Trapping Entomopathogenic Fungi from Vine Terroir Soil Samples with Insect Baits for Controlling Serious Pests
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
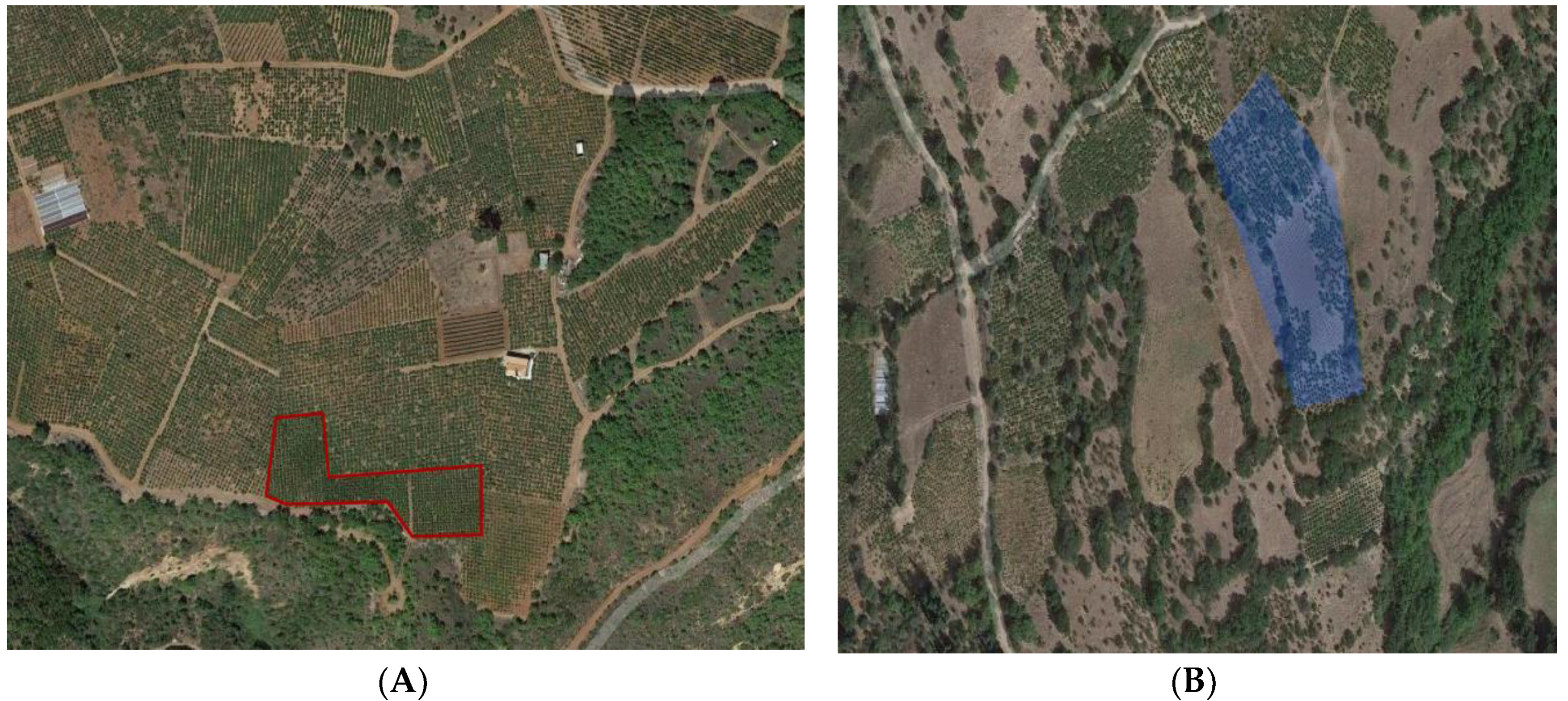
2.2. Sampling—Entrapment of Entomopathogenic Fungi
2.3. Isolation of Entomopathogenic Fungi and Conidia Identification Method
2.4. Colony Forming Units of Fungi in Vine Terroir Soil
2.5. Statistical Analysis
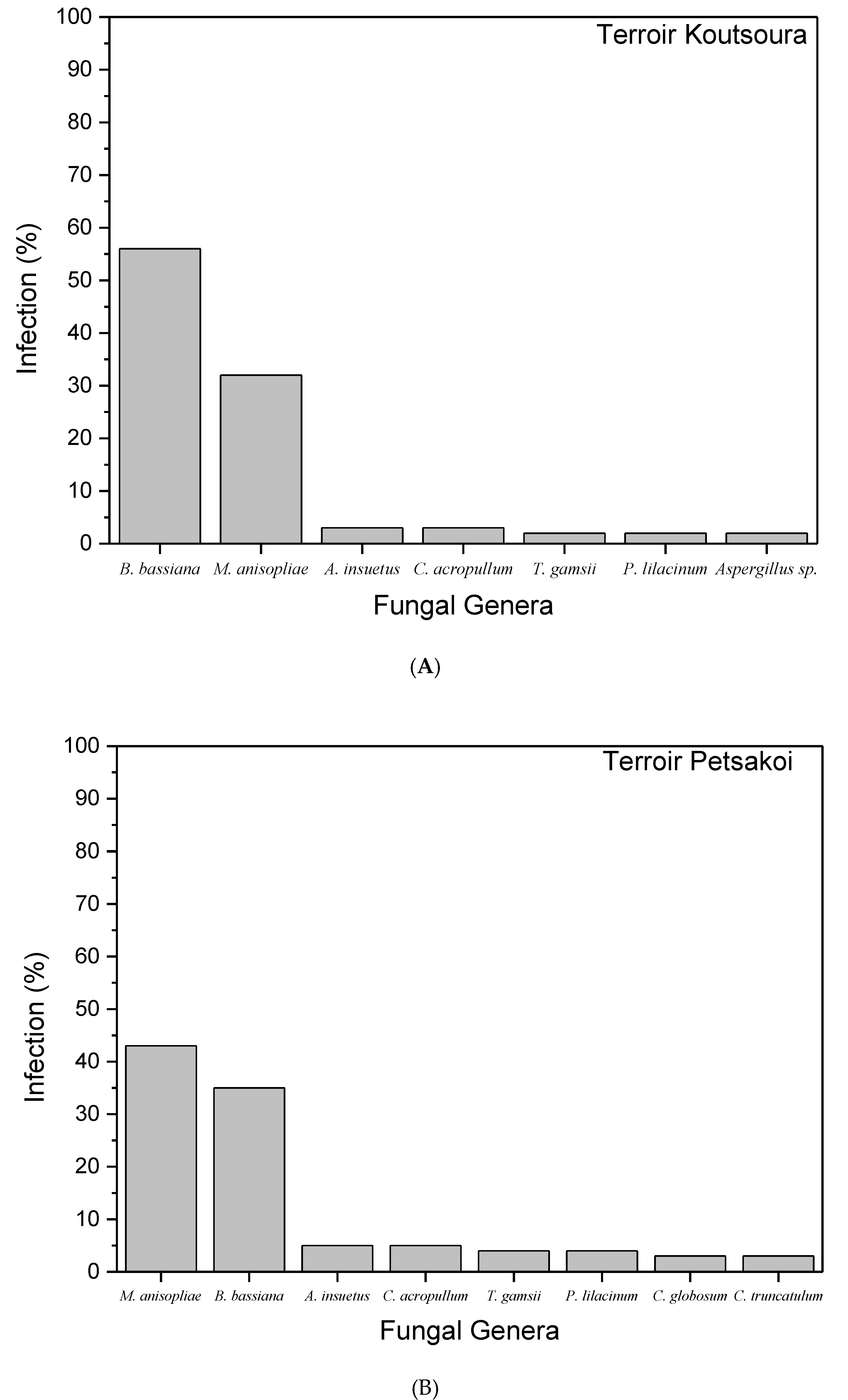
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mantzoukas, S.; Lagogiannis, I.; Mpekiri, M.; Pettas, I.; Eliopoulos, P. Insecticidal action of several isolates of entomopathogenic fungi against the granary weevil Sitophilus granarius. Agriculture 2019, 9, 222. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Eliopoulos, P. Endophytic Entomopathogenic Fungi: A Valuable Biological Control Tool against Plant Pests. Applied Science, SI: Endophytic Entomopathogenic Fungi: New approach for controlling serious pests. Appl. Sci. 2010, 10, 370. [Google Scholar] [CrossRef]
- Vega, F.E.; Goettel, M.S.; Blackwell, M.; Chandler, D.; Jackson, M.A.; Keller, S.; Koike, M.; Maniania, N.K.; Monzo´n, A.; Ownley, B.H.; et al. Fungal ento- mopathogens: New insights on their ecology. Fungal. Ecol. 2009, 2, 149–159. [Google Scholar] [CrossRef]
- Tkaczuk, C.; Mietkiewski, R. Occurrence of entomopathogenic fungi in different kinds of soil. Rocz. Nauk. Rol. Ser. E 1996, 25, 43–48. [Google Scholar]
- Krysa, A.; Ropek, D.; Kuźniar, T. The occurrence of entomopathogenic fungi depending on season in selected organic farm. J. Res. Appl. Agric. Eng. 2012, 57, 226–230. [Google Scholar]
- Tkaczuk, C. Występowanie i potencjał infek-cyjny grzybów owadobójczych w glebach agroce-noza i środowisk seminaturalnych w krajobrazie rolniczym. (Occurrence and infective potential of entomopathogenic fungi in the soils of agrocenoses and semi-natural habitats in agricultural landscape). Rozpr. Nauk. Nr. 2008, 94, 160. [Google Scholar]
- Mantzoukas, S.; Pettas, I.; Lagogiannis, I. Stored product pests as models for trapping entomopathogenic fungi from olive tree orchards in Western Greece. J. Stored Prod. Res. 2020, 87, 101584. [Google Scholar] [CrossRef]
