A Dynamic Multiple Reaction Monitoring Analytical Method for the Determination of Fungicide Residues in Drinking Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
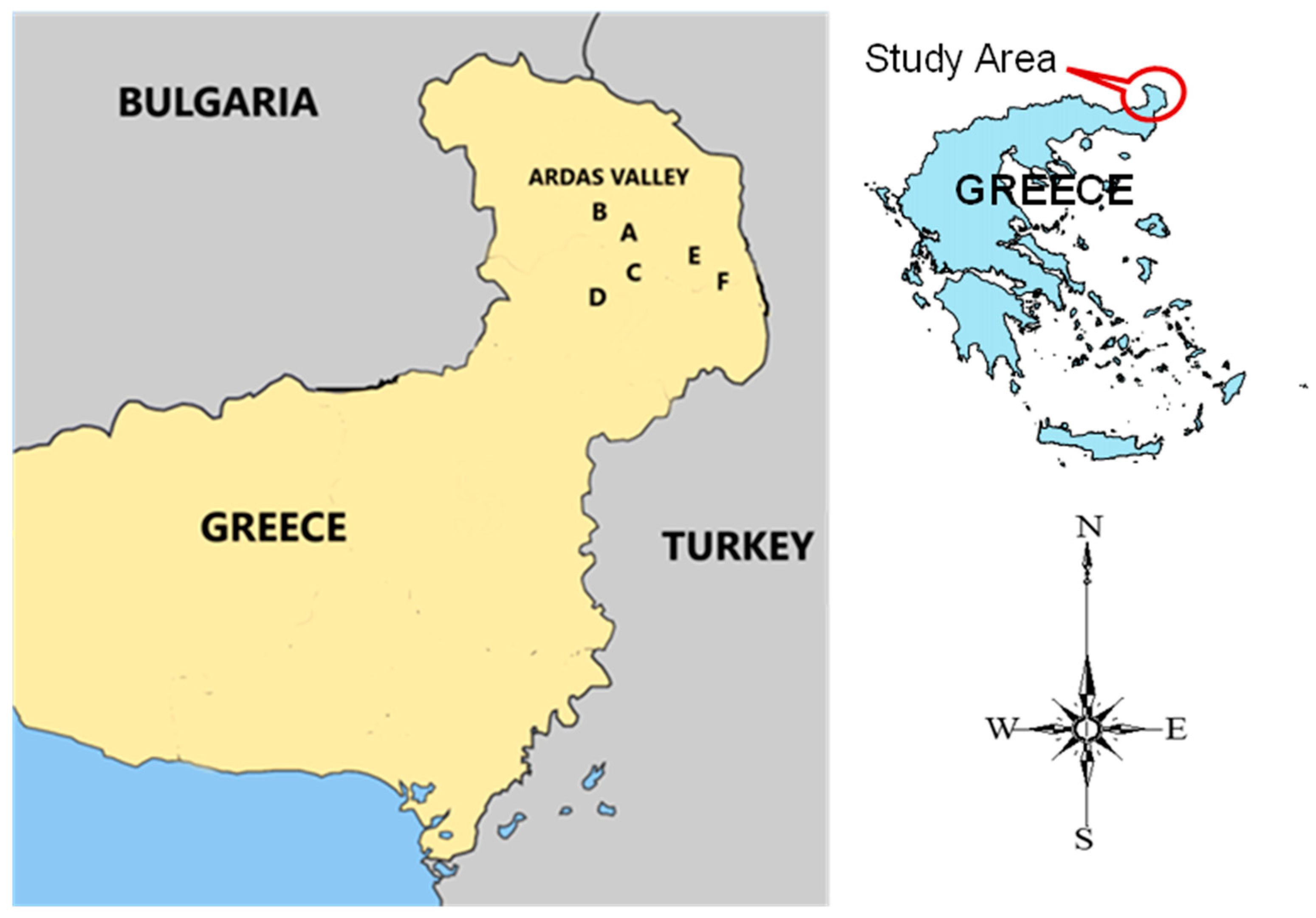
2.2. Study Area Target Fungicides Selection and Sampling
2.3. Sample Preparation and Instrumental Analysis
2.4. Method Validation
2.5. Human Health Risk Assessment
2.5.1. Chronic Daily Intake
2.5.2. Hazard Quotient (Non-Carcinogenic Risk Assessment)
2.5.3. Carcinogenic Risk Assessment
3. Results and Discussion
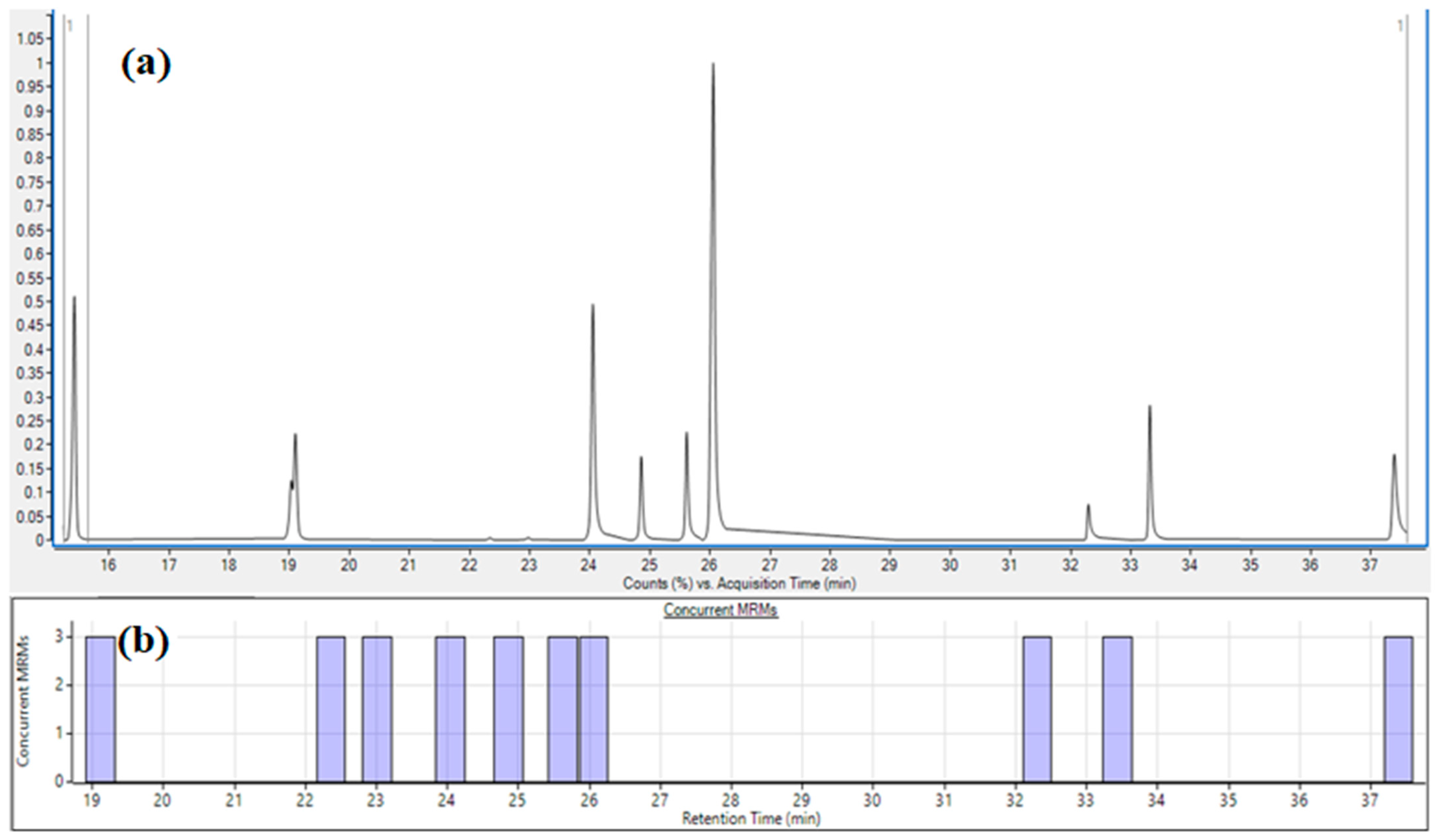
3.1. Development of the Multiple Reaction Monitoring Method for Fungicide Determination
3.2. Separation Distribution, Peak Shape, and Sensitivity Using the Dynamic Multiple Reaction Monitoring Mode
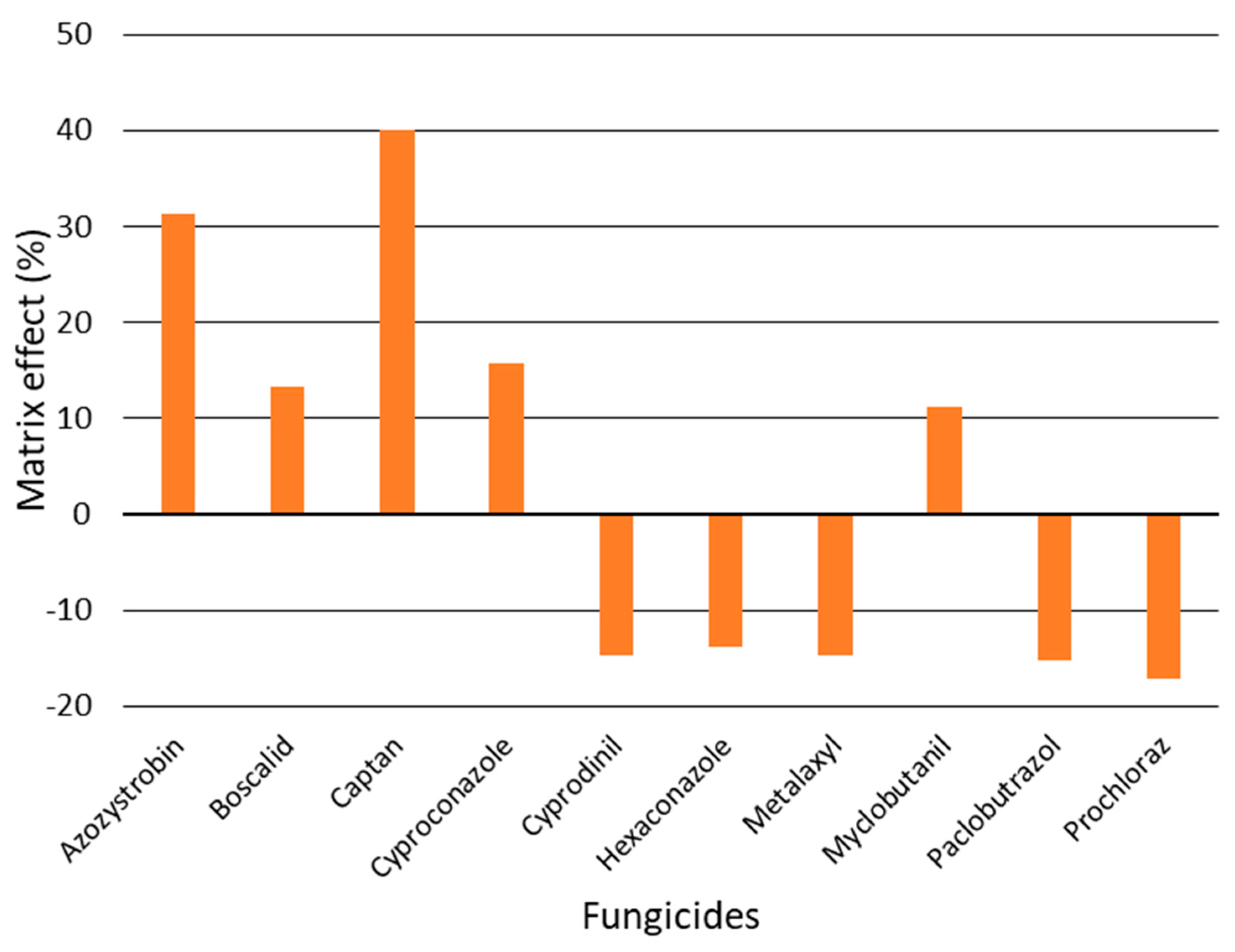
3.3. Dynamic Multiple Reaction Monitoring Method Validation and Matrix Effect
3.4. Application on Real Samples
3.5. Human Health Risk Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Javaid, Z.; Ghazala; Ibrahim, M.; Mahmood, A.; Bajwa, A.A. Pesticide Contamination of Potable Water and Its Correlation with Water Quality in Different Regions of Punjab, Pakistan. Water 2023, 15, 543. [Google Scholar] [CrossRef]
- Mas, L.I.; Aparicio, V.C.; De Gerónimo, E.; Costa, J.L. Pesticides in water sources used for human consumption in the semiarid region of Argentina. SN Appl. Sci. 2020, 2, 691. [Google Scholar] [CrossRef]
