The Effect of Grain Type on Virulence of Entomopathogenic Fungi Against Stored Product Pests
Abstract
1. Introduction
2. Material and Methods
2.1. Insect Rearing
2.2. Entomopathogenic Fungi
2.3. Bioassays
2.4. Statistical Analysis
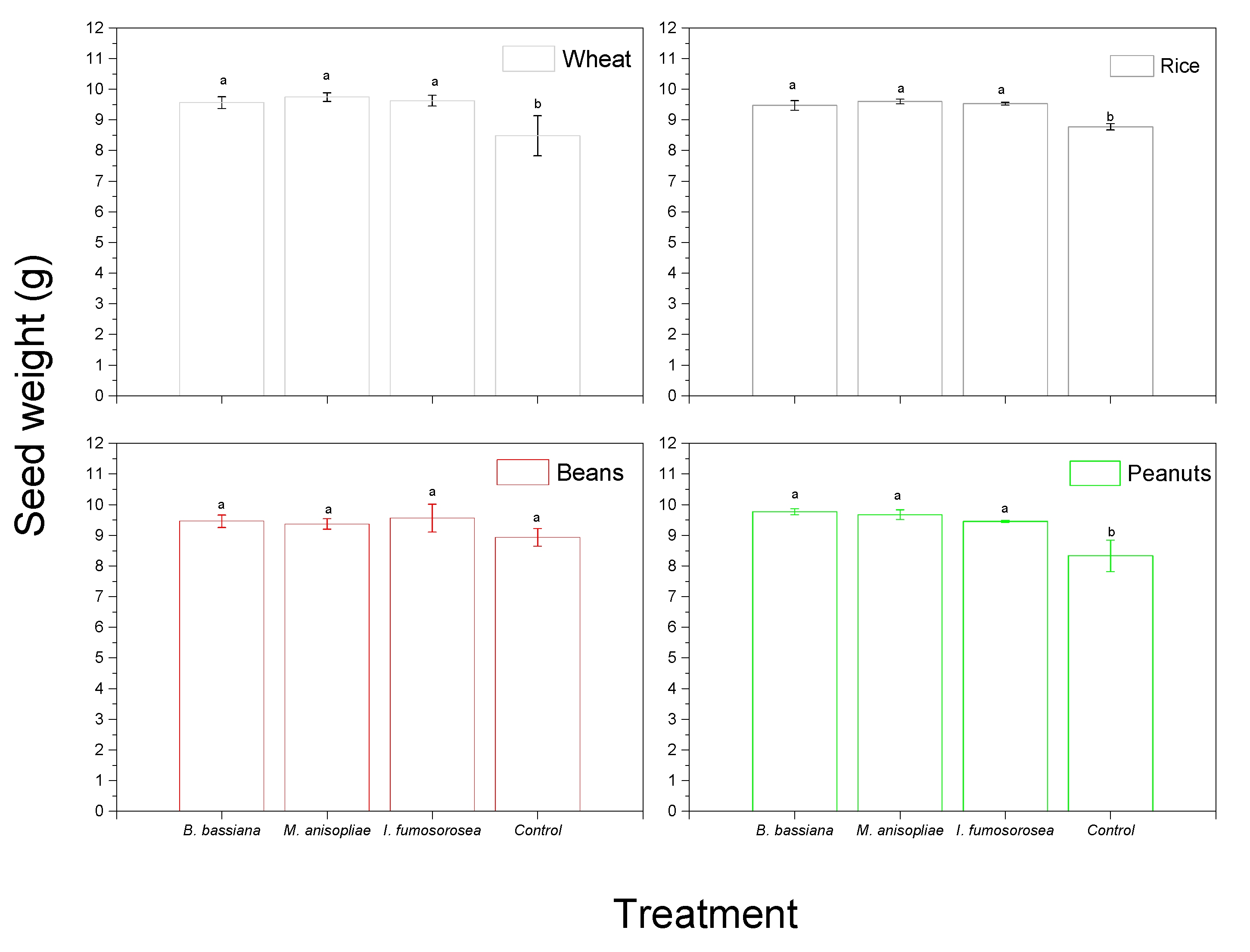
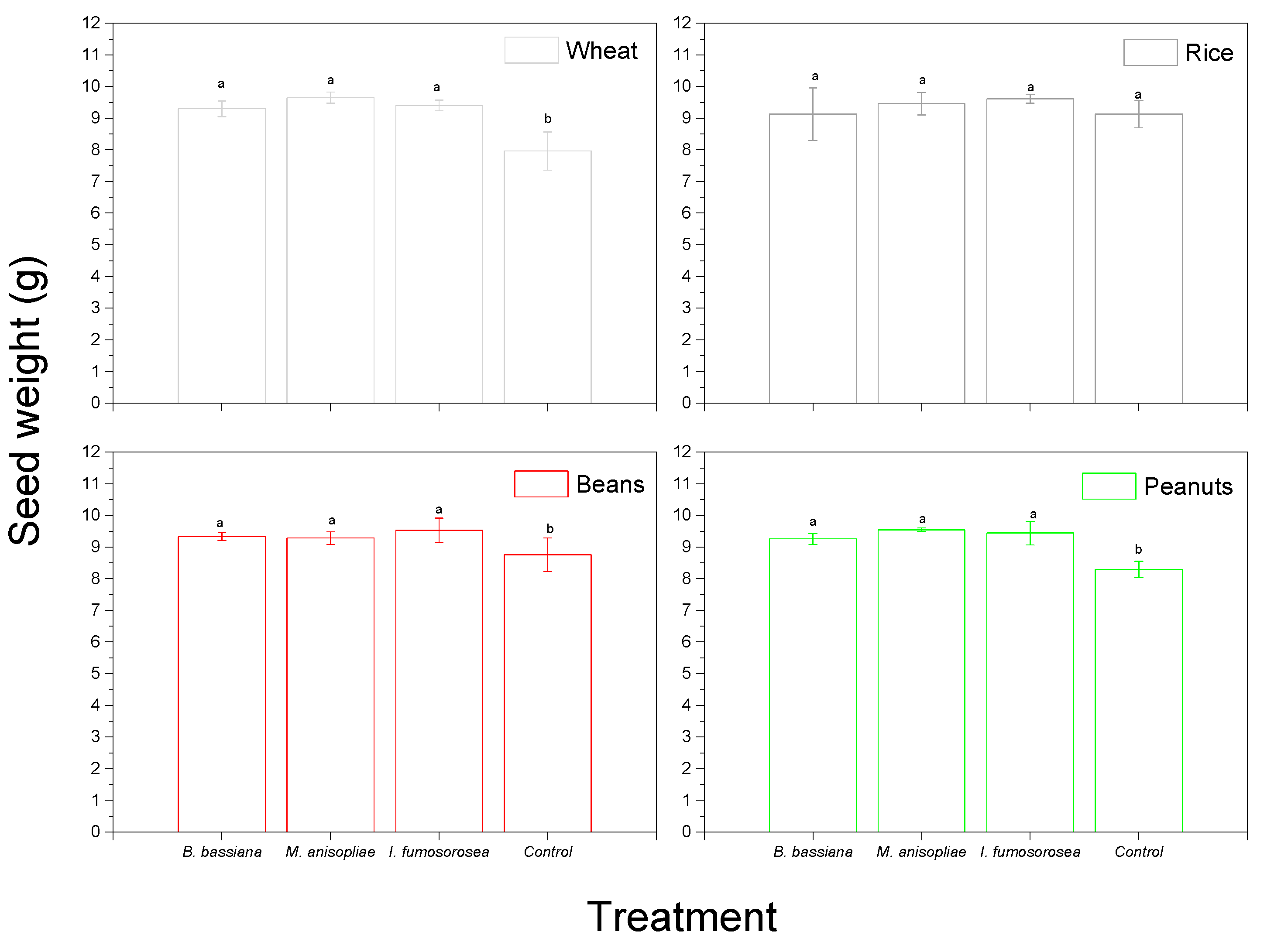
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flinn, P.; Hagstrum, D.; Phillips, T.; Armitage, D.M.; Bell, C.H.; Cogan, P.M.; Highley, E.; Reed, C.; Credland, P.F. Areawide integrated pest management program for commercial grain stores. In Advances in Stored Product Protection. Proceedings of the 8th International Working Conference on Stored Product Protection, York, UK, 22-26 July 2002; CABI Publishing: Wallingford, UK, 2003; pp. 99–102. [Google Scholar]
- Stejskal, V.; Hubert, J.; Aulicky, R.; Kucerova, Z. Overview of present and past and pest-associated risks in stored food and feed products: European perspective. J. Stored Prod. Res. 2015, 64, 122–132. [Google Scholar] [CrossRef]
- Phillips, T.W.; Throne, J.E. Biorational Approaches to Managing Stored-Product Insects. Annu. Rev. Èntomol. 2010, 55, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S. Insect Pest Management in Stored Products. Outlooks Pest Manag. 2020, 31, 24–35. [Google Scholar] [CrossRef]
- Subramanyam, B.; Hagstrum, D.W. (Eds.) Alternatives to Pesticides in Stored-Product IPM; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Canhilal, R. The use of entomopathogens in the controlling of insect pests of stored product. Sci. Pap. Ser. A Agron. 2016, 59, 235–240. [Google Scholar]
- Schöller, M.; Prozell, S.; Al-Kirshi, A.-G.; Reichmuth, C. Towards biological control as a major component of integrated pest management in stored product protection. J. Stored Prod. Res. 1997, 33, 81–97. [Google Scholar] [CrossRef]
- Wakil, W.; Ghazanfar, M.U.; Yasin, M. Naturally occurring entomopathogenic fungi infecting stored grain insect species in Punjab, Pakistan. J. Insect Sci. 2014, 14, 182. [Google Scholar] [CrossRef]
- Batta, Y.; Kavallieratos, N.G. The use of entomopathogenic fungi for the control of stored-grain insects. Int. J. Pest Manag. 2017, 64, 77–87. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Lagogiannis, I.; Mpekiri, M.; Pettas, I.; Eliopoulos, P.A. Insecticidal Action of Several Isolates of Entomopathogenic Fungi against The Granary Weevil Sitophilus granarius. Agriculture 2019, 9, 222. [Google Scholar] [CrossRef]
