A.; Kucharska, A.Z. Characteristics of organic acids in the fruit of different pumpkin species. Food Chem. 2014, 148, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.; Lee, C.; Song, G.; Kim, Y. Characterization of the pectic polysaccharides from pumpkin peel. LWT-Food Sci. Technol. 2006, 39, 554–561. [Google Scholar] [CrossRef]
- Adams, G.G.; Imran, S.; Wang, S.; Mohammad, A.; Kok, S.; Gray, D.A.; Channell, G.A.; Morris, G.A.; Harding, S.E. The hypoglycaemic effect of pumpkins as anti-diabetic and functional medicines. Food Res. Int. 2011, 44, 862–867. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Dong, L. Inhibitory effect of polysaccharides from pumpkin on advanced glycation end-products formation and aldose reductase activity. Food Chem. 2012, 130, 821–825. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Hall, R.D.; Capanoglu, E. A Review on the Effect of Drying on Antioxidant Potential of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2016, 56, S110–S129. [Google Scholar] [CrossRef]
- Asami, D.K.; Hong, Y.J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef]
- Gündeşli, M.A.; Korkmaz, N.; Okatan, V. Polyphenol content and antioxidant capacity of berries: A review. Int. J. Agric. For. Life Sci. 2019, 3, 350–361. [Google Scholar]
- Raudone, L.; Raudonis, R.; Liaudanskas, M.; Viskelis, J.; Pukalskas, A.; Janulis, V. Phenolic profiles and contribution of individual compounds to antioxidant activity of apple powders. J. Food Sci. 2016, 81, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.C.; do Nascimento da Silva, E.; de Souza, A.O.; Vieira, M.A.; Ribeiro, A.S.; Cadore, S. Evaluation of the bioaccessibility of minerals from blackberries, raspberries, blueberries and strawberries. J. Food Compos. Anal. 2018, 68, 73–78. [Google Scholar] [CrossRef]
- Moazzem, M.S.; Sikder, M.B.H.; Zzaman, W. Shelf-Life Extension of Wood Apple Beverages Maintaining Consumption-Safe Parameters and Sensory Qualities. Beverages 2019, 5, 25. [Google Scholar] [CrossRef]
- Sobota, A.; Rzedzicki, Z.; Zarzycki, P.; Kuzawińska, E. Application of common wheat bran for the industrial production of high-fibre pasta. Int. J. Food Sci. Technol. 2015, 50, 111–119. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrì, F.; Boutrou, R.; Corredig, F.M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Bochnak, J.; Świeca, M. Potentially bioaccessible phenolics, antioxidant capacities and the colour of carrot, pumpkin and apple powders—Effect of drying temperature and sample structure. Int. J. Food Sci. Technol. 2020, 55, 136–145. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Campos, F.M.; Ribeiro, S.M.R.; Della Lucia, C.M.; Pinheiro-Sant’ana, H.M. Optimization of methodology to analyze ascorbic and dehydroascorbic acid in vegetables. Quim. Nova 2009, 32, 87–91. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 2002, 27, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Ogunjobi, M.A.K.; Ogunwolu, S.O. Physicochemical and sensory properties of cassava flour biscuits suplemented with cashew apple powder. J. Food Technol. 2010, 8, 24–29. [Google Scholar] [CrossRef]
- Ajila, C.M.; Leelavathi, K.; Prasada Rao, U.J.S. Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peel powder. J. Cereal Sci. 2008, 48, 319–326. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, 6–15. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U. Changes in the antioxidant activities of vegetables as a consequence of interactions between active compounds. J. Funct. Foods 2012, 4, 872–882. [Google Scholar] [CrossRef]
- Wang, S.; Meckling, K.A.; Marcone, M.F.; Kakuda, Y.; Tsao, R. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J. Agric. Food Chem. 2011, 59, 960–968. [Google Scholar] [CrossRef]
- Roongruangsri, W.; Bronlund, J.E. A review of drying processes in the production of pumpkin powder. Int. J. Food Eng. 2015, 11, 789–799. [Google Scholar] [CrossRef]
- Priecina, L.; Karklina, D. Composition of major organic acids in vegetables and spices. CBU Int. Conf. Proc. 2015, 3, 447–454. [Google Scholar] [CrossRef]
- Jirasatid, S.; Chaikham, P.; Nopharatana, M. Thermal degradation kinetics of total carotenoids and antioxidant activity in banana-pumpkin puree using Arrhenius, Eyring-Polanyi and Ball models. Int. Food Res. J. 2018, 25, 1912–1919. [Google Scholar]
- Sikora, M.; Złotek, U.; Świeca, M. Effect of basil leaves and wheat bran water extracts on enzymatic browning of shredded storage iceberg lettuce. Int. J. Food Sci. Technol. 2020, 55, 1318–1325. [Google Scholar] [CrossRef]
- Funamoto, Y.; Yamauchi, N.; Shigyo, M. Involvement of peroxidase in chlorophyll degradation in stored broccoli (Brassica oleracea L.) and inhibition of the activity by heat treatment. Postharvest Biol. Technol. 2003, 28, 39–46. [Google Scholar] [CrossRef]
- Parpinello, G.P.; Chinnici, F.; Versari, A.; Riponi, C. Preliminary study on glucose oxidase–catalase enzyme system to control the browning of apple and pear purees. LWT-Food Sci. Technol. 2002, 35, 239–243. [Google Scholar] [CrossRef]
- Mridula, D.; Sharma, M. Development of non-dairy probiotic drink utilizing sprouted cereals, legume and soymilk. LWT-Food Sci. Technol. 2015, 62, 482–487. [Google Scholar] [CrossRef]
- Chavan, M.; Gat, Y.; Harmalkar, M.; Waghmare, R. Development of non-dairy fermented probiotic drink based on germinated and ungerminated cereals and legume. LWT-Food Sci. Technol. 2018, 91, 339–344. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D.; Baraniak, B.; Tomiło, J.; Czyz, J. Quality and antioxidant properties of breads enriched with dry onion (Allium cepa L.) skin. Food Chem. 2013, 138, 1621–1628. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin—Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Del Pino-García, R.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; García-Lomillo, J.; Muñiz, P. Total antioxidant capacity of new natural powdered seasonings after gastrointestinal and colonic digestion. Food Chem. 2016, 211, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Świeca, M.; Sȩczyk, Ł.; Gawlik-Dziki, U.; Dziki, D. Bread enriched with quinoa leaves – the influence of protein—Phenolics interactions on the nutritional and antioxidant quality. Food Chem. 2014, 162, 54–62. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).