- Parlakidis, P.; Rodriguez, M.S.; Gikas, G.D.; Alexoudis, C.; Perez-Rojas, G.; Perez-Villanueva, M.; Carrera, A.P.; Fernández-Cirelli, A.; Vryzas, Z. Occurrence of Banned and Currently Used Herbicides, in Groundwater of Northern Greece: A Human Health Risk Assessment Approach. Int. J. Environ. Res. Public Health 2022, 19, 8877. [Google Scholar] [CrossRef] [PubMed]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An overlooked pesticide class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef] [PubMed]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in Drinking Water—A Review. Int. J. Environ. Res. 2021, 18, 468. [Google Scholar] [CrossRef] [PubMed]
- Gikas, G.D.; Parlakidis, P.; Mavropoulos, T.; Vryzas, Z. Particularities of Fungicides and Factors Affecting Their Fate and Removal Efficacy: A Review. Sustainability 2022, 14, 4056. [Google Scholar] [CrossRef]
- Liu, J.; Xia, W.; Wan, Y.; Xu, S. Azole and strobilurin fungicides in source, treated, and tap water from Wuhan, central China: Assessment of human exposure potential. Sci. Total Environ. 2021, 801, 149733. [Google Scholar] [CrossRef]
- Eissa, F.; Al-Sisi, M.; Ghanem, K. Occurrence, human health, and ecotoxicological risk assessment of pesticides in surface waters of the River Nile’s Rosetta Branch, Egypt. Environ. Sci. Pollut. 2021, 28, 55511–55525. [Google Scholar] [CrossRef]