- Strasser, H.; Vey, A.; Butt, T. Are There any Risks in Using Entomopathogenic Fungi for Pest Control, with Particular Reference to the Bioactive Metabolites of Metarhizium, Tolypocladium and Beauveria species? Biocontrol Sci. Technol. 2000, 10, 717–735. [Google Scholar] [CrossRef]
- Zimmermann, G. Review on safety of the entomopathogenic fungusMetarhizium anisopliae. Biocontrol Sci. Technol. 2007, 17, 879–920. [Google Scholar] [CrossRef]
- Zimmermann, G. Review on safety of the entomopathogenic fungiBeauveria bassianaandBeauveria brongniartii. Biocontrol Sci. Technol. 2007, 17, 553–596. [Google Scholar] [CrossRef]
- Thomas, M.B.; Langewald, J.; Wood, S.N. Evaluating the Effects of a Biopesticide on Populations of the Variegated Grasshopper, Zonocerus variegatus. J. Appl. Ecol. 1996, 33, 1509. [Google Scholar] [CrossRef]
- Thomas, M.B.; Wood, S.N.; Langewald, J.; Lomer, C.J. Persistence ofMetarhizium flavovirideand Consequences for Biological Control of Grasshoppers and Locusts. Pestic. Sci. 1997, 49, 47–55. [Google Scholar] [CrossRef]
- Moore, D.; Lord, J.C.; Smith, S.M. Pathogens. In Alternatives to Pesticides in Stored-Product IPM; Springer: Boston, MA, USA, 2000; pp. 193–227. [Google Scholar]
- Samson, R.A.; Evans, H.C.; Latgé, J.P. Atlas of Entomopathogenic Fungi; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Shah, P.A.; Pell, J.K. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 2003, 61, 413–423. [Google Scholar] [CrossRef]
- McGuire, A.V.; Northfield, T.D. Tropical Occurrence and Agricultural Importance of Beauveria bassiana and Metarhizium anisopliae. Front. Sustain. Food Syst. 2020, 4, 6. [Google Scholar] [CrossRef]
- Mora, J.R.C.R.M.A.E. Occurrence of entomopathogenic fungi in atlantic forest soils. Microbiol. Discov. 2016, 4, 1. [Google Scholar] [CrossRef][Green Version]
- Akbar, W.; Lord, J.C.; Nechols, J.R.; Howard, R.W. Diatomaceous earth increases the efficacy of Beauveria bassiana against Tribolium castaneum larvae and increases conidia attachment. J. Econ. Entomol. 2004, 97, 273–280. [Google Scholar] [CrossRef]
- Sabbour, M.; El-Aziz, S.A.; Sherief, M. Efficacy of Three Entomopathogenic Fungi Alone or in Combination With Diatomaceous Earth Modifications for the Control of Three Pyralid Moths in Stored Grains. J. Plant Prot. Res. 2012, 52, 359–363. [Google Scholar] [CrossRef]
