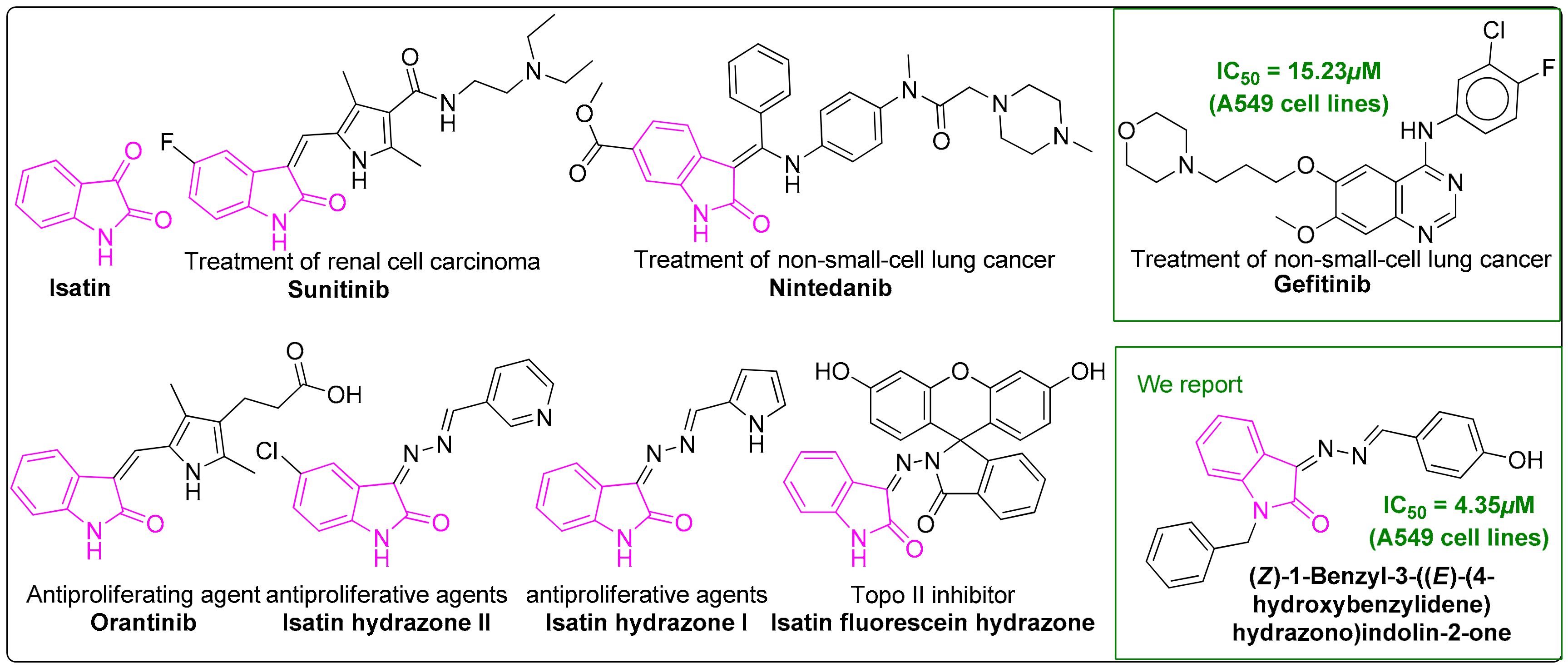
Synthesis of Novel Potent Biologically Active N-Benzylisatin-Aryl Hydrazones in Comparison with Lung Cancer Drug ‘Gefitinib’
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents Were of Commercial Reagent Grade (Sigma-Aldrich, St. Louis, MO, USA) and Used without Further Purification
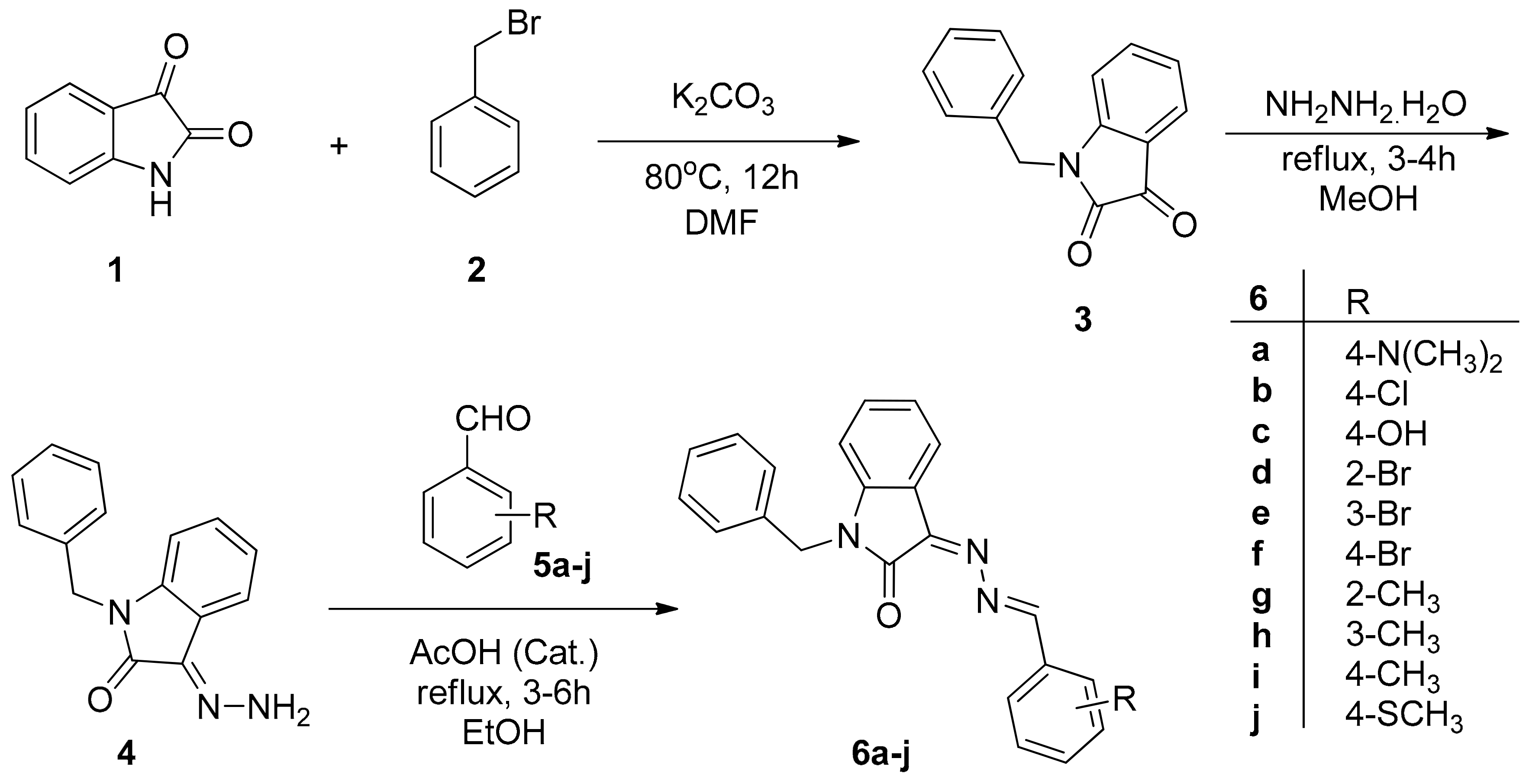
2.2. Preparation and Characterization of Target Compounds
2.2.1. 1-Benzylindoline-2,3-dione (3)
2.2.2. (Z)-1-Benzyl-3-hydrazonoindolin-2-one (4)
2.2.3. General Procedure for the Synthesis of 6a–j
1-Benzyl-3-((4-(dimethylamino) benzylidene)hydrazono)indolin-2-one (6a)
1-Benzyl-3-(((4-chlorocyclohexa−1, 5-dien-1-yl) methylene)hydrazono)indolin-2-one (6b)
1-Benzyl-3-((4-hydroxybenzylidene)hydrazono)indolin-2-one (6c)
1-Benzyl-3-((2-bromobenzylidene)hydrazono)indolin-2-one (6d)
1-Benzyl-3-((E)-(3-bromobenzylidene)hydrazono)indolin-2-one (6e)
1-Benzyl-3-((4-bromobenzylidene)hydrazono)indolin-2-one (6f)
1-Benzyl-3-((2-methyl benzylidene)hydrazono)indolin-2-one (6g)
1-Benzyl-3-((3-methylbenzylidene)hydrazono)indolin-2-one (6h)
1-Benzyl-3-((4-methylbenzylidene)hydrazono)indolin-2-one (6i)
1-Benzyl-3-((4-(methylthio)benzylidene)hydrazono)indolin-2-one (6j)
2.3. Antimicrobial Evaluation
2.4. Antiproliferative Assay
2.5. In Silico ADME Prediction Analysis
3. Results and Discussion
3.1. Synthesis of 6a–j
3.2. Biological Evaluation
3.2.1. Antimicrobial Evaluation of 6a–j
3.2.2. Antiproliferative Activity of 6a–j
3.3. Structure Activity Relationships (SARs) of 6a–j
3.4. In Silico Drug Likeness Property Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crespo-Ortiz, M.P.; Wei, M.Q. Antitumor activity of artemisinin and its derivatives: From a well-known antimalarial agent to a potential anticancer drug. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. The Drug Development Paradigm in Oncology: Proceedings of a Workshop; The National Academies Press: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- Burotto, M.; Manasanch, E.E.; Wilkerson, J.; Fojo, T. Gefitinib and erlotinib in metastatic non-small cell lung cancer: A meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist 2015, 20, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Hsiue, E.H.