- Ganaie, M.I.; Jan, I.; Mayer, A.N.; Dar, A.A.; Mayer, I.A.; Ahmed, P.; Sofi, J.A. Health Risk Assessment of Pesticide Residues in Drinking Water of Upper Jhelum Region in Kashmir Valley-India by GC-MS/MS. Int. J. Anal. Chem. 2023, 27, 6802782. [Google Scholar] [CrossRef]
- Campanale, C.; Losacco, D.; Triozzi, M.; Massarelli, C.; Uricchio, V.F. An Overall Perspective for the Study of Emerging Contaminants in Karst Aquifers. Resources 2022, 11, 105. [Google Scholar] [CrossRef]
- Jeon, S.H.; Kim, Y.P.; Kho, Y.; Shin, J.H.; Ji, W.H.; Ahn, Y.G. Development and validation of gas chromatography-triple quadrupole mass spectrometric method for quantitative determination of regulated plasticizers in medical infusion sets. J. Anal. Methods Chem. 2018, 2018, 9470254. [Google Scholar] [CrossRef] [PubMed]
- Belarbi, S.; Vivier, M.; Zaghouani, W.; Sloovere, A.; Agasse-Peulon, V.; Cardinael, P. Comparison of new approach of GC-HRMS (Q-Orbitrap) to GC-MS/MS (triple-quadrupole) in analyzing the pesticide residues and contaminants in complex food matrices. Food Chem. 2021, 359, 129932. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.C.; Monteiro, S.H.; Francisco, J.G.; Figueiredo, L.A.; Botelho, R.G.; Tornisielo, V.L. Liquid chromatography-electrospray ionization tandem mass spectrometry and dynamic multiple reaction monitoring method for determining multiple pesticide residues in tomato. Food Chem. 2015, 175, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Škufca, D.; Kovačič, A.; Griessler; Bulc, T.; Heath, E. Determination of 18 bisphenols in aqueous and biomass phase of high rate algal ponds: Development, validation and application. Chemosphere 2021, 271, 129786. [Google Scholar] [CrossRef] [PubMed]
- European Commission. SANTE/12682/2019. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. European Commission Directorate-General for Health and Food Safety. (rev.0). 2019. Available online: https://www.eurlpesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf (accessed on 7 September 2023).
- Vryzas, Z.; Papadakis, E.N.; Vassiliou, G.; Papadopoulou-Mourkidou, E. Occurrence of pesticides in transboundary aquifers of North-eastern Greece. Sci. Total Environ. 2012, 441, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Vryzas, Z.; Papadakis, E.N.; Papadpoulou-Mourkidou, E. Leaching of Br-, metolachlor, alachlor, atrazine, deethylatrazine and deisopropylatrazine in clayey vadoze zone: A field scale experiment in north-east Greece. Water Res. 2012, 46, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- PPDB (Pesticide Properties Data Base). Pyraclostrobin General Information, University of Hertfordshire UK 2023. Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/564.htm (accessed on 20 September 2023).
- Koçyiğit, H.; Sinanoğlu, F. Method validation for the analysis of pesticide residue in aqueous environment. Environ. Monit. Assess. 2020, 192, 567. [Google Scholar] [CrossRef]