- Barra, P.; Rosso, L.; Nesci, A.; Etcheverry, M. Isolation and identification of entomopathogenic fungi and their evaluation against Tribolium confusum, Sitophilus zeamais, and Rhyzopertha dominica in stored maize. Journal of Pest Science 2012, 86, 217–226. [Google Scholar] [CrossRef]
- Batta, Y. Recent Advances in Formulation and Application of Entomopathogenic Fungi for Biocontrol of Stored-grain Insects. Biocontrol Sci. Technol. 2016, 26, 1–28. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Zikou, A.; Triantafillou, V.; Lagogiannis, I.; Eliopoulos, P.A. Ιnteractions between Beauveria bassiana and Isaria fumosorosea and Their Hosts Sitophilus granarius (L.) and Sitophilus oryzae (L.) (Coleoptera: Curculionidae). Insects 2019, 10, 362. [Google Scholar] [CrossRef]
- Ignoffo, C.M. The fungus Nomureae rileyi. Microb. Control Pest Plant Dis. 1981, 1970–1980, 523–538. [Google Scholar]
- Ignoffo, C.M.; Hostetter, D.L.; Sikorowski, P.P.; Sutter, G.; Brooks, W.M. Inactivation of Representative Species of Entomopathogenic Viruses, a Bacterium, Fungus, and Protozoan by an Ultraviolet Light Source. Environ. Èntomol. 1977, 6, 411–415. [Google Scholar] [CrossRef]
- Kish, L.P.; Allen, G.E. The biology and ecology of Nomuraea rileyi and a program for predicting its incidence on Anticarsia gemmatalis in soybean. Bulletin. Fla. Agric. Exp. Stn. 1978, 48, 795. [Google Scholar]
- Humber, A.R. Identification of entomopathogenic fungi. In Manual of Techniques in Invertebrate Pathology, 2nd ed.; Springer: Boston, MA, USA, 2012; pp. 151–187. [Google Scholar]
- Abbott, W.S. A method for computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- FAO. Prevention of Postharvest Food Losses; Training Series No 10; Food and Agricultural Organization: Rome, Italy, 1985; p. 122. [Google Scholar]
- Batta, Y. Control of rice weevil (Sitophilus oryzae L., Coleoptera: Curculionidae) with various formulations of Metarhizium anisopliae. Crop. Prot. 2004, 23, 103–108. [Google Scholar] [CrossRef]
- Cherry, A.; Abalo, P.; Hell, K. A laboratory assessment of the potential of different strains of the entomopathogenic fungi Beauveria bassiana (Balsamo) Vuillemin and Metarhizium anisopliae (Metschnikoff) to control Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) in stored cowpea. J. Stored Prod. Res. 2005, 41, 295–309. [Google Scholar] [CrossRef]