- Oliveira, I.; Pereira, J.A.; Quesada-Moraga, E.; Lino-Neto, T.; Bento, A.; Baptista, P. Effect of soil tillage on natural occurence of fungal entomopathogens associated to Prays oleae Bern (Lepidoptera, Plutellidae). Sci. Hortic. 2013, 159, 190–196. [Google Scholar] [CrossRef]
- Jabbour, R.; Barbercheck, M.E. Soil management effects on entomopathogenic fungi during the transition to organic agriculture in a feed grain rotation. Biol. Control 2009, 51, 435–443. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Navas-Cortes, J.A.; Maranhao, A.A.; Ortiz-Urquiza, A.; Santiago-Alvarez, C. Factors affecting the occurence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycol. Res. 2007, 111, 947–966. [Google Scholar] [CrossRef]
- Hummel, R.L.; Walegenbach, J.F.; Barbercheck, M.E.; Kennedy, G.G.; Hoyt, G.D.; Arellano, C. Effects of production practices on soil—Borne entomopathogens in western North Karolina vegetable systems. Environ. Entomolol. 2002, 31, 84–91. [Google Scholar] [CrossRef]
- Meyling, N.V.; Eilenberg, J. Occurrence and distribution of soil borne entomopathogenic fungi within a single organic agroecosystem. Agric. Ecosyst. Environ. 2006, 113, 336–341. [Google Scholar] [CrossRef]
- Keller, S.; Zimmerman, G. Mycopathogens of soil insects. In Insect–Fungus Interactions; Wilding, N., Collins, N.M., Hammond, P.M., Webber, J.F., Eds.; Academic Press: London, UK, 1989; pp. 240–270. [Google Scholar]
- Toledo, J.; Liedo, P.; Flores, S.; Campos, S.E.; Villaseñor, A.; Montoya, P.; Sugayama, R.; Zucchi, R.; Ovruski, S.; Sivinski, J. Use of Beauveria bassiana and Metarhizium anisopliae for fruit fly control: A novel approach. In Proceedings of the 7th International Symposium on Fruit Flies of Economic Importance, Salvador, Brazil, 10–15 September 2006; Research Organizations: Biofabrica Moscamed Brasil, Juazeiro, Brazil, 2006; pp. 127–132. [Google Scholar]
- Vänninen, I.; Husberg, G.; Hokkanen, H. Occurrence of entomopathogenic fungi and entomoparasitic nematodes in cultivated soils in Finland. Acta Entomol. Fenn. 1989, 53, 65–67. [Google Scholar]