-C.; Lee, J.-H.; Lin, C.-C.; Yang, J.C.-H. Safety of gefitinib in non-small cell lung cancer treatment. Expert Opin. Drug Saf. 2016, 15, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.N.; Lord, S.J.; Gebski, V.; Links, M.; Bray, V.; Gralla, R.J.; Yang, J.C.-H.; Lee, C.K. Risk of treatment-related toxicities from EGFR tyrosine kinase inhibitors: A meta-analysis of clinical trials of gefitinib, erlotinib, and afatinib in advanced EGFR-mutated non-small cell lung cancer. J. Thorac. Oncol. 2016, 12, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Atagi, S.; Goto, K.; Hida, T.; Horai, T.; Inoue, A.; Ichinose, Y.; Koboyashi, K.; Takeda, K.; Kiura, K.; et al. LUX-Lung 4: A phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J. Clin. Oncol. 2013, 31, 3335–3341. [Google Scholar] [CrossRef]
- Roy, S.; Hagen, K.D.; Maheswari, P.U.; Lutz, M.; Spek, A.L.; Reedijk, J.; van Wezel, G.P. Phenanthroline derivatives with improved selectivity as DNA-targeting anticancer or antimicrobial drugs. ChemMedChem 2008, 3, 1427–1434. [Google Scholar] [CrossRef]
- O’Shea, R.; Moser, H.E. Physicochemical properties of antibacterial compounds: Implications for drug discovery. J. Med. Chem. 2008, 51, 2871–2878. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Vine, K.L.; Matesic, L.; Locke, J.M.; Skropeta, D. Recent highlights in the development of isatin-based anticancer agents. Adv. Anticancer Agents Med. Chem. 2013, 2, 254–312. [Google Scholar] [CrossRef]
- Hajare, R.; Kulkarni, S.; Thakar, M.; Paranjape, R. Isatin anti-HIV agent: A review. World J. Pharm. Pharm. Sci. 2016, 5, 569–575. [Google Scholar] [CrossRef]
- Pakravan, P.; Kashanian, S.; Khodaei, M.M.; Harding, F.J. Biochemical and pharmacological characterization of isatin and its derivatives: From structure to activity. Pharmacol. Rep. 2013, 65, 313–335. [Google Scholar] [CrossRef]
- Li, C.; Zhang, F. Single step incorporation of isatin to enaminone: A recyclable catalyst towards assembly of diverse four ring fused pyrrolo[2,3,4-kl]acridin-1-ones. RSC Adv. 2016, 6, 75359–75364. [Google Scholar] [CrossRef]
- Loloiu, G.; Loloiu, T.; Maior, O. Chemistry of isatin. Synthesis of 2,3-oxo-1H-pyrrolo[2,3-b]dibenzo-p-dioxins. Rev. Chim. (Buchar.) 1998, 49, 861–864. [Google Scholar]