- Song, N.-E.; Jung, Y.S.; Choi, J.Y.; Koo, M.; Choi, H.-K.; Seo, D.-H.; Lim, T.-G.; Nam, T.G. Development and Application of a Multi-Residue Method to Determine Pesticides in Agricultural Water Using QuEChERS Extraction and LC-MS/MS Analysis. Separations 2020, 7, 52. [Google Scholar] [CrossRef]
- Kim, H.H.; Lim, Y.W.; Yang, J.Y.; Shin, D.C.; Ham, H.S.; Choi, B.S.; Lee, J.Y. Health risk assessment of exposure to chlorpyrifos and dichlorvos in children at childcare facilities. Sci. Total Environ. 2013, 444, 441–450. [Google Scholar] [CrossRef]
- Muhammad, S.; Shah, M.T.; Khan, S. Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, northern Pakistan. Microchem. J. 2011, 98, 334–343. [Google Scholar] [CrossRef]
- Ali, N.; Kalsoom; Khan, S.; Ihsanullah; Rahman, I.; Said, M. Human Health Risk Assessment Through Consumption of Organophosphate Pesticide Contaminated Water of Peshawar Basin, Pakistan. Expos. Health 2018, 10, 259–272. [Google Scholar] [CrossRef]
- USEPA. Definitions and General Principles for Exposure Assessment: Guidelines for Exposure Assessment; Office of Pesticide Programs: Washington, DC, USA, 1999.
- IRIS (Integrated Risk Information System). Oral Chronic Reference Dose Integrate Risk Information System Database; Toxicity and Chemical Specific Factor Database. Available online: www.epa.gov/iris (accessed on 21 September 2023).