- Kleespies, R.; Bathon, H.; Zimmermann, G. Untersuchen zum naturlichen Vorkommen von entomopathogenen Pilzen und Nematoden in verschiedenen Boden in der Umgebung von Darmstad. Gesunde Pflanz. 1989, 41, 350–355. [Google Scholar]
- Mietkiewski, R.; Tkaczuk, C.; Badowska-Czubik, T. Entomogenous fungi isolated from strawberry plantation soil infested by Otiorhynchus ovatus L. (Coleoptera, Curculionidae). Rocz. Nauk Rol. Ser. E 1993, 22, 39–46. [Google Scholar]
- Steenberg, T. Natural Occurrence of Beauveria bassiana (Bals.) Vuill. with Focus on Infectivity to Sitona Species and Other Insects in Lucerne. Ph.D. Thesis, The Royal Veterinary and Agricultural University, Frederiksberg, Denmark, 1995. [Google Scholar]
- Pilz, C.; Wegensteiner, R.; Keller, S. Natural occurrence of insect pathogenic fungi and insect parasitic nematodes in Diabrotica virgifera virgifera populations. Biocontrol 2006, 53, 353–359. [Google Scholar] [CrossRef]
- Oreste, M.; Bubici, G.; Poliseno, M.; Triggiani, O.; Tarasco, E. Pathogenicity of Beauveria bassiana (Bals.-Criv.) Vuill. and Metarhizium anisopliae (Metschn.) Sorokin against Galleria mellonella L. and Tenebrio molitor L. in laboratory assays. Redia 2012, 95, 43–48. [Google Scholar]
- Vaudour, E. The Quality of Grapes and Wine in Relation to Geography: Notions of Terroir at Various Scales. J. Wine Res. 2002, 13, 117–141. [Google Scholar] [CrossRef]
- Knight, S.; Klaere, S.; Fedrizzi, B.; Goddard, M.R. Regional microbial signatures positively correlate with differential wine phenotypes: Evidence for a microbial aspect to terroir. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Balafoutis, A.T.; Koundouras, S.; Anastasiou, E.; Fountas, S.; Konstantinos, A. Life Cycle Assessment of Two Vineyards after the Application of Precision Viticulture Techniques: A Case Study. Sustainability 2017, 9, 1997. [Google Scholar] [CrossRef]
- Cookson, W.R.; Marschner, P.; Clark, I.M.; Milton, N.; Smirk, M.N.; Murphy, D.V.; Osman, M.; Stockdale, E.A.; Hirsch, P.R. The influence of season, agricultural management, and soil properties on gross nitrogen transformations and bacterial community structure. Aust. J. Soil Res. 2006, 44, 453–465. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Meyling, N.V.; Hajek, A.E. Principles from community and metapopulation ecology: Application to fungal entomopathogens. BioControl 2010, 55, 39–54. [Google Scholar] [CrossRef]