- Popp, F.D. Chemistry of isatin. Adv. Heterocycl. Chem. 1975, 18, 1–58. [Google Scholar]
- Rajopadhye, M. The chemistry of isatin and isatin derivatives: Synthesis of novel ring systems. Diss. Abstr. Int. B 1988, 48, 2325–2326. [Google Scholar]
- Sumpter, W.C. The chemistry of isatin. Chem. Rev. 1944, 34, 393–434. [Google Scholar] [CrossRef]
- Da Silva, J.F.M.; Garden, S.J.; Pinto, A.C. The chemistry of isatins: A review from 1975 to 1999. J. Braz. Chem. Soc. 2001, 12, 273–324. [Google Scholar] [CrossRef]
- Lashgari, N.; Mohammadi Ziarani, G. Synthesis of Heterocyclic Compounds Based on Isatin Through 1,3-Dipolar Cycloaddition Reactionsp; ARKIVOC: Gainesville, FL, USA, 2012. [Google Scholar]
- Medvedev, A.; Igosheva, N.; Crumeyrolle-Arias, M.; Glover, V. Isatin: Role in stress and anxiety. Stress 2005, 8, 175–183. [Google Scholar] [CrossRef]
- Vine, K.L.; Matesic, L.; Locke, J.M.; Ranson, M.; Skropeta, D. Cytotoxic and anticancer activities of isatin and its derivatives: A comprehensive review from 2000–2008. Anti-Cancer Agents Med. Chem. 2009, 9, 397–414. [Google Scholar] [CrossRef]
- Matesic, L.; Locke, J.M.; Bremner, J.B.; Pyne, S.G.; Skropeta, D.; Ranson, M.; Vine, K.L. N-Phenethyl and N-naphthylmethyl isatins and analogues as in vitro cytotoxic agents. Bioorg. Med. Chem. 2008, 16, 3118–3124. [Google Scholar] [CrossRef]
- Han, K.; Zhou, Y.; Liu, F.; Guo, Q.; Wang, P.; Yang, Y.; Song, B.; Liu, W.; Yao, Q.; Teng, Y.; et al. Design, synthesis and in vitro cytotoxicity evaluation of 5-(2-carboxyethenyl)isatin derivatives as anticancer agents. Bioorg. Med. Chem. Lett. 2014, 24, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.S.; Abou-seri, S.M.; Ismail, N.S.M.; Elaasser, M.M.; Aly, M.H.; Abdel-Aziz, H.A. Bis-isatin hydrazones with novel linkers: Synthesis and biological evaluation as cytotoxic agents. Eur. J. Med. Chem. 2016, 108, 415–422. [Google Scholar] [CrossRef]
- Pervez, H.; Ahmad, M.; Zaib, S.; Yaqub, M.; Naseer, M.M.; Iqbal, J. Synthesis, cytotoxic and urease inhibitory activities of some novel isatin-derived bis-Schiff bases and their copper(II) complexes. MedChemComm 2016, 7, 914–923. [Google Scholar] [CrossRef]