- Lee, S.H.; Kwak, S.Y.; Sarker, A.; Moon, J.-K.; Kim, J.-E. Optimization of a Multi-Residue Analytical Method during Determination of Pesticides in Meat Products by GC-MS/MS. Foods 2022, 11, 2930. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.N.; Qin, Y.Q.; Chen, M.T.; Liu, H.; Liu, S.; Sun, X.; Wang, X.; Chen, L.; Xie, F.; Cui, H.; et al. Accurate identification and quantification of ultra-multi-target flavours in essential oils through a combination of retention index distribution-based parallel dual-column GC-MS/MS and analyte protectants. Ind. Crops Prod. 2023, 193, 116186. [Google Scholar] [CrossRef]
- Gilbert-López, B.; García-Reyes, J.F.; Lozano, A.; Fernández-Alba, A.R.; Molina-Díaz, A. Large-scale pesticide testing in olives by liquid chromatography-electrospray tandem mass spectrometry using two sample preparation methods based on matrix solid-phase dispersion and QuEChERS. J. Chromatogr. A 2010, 1217, 6022–6035. [Google Scholar] [CrossRef] [PubMed]
- EC Council directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off. J. Eur. Communities 1998, 330, 1–23.
- OECD. Series on Testing and Assessment no. 72 and Series on Pesticides No. 39; Guidance Document on Pesticide Residue Analytical Methods, ENV/JM/MONO (2007); OECD: Paris, France, 2007. [Google Scholar]
- Song, N.E.; Seo, D.-H.; Choi, J.Y.; Yoo, M.; Koo, M.; Nam, T.G. Dispersive Solid–Liquid Extraction Coupled with LC-MS/MS for the Determination of Sulfonylurea Herbicides in Strawberries. Foods 2019, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Brunete, C.; Albero, B.; Martín, G.; Tadeo, J.L. Determination of pesticide residues by GC-MS using analyte protectants to counteract the matrix effect. Anal Sci. 2005, 21, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, E.; Łozowicka, B.; Kaczyński, P. Compensation of matrix effects in seed matrices followed by gas chromatography-tandem mass spectrometry analysis of pesticide residues. J. Chromatogr. A 2020, 1614, 460738. [Google Scholar] [CrossRef]
- Rutkowska, E.; Łozowicka, B.; Kaczyński, P. Three approaches to minimize matrix effects in residue analysis of multiclass pesticides in dried complex matrices using gas chromatography tandem mass spectrometry. Food Chem. 2019, 279, 20–29. [Google Scholar] [CrossRef]
- Bloomfield, J.P.; Williams, R.J.; Gooddy, D.C.; Cape, J.N.; Guha, P. Impacts of climate change on the fate and behaviour of pesticides in surface and groundwater—A UK perspective. Sci. Total Environ. 2006, 369, 163–177. [Google Scholar] [CrossRef]
- Pimentel, D.; Levitan, L. Pesticides: Amounts applied and amounts reaching pests. BioScience 1986, 36, 86–91. [Google Scholar] [CrossRef]
- Parlakidis, P.; Mavropoulos, T.; Vryzas, Z.; Gikas, G.D. Fluopyram removal from agricultural equipment rinsing water using HSF pilot-scale constructed wetlands. Environ. Sci. Pollut. Res. 2022, 29, 29584–29596. [Google Scholar] [CrossRef] [PubMed]
- Kapsi, M.; Tsoutsi, C.; Paschalidou, A.; Albanis, T. Environmental monitoring and risk assessment of pesticide residues in surface waters of the Louros River (N.W. Greece). Sci. Total Environ. 2019, 650, 2188–2198. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Vryzas, Z.; Alexoudis, C.; Vassiliou, G.; Galanis, K.; Papadopoulou-Mourkidou, E. Determination and aquatic risk assessment of pesticide residues in riparian drainage canals in northeastern Greece. Ecotoxicol. Environ. Saf. 2011, 74, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Nouma, B.B.; Rezig, M.; Bahrouni, H. Best Irrigation Practices Designed for Pesticides Use to Reduce Environmental Impact on Groundwater Resource in the Tunisian Context. J. Agric. Sci. 2016, 8, 142–152. [Google Scholar] [CrossRef]