- Zimmermann, G. The Galleria bait method for detection of entomopathogenic fungi in soil. J. Appl. Entomol. 1986, 102, 213–215. [Google Scholar] [CrossRef]
- Lingg, A.J.; Donaldson, M.D. Biotic and abiotic factors affecting stability of Beauveria bassiana conidia in soil. J. Invertebr. Pathol. 1981, 38, 191–200. [Google Scholar] [CrossRef]
- Smith, R.J.; Grula, E.A. Toxic components on the larval surface of the corn earworm (Heliothis zea) and their effects on germination and growth of Beauveria bassiana. J. Invertebr. Pathol. 1982, 39, 15–22. [Google Scholar] [CrossRef]
- Bidochka, M.J.; Kasperski, J.E.; Wild, G.A. Occurrence of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana in soils from temperate and nearnorthern habitats. Can. J. Bot. Rev. Can. Bot. 1998, 76, 1198–1204. [Google Scholar] [CrossRef]
- Raid, R.N.; Cherry, R.H. Pathogenicity of Metarhizium anisopliae var. major (Metschnikoft) Sorokin to a Sugarcane Grub Ligyrus subtropicus (Blatchley) (Coleoptera: Scarabaeidae). Agric. Entomolol. 1992, 9, 11–16. [Google Scholar]
- Meyling, N.V.; Eilenberg, J. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biol. Control 2007, 43, 145–155. [Google Scholar] [CrossRef]
- St. Leger, R.J. Studies on adaptations of Metarhizium anisopliae to life in the soil. J. Invertebr. Pathol. 2008, 98, 271–276. [Google Scholar] [CrossRef]
- Pava-Ripoll, M.; Angelini, C.; Fang, W.; Wang, S.; Posada, F.J.; St. Leger, R. The rhizosphere-competent entomopathogen Metarhizium anisopliae expresses a specific subset of genes in plant root exudate. Microbiology 2011, 157, 47e55. [Google Scholar] [CrossRef] [PubMed]
- Eilenberg, J.; Hajek, A.; Lomer, C. Suggestions for unifying the terminology in biological control. Biocontrol 2001, 46, 387–400. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Mantzoukas, S.; Lagogiannis., I. Endophytic Colonization of Pepper (Capsicum anuum) controls Aphids (Myzus persicae Sulzer). Appl. Sci. 2019, 9, 2239. [Google Scholar] [CrossRef]