- Ganguly, S.; Debnath, B. Molecular docking studies and ADME prediction of novel isatin analogs with potent anti-EGFR activity. Med. Chem. (Los Angeles, CA, USA) 2014, 4, 558–568. [Google Scholar] [CrossRef]
- Rahman, A.F.M.M.; Park, S.-E.; Kadi, A.A.; Kwon, Y. Fluorescein hydrazones as novel nonintercalative topoisomerase catalytic inhibitors with low DNA toxicity. J. Med. Chem. 2014, 57, 9139–9151. [Google Scholar] [CrossRef]
- Dweedar, H.E.; Mahrous, H.; Ibrahim, H.S.; Abdel-Aziz, H.A. Analogue-based design, synthesis and biological evaluation of 3-substituted-(methylenehydrazono)indolin-2-ones as anticancer agents. Eur. J. Med. Chem. 2014, 78, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Altoukhy, A.; Mahrous, H.; Abdel-Aziz, H.A. Design, synthesis and QSAR study of certain isatin-pyridine hybrids as potential anti-proliferative agents. Eur. J. Med. Chem. 2015, 90, 684–694. [Google Scholar] [CrossRef]
- Islam, M.S.; Al-Majid, A.M.; El-Senduny, F.F.; Badria, F.A.; Rahman, A.F.M.M.; Barakat, A.; Elshaier, Y.A.M.M. Synthesis, anticancer activity, and molecular modeling of new halogenated spiro[pyrrolidine-thiazolo-oxindoles] derivatives. Appl. Sci. 2020, 10, 2170. [Google Scholar] [CrossRef]
- Kadi, A.A.; Al-Shakliah, N.S.; Rahman, A.F.M.M. Synthesis and fragmentation behavior study of n-alkyl/benzyl Isatin derivatives present in small/complex molecules: Precursor for the preparation of biological active heterocycles. Mass Spectrom. Lett. 2015, 6, 65–70. [Google Scholar] [CrossRef]
- Coffey, K.E.; Moreira, R.; Abbas, F.Z.; Murphy, G.K. Synthesis of 3,3-dichloroindolin-2-ones from isatin-3-hydrazones and (dichloroiodo)benzene. Org. Biomol. Chem. 2015, 13, 682–685. [Google Scholar] [CrossRef]
- Popp, F.D. Potential anticonvulsants. IX. Some isatin hydrazones and related compounds. J. Heterocycl. Chem. 1984, 21, 1641–1645. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Stewart, M.J.; Watson, I.D. Standard units for expressing drug concentrations in biological fluids. Br. J. Clin. Pharmacol. 