- Sewify, G.H.; El Shabrawy, H.A.; Eweis, M.E.; Naroz, M.H. Efficacy of entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae for controlling certain stored product insects. Egypt. J. Biol. Pest Control 2014, 24, 191. [Google Scholar]
- Fargues, J.; Goettel, M.S.; Smits, N.; Ouedraogo, A.; Vidal, C.; Lacey, L.A.; Lomer, C.J.; Rougier, M. Variability in susceptibility to simulated sunlight of conidia among isolates of entomopathogenic Hyphomycetes. Mycopathol. 1996, 135, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.D.; Wakefield, M.E.; Price, N.; Wildey, K.B.; Chambers, J.; Moore, D.; Aquino De Muro, M.; Bell, B.A. The potential use of insect-specific fungi to control grain storage pests in empty grain stores. HGCA Proj. Rep. 2004, 341, 1–49. [Google Scholar]
- Rice, W.C.; Cogburn, R.R. Activity of the Entomopathogenic Fungus Beauveria bassiana (Deuteromycota: Hyphomycetes) Against Three Coleopteran Pests of Stored Grain. J. Econ. Èntomol. 1999, 92, 691–694. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Rumbos, C.I.; Kontodimas, D.C. Influence of Temperature and Relative Humidity on the Insecticidal Efficacy of Metarhizium anisopliae against Larvae of Ephestia kuehniella (Lepidoptera: Pyralidae) on Wheat. J. Insect Sci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Draganova, S.; Markova, E. Bioassays with Isolates of Entomopathogenic Fungi against Ephestia kuhniella Zell.(Lepidoptera: Pyralidae). Bulg. J. Agric. Sci. 2006, 12, 637. [Google Scholar]
- Padı́n, S.; Bello, G.D.; Fabrizio, M. Grain loss caused by Tribolium castaneum, Sitophilus oryzae and Acanthoscelides obtectus in stored durum wheat and beans treated with Beauveria bassiana. J. Stored Prod. Res. 2002, 38, 69–74. [Google Scholar] [CrossRef]
- Adane, K.; Moore, D.; Archer, S. Preliminary studies on the use of Beauveria bassiana to control Sitophilus zeamais (Coleoptera: Curculionidae) in the laboratory. J. Stored Prod. Res. 1996, 32, 105–113. [Google Scholar] [CrossRef]
- Steinkraus, D.C.; Tugwell, N.P. Beauveria bassiana (Deuteromycotina: Moniliales) Effects on Lygus lineolaris (Hemiptera: Miridae). J. Èntomol. Sci. 1997, 32, 79–90. [Google Scholar] [CrossRef]