- Mantzoukas, S. Endophytic colonization of Solanum tuberosum L. (Solanales: Solanaceae) plants can affect the infestation of serious pests. Appl. Microbiol. 2020, 1, 1–7. [Google Scholar]
- Manoussopoulos, Y.; Mantzoukas, S.; Lagogiannis, I.; Goudoudaki, S.; Kambouris, M. Effect of three strawberry endophytic entomopathogenic fungi on the prefeeding behavior of the aphid Myzus persicae. J. Insect Behav. 2019, 32, 99–108. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Grammatikopoulos, G. The effect of three entomopathogenic endophytes of the sweet sorghum on the growth and feeding performance of its pest, Sesamia nonagrioides larvae, and their efficacy under field conditions. Crop Prot. 2019, 127, 104952. [Google Scholar] [CrossRef]
| Mortality Factor | Vine Terroir | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Koutsoura | Petsakoi | |||||||||||
| Insect baits | ||||||||||||
| P.t | R.d | T.c | C.f | S.z | S.o | P.t | R.d | T.c | C.f | S.z | S.o | |
| Entomopathogenic fungi | ||||||||||||
| B. bassiana | 83 ± 2 a | 80 ± 3 a | 86 ± 3 a | 82 ± 2 a | 53 ± 3 d | 53 ± 7 d | 34 ± 5 e | 32 ± 2 e | 28 ± 4 e | 0 | 0 | 0 |
| M. anisopliae | 0 | 10 ± 2 g | 0 | 18 ± 4 f | 45 ± 2 d | 36 ± 2 e | 62 ± 1 c | 56 ± 3 d | 65 ± 4 c | 72 ± 2 b | 78 ± 2 a | 72 ± 3 b |
| Fungi of unproved entomopathogenic abilities | ||||||||||||
| A. insuetus. | 3 ± 3 g | 2 ± 2 g | 4 ± 1 g | 0 | 0 | 1 ± 3 g | 0 | 4 ± 2 g | 0 | 3 ± 5 g | 6 ± 1 g | 5 ± 1 g |
| C. acropullum. | 3 ± 3 g | 0 | 2 ± 1g | 0 | 2 ± 4 g | 2 ± 3 g | 0 | 0 | 2 ± 3 g | 4 ± 2 g | 2 ± 3g | 5 ± 4 g |
| T. gamsii | 4 ± 2 g | 2 ± 2 g | 3 ± 3 g | 0 | 0 | 3 ± 3 g | 0 | 0 | 0 | 4 ± 2 g | 0 | 5 ± 4 g |
| P. lilacinum. | 6 ± 1 g | 5 ± 3 g | 2 ± 2 g | 0 | 0 | 4 ± 3 g | 0 | 0 | 2 ± 2 g | 0 | 2 ± 1 g | 6 ± 2 g |
| C. globosum | 0 | 0 | 0 | 0 | 0 | 0 | 2 ± 4 g | 6 ± 3 g | 3 ± 5 g | 4 ± 6 g | 2 ± 3 g | 3 ± 2 g |
| C. truncatulum | 0 | 0 | 0 | 0 | 0 | 0 | 2 ± 2 g | 2 ± 1 g | 0 | 2 ± 4 g | 2 ± 1 g | 3 ± 3 g |
| Aspergillus sp. | 1 ± 3 g | 1 ± 1 g | 1 ± 3 g | 0 | 0 | 1 ± 1 g | 0 | 0 | 0 | 0 | 0 | 0 |
| Other causes | ||||||||||||
| Unspecified causes | 0 | 0 | 4 ± 3 g | 0 | 0 | 0 | 0 | 0 | 0 | 1 ± 4 g | 8 ± 2 g | 1 ± 4 g |
| Dependent Variable: Mortality | |||
|---|---|---|---|