- Gustafson, D.I. Groundwater ubiquity score: A simple method for assessing pesticide leachability. Environ. Toxicol. Chem. 1989, 8, 339–357. [Google Scholar] [CrossRef]
- Hyun, H.N.; Jang, G.M.; Oh, S.S.; Chung, J.B. Evaluation of groundwater contamination potential of pesticides using groundwater ubiquity score in Jeju Island soils. Korean J. Pestic. Sci. 2007, 11, 144–153. [Google Scholar]
- Dusek, J.; Sanda, M.; Loo, B.; Ray, C. Field leaching of pesticides at five test sites in Hawaii: Study description and results. Pest. Manag. Sci. 2010, 66, 596–611. [Google Scholar] [CrossRef]
- Parlakidis, P.; Gounari, I.; Georgiou, A.; Adamidis, G.; Vryzas, Z.; Gikas, G.D. Removal of Two Triazole Fungicides from Agricultural Wastewater in Pilot-Scale Horizontal Subsurface Flow Constructed Wetlands. Agronomy 2023, 13, 265. [Google Scholar] [CrossRef]
- Qian, L.; Qi, S.; Cao, F.; Zhang, J.; Zhao, F.; Li, C.; Wang, C. Toxic effects of boscalid on the growth, photosynthesis, antioxidant system and metabolism of Chlorella vulgaris. Environ. Pollut. 2018, 242, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Kunz, J.; Ingersoll, C.; Smalling, K.; Elskus, A.; Kuivila, K. Chronic toxicity of azoxystrobin to freshwater amphipods, midges, cladocerans and mussels in water-only exposures. Environ. Toxicol. Chem. 2017, 36, 2308–2315. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhu, Y.; Laziyan, Y.; Yang, C.; He, C.; Zuo, Z. Long-term exposure to cyprodinil causes abnormal zebrafish aggressive and antipredator behavior through the hypothalamic-pituitary-interrenal axis. Aquat. Toxicol. 2021, 241, 106002. [Google Scholar] [CrossRef] [PubMed]
- Caldas, S.S.; Arias, J.L.O.; Rombaldi, C.; Mello, L.L.; Cerqueira, M.B.; Martins, A.F.; Primel, E.G. Occurrence of Pesticides and PPCPs in Surface and Drinking Water in Southern Brazil: Data on 4-Year Monitoring. J. Braz. Chem. Soc. 2019, 30, 71–80. [Google Scholar] [CrossRef]
- De Sousa, A.; AbdElgawad, H.; Asard, H.; Pinto, A.; Soares, C.; Branco-Neves, S.; Braga, T.; Azenha, M.; Selim, S.; Al Jaouni, S.; et al. Metalaxyl Effects on Antioxidant Defenses in Leaves and Roots of Solanum nigrum L. Front. Plant Sci. 2017, 8, 1967. [Google Scholar] [CrossRef]
- Hussain, A.; Audira, G.; Siregar, P.; Lin, Y.-C.; Villalobos, O.; Villaflores, O.; Wang, W.-D.; Hsiao, C.D. Waterborne Exposure of Paclobutrazol at Environmental Relevant Concentration Induce Locomotion Hyperactivity in Larvae and Anxiolytic Exploratory Behavior in Adult Zebrafish. Int. J. Environ. Res. Public Health 2020, 17, 4632. [Google Scholar] [CrossRef]
- Kuzmanović, M.; Ginebreda, A.; Petrović, M.; Barceló, D. Risk assessment based prioritization of 200 organic micropollutants in 4 Iberian rivers. Sci. Total Environ. 2015, 503–504, 289–299. [Google Scholar] [CrossRef]
- Papadakis, E.N.; Tsaboula, A.; Vryzas, Z.; Kotopoulou, A.; Kintzikoglou, K.; Papadopoulou-Mourkidou, E. Pesticides in the