1983, 16, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Arifuzzaman, M.; Mitra, S.; Jahan, S.I.; Jakaria, M.; Abeda, T.; Absar, N.; Dash, R. A Computational workflow for the identification of the potent inhibitor of type II secretion system traffic ATPase of Pseudomonas aeruginosa. Comput. Biol. Chem. 2018, 76, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Murukan, B.; Kumari, B.S.; Mohanan, K. Synthesis, spectral, electrochemical and antibacterial studies of copper(II) complexes with isatin derived bishydrazone and different co-ligands. J. Coord. Chem. 2007, 60, 1607–1617. [Google Scholar] [CrossRef]
- Ekins, S.; Waller, C.L.; Swaan, P.W.; Cruciani, G.; Wrighton, S.A.; Wikel, J.H. Progress in predicting human ADME parameters in silico. J. Pharmacol. Toxicol. Methods 2000, 44, 251–272. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Duffy, E.M. Prediction of drug solubility from structure. Adv. Drug Deliv. Rev. 2002, 54, 355–366. [Google Scholar] [CrossRef]
- Chemi, G.; Gemma, S.; Campiani, G.; Brogi, S.; Butini, S.; Brindisi, M. Computational tool for fast in silico evaluation of hERG K+ channel affinity. Front. Chem. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Han, Y.; Hopfinger, A.J. Predicting Caco-2 cell permeation coefficients of organic molecules using membrane-interaction QSAR. J. Chem. Inf. Comput. Sci. 2002, 42, 331–342. [Google Scholar] [CrossRef]
- Clark, D.E. In silico prediction of blood-brain barrier permeation. Drug Discov. Today 2003, 8, 927–933. [Google Scholar] [CrossRef]
- Lionta, E.; Spyrou, G.; Vassilatis, D.K.; Cournia, Z. Structure-based virtual screening for drug discovery: Principles, applications and recent advances. Curr. Top. Med. Chem. 2014, 14, 1923–1938. [Google Scholar] [CrossRef] [PubMed]
| Compound | 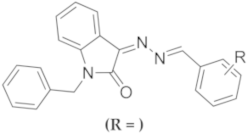 | IC50 ( µM) | |
|---|---|---|---|
| A549 | Hela | ||
| 4 | – | 14 ± 2.15 | 46 ± 1.30 |
| 6a | 4-N(CH3)2 | 23.7 ± 0.69 | 25.1 ± 0.95 |
| 6b | 4-Cl | 22 ± 1.11 | 14.8 ± 0.94 |
| 6c | 4-OH | 4.35 ± 0.05 | 4 ± 0.09 |
| 6d | 2-Br | 7.3 ± 0.52 | 6.7 ± 0.17 |
| 6e | 3-Br | 41 ± 1.20 | 9.1 ± 0.35 |
| 6f | 4-Br | 7.30 ± 0.03 | 7.4 ± 0.20 |
| 6g | 2-CH3 | 7.5 ± 0.11 | 7.7 ± 0.21 |
| 6h | 3-CH3 | 8.4 ± 0.25 | 13.4 ± 0.35 |
| 6i | 4-CH3 | 17 ± 0.70 | 18.5 ± 0.65 |
| 6j | 4-SCH3 | 14.6 ± 0.53 | 9 ± 0.86 |
| Gefitinib | – | 15.2 ± 0.48 | 7.3 ± 0.42 |
| No. | MW b | HBD c | HBA d | logPo/w e | logS f | logP | HERGg | Caco-2 h | BBB i | MDCKj | HOA(%) k |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6a | 382 | 0 | 6.5 | 4.77 | −5.76 | 10.2 | −7.35 | 3189 | −0.36 | 1733.1 | 100 |