| Source | df | F | p-Value |
| Bait Species | 5 | 21,758 | 0.000 |
| Terroir | 1 | 3693 | 0.001 |
| Time of experiment | 6 | 11,349 | 0.000 |
| Bait * Terroir | 5 | 2754 | 0.001 |
| Bait * Time of experiment | 30 | 4749 | 0.000 |
| Terroir * Time of experiment | 6 | 1976 | 0.044 |
| Bait * Terroir * Time of experiment | 30 | 1668 | 0.010 |
| Error | 190 | ||
| Total | 289 | ||
| Corrected Total | 288 | ||
| Fungal Species | Vine Terroir | |
|---|---|---|
| Koutsoura | Petsakoi | |
| B. bassiana | 7.7 ± 2.1 a | 5.8 ± 1.2 a |
| M. anisopliae | 3.2 ± 2 a | 7.8 ± 1.6 a |
| A. insuetus | 1.4 ± 0.8 b | 1 ± 1 b |
| C. acropullum | 0.5 ± 0.2 b | 0.7 ± 0.2 b |
| T. gamsii | 0.5 ± 0.4 b | 0.6 ± 0.2 b |
| P. lilacinum | 1.2 ± 0.8 b | 1.1 ± 0.2 b |
| C. globosum | Not present | 0.7 ± 0.3 b |
| C. truncatulum | Not present | 0.7 ± 0.3 b |
| Aspergillus sp. | 0.2 ± 0.4 b | Not present |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantzoukas, S.; Lagogiannis, I.; Ntoukas, A.; Eliopoulos, P.A.; Kouretas, D.; Karpouzas, D.G.; Poulas, K. Trapping Entomopathogenic Fungi from Vine Terroir Soil Samples with Insect Baits for Controlling Serious Pests. Appl. Sci. 2020, 10, 3539. https://doi.org/10.3390/app10103539
Mantzoukas S, Lagogiannis I, Ntoukas A, Eliopoulos PA, Kouretas D, Karpouzas DG, Poulas K. Trapping Entomopathogenic Fungi from Vine Terroir Soil Samples with Insect Baits for Controlling Serious Pests. Applied Sciences. 2020; 10(10):3539. https://doi.org/10.3390/app10103539
Chicago/Turabian StyleMantzoukas, Spiridon, Ioannis Lagogiannis, Aristeidis Ntoukas, Panagiotis A. Eliopoulos, Demetrios Kouretas, Dimitrios G. Karpouzas, and Konstantinos Poulas. 2020. "Trapping Entomopathogenic Fungi from Vine Terroir Soil Samples with Insect Baits for Controlling Serious Pests" Applied Sciences 10, no. 10: 3539. https://doi.org/10.3390/app10103539
APA StyleMantzoukas, S., Lagogiannis, I., Ntoukas, A., Eliopoulos, P. A., Kouretas, D., Karpouzas, D. G., & Poulas, K. (2020). Trapping Entomopathogenic Fungi from Vine Terroir Soil Samples with Insect Baits for Controlling Serious Pests. Applied Sciences, 10(10), 3539. https://doi.org/10.3390/app10103539