- Moino, A.; Alves, S.B.; Pereira, R.M. Efficacy ofBeauveria bassiana(Balsamo) Vuillemin isolates for control of stored-grain pests. J. Appl. Èntomol. 1998, 122, 301–305. [Google Scholar] [CrossRef]
- Hidalgo, E.; Moore, D.; Le Patourel, G. The effect of different formulations of Beauveria bassiana on Sitophilus zeamaisin stored maize. J. Stored Prod. Res. 1998, 34, 171–179. [Google Scholar] [CrossRef]


| (A) Variable: Mortality | |||
| Factor | df | F | Sig. |
| Insect | 2 | 5.665 | 0.000 |
| Product | 3 | 14.818 | 0.000 |
| Fungi | 3 | 50.484 | 0.000 |
| Insect * Product | 6 | 1.438 | 0.196 |
| Insect * Fungi | 6 | 1.576 | 0.150 |
| Product * Fungi | 9 | 3.834 | 0.000 |
| Insect * Product * Fungi | 18 | .971 | 0.491 |
| Error | 1392 | ||
| Total | 1440 | ||
| Corrected Total | 1439 | ||
| (B) Variable: Seed Weight | |||
| Factor | df | F | Sig. |
| Product | 3 | 1.799 | 0.153 |
| Insect | 2 | 12.341 | 0.000 |
| Fungi | 3 | 106.072 | 0.000 |
| Product * Insect | 6 | 1.515 | 0.181 |
| Product * Fungi | 9 | 3.976 | 0.000 |
| Insect * Fungi | 6 | 1.294 | 0.267 |
| Product * Insect * Fungi | 18 | 1.217 | 0.264 |
| Error | 96 | ||
| Total | 144 | ||
| Corrected Total | 143 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantzoukas, S.; Lagogiannis, I.; Karmakolia, K.; Rodi, A.; Gazepi, M.; Eliopoulos, P.A. The Effect of Grain Type on Virulence of Entomopathogenic Fungi Against Stored Product Pests. Appl. Sci. 2020, 10, 2970. https://doi.org/10.3390/app10082970
Mantzoukas S, Lagogiannis I, Karmakolia K, Rodi A, Gazepi M, Eliopoulos PA. The Effect of Grain Type on Virulence of Entomopathogenic Fungi Against Stored Product Pests. Applied Sciences. 2020; 10(8):2970. https://doi.org/10.3390/app10082970
Chicago/Turabian StyleMantzoukas, Spiridon, Ioannis Lagogiannis, Katerina Karmakolia, Anastasia Rodi, Maria Gazepi, and Panagiotis A. Eliopoulos. 2020. "The Effect of Grain Type on Virulence of Entomopathogenic Fungi Against Stored Product Pests" Applied Sciences 10, no. 8: 2970. https://doi.org/10.3390/app10082970
APA StyleMantzoukas, S., Lagogiannis, I., Karmakolia, K., Rodi, A., Gazepi, M., & Eliopoulos, P. A. (2020). The Effect of Grain Type on Virulence of Entomopathogenic Fungi Against Stored Product Pests. Applied Sciences, 10(8), 2970. https://doi.org/10.3390/app10082970