| 6b | 374 | 0 | 5.5 | 4.86 | −5.60 | 9.4 | −7.16 | 3720 | −0.02 | 5054.6 | 100 |
| 6c | 355 | 1 | 6.25 | 3.82 | −5.07 | 11.8 | −7.13 | 1102 | −0.83 | 549.7 | 100 |
| 6d | 418 | 0 | 5.5 | 4.87 | −5.58 | 9.5 | −7.19 | 3656 | −0.04 | 4745.7 | 100 |
| 6e | 418 | 0 | 5.5 | 4.93 | −5.74 | 9.4 | −7.20 | 3637 | −0.02 | 5296.9 | 100 |
| 6f | 418 | 0 | 5.5 | 4.93 | −5.72 | 9.4 | −7.19 | 3719 | −0.01 | 5433.8 | 100 |
| 6 g | 353 | 0 | 5.5 | 4.61 | −5.30 | 9.4 | −7.14 | 3754 | −0.19 | 2067.3 | 100 |
| 6 h | 353 | 0 | 5.5 | 4.67 | −5.46 | 9.4 | −7.17 | 3638 | −0.21 | 1998.3 | 100 |
| 6i | 353 | 0 | 5.5 | 4.67 | −5.44 | 9.3 | −7.16 | 3734 | −0.20 | 2054.9 | 100 |
| 6j | 385 | 0 | 6.0 | 5.01 | −5.93 | 9.7 | −7.38 | 3479 | −0.21 | 3246.6 | 100 |
| Gl | 447 | 1 | 7.7 | 4.37 | −5.23 | 10.8 | −7.12 | 1053 | 0.31 | 2313.1 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Salem, H.S.; Abuelizz, H.A.; Issa, I.S.; Mahmoud, A.Z.; AlHoshani, A.; Arifuzzaman, M.; Rahman, A.F.M.M. Synthesis of Novel Potent Biologically Active N-Benzylisatin-Aryl Hydrazones in Comparison with Lung Cancer Drug ‘Gefitinib’. Appl. Sci. 2020, 10, 3669. https://doi.org/10.3390/app10113669
Al-Salem HS, Abuelizz HA, Issa IS, Mahmoud AZ, AlHoshani A, Arifuzzaman M, Rahman AFMM. Synthesis of Novel Potent Biologically Active N-Benzylisatin-Aryl Hydrazones in Comparison with Lung Cancer Drug ‘Gefitinib’. Applied Sciences. 2020; 10(11):3669. https://doi.org/10.3390/app10113669
Chicago/Turabian StyleAl-Salem, Huda S., Hatem A. Abuelizz, Iman S. Issa, Amany Z. Mahmoud, Ali AlHoshani, Md Arifuzzaman, and A. F. M. Motiur Rahman. 2020. "Synthesis of Novel Potent Biologically Active N-Benzylisatin-Aryl Hydrazones in Comparison with Lung Cancer Drug ‘Gefitinib’" Applied Sciences 10, no. 11: 3669. https://doi.org/10.3390/app10113669
APA StyleAl-Salem, H. S., Abuelizz, H. A., Issa, I. S., Mahmoud, A. Z., AlHoshani, A., Arifuzzaman, M., & Rahman, A. F. M. M. (2020). Synthesis of Novel Potent Biologically Active N-Benzylisatin-Aryl Hydrazones in Comparison with Lung Cancer Drug ‘Gefitinib’. Applied Sciences, 10(11), 3669. https://doi.org/10.3390/app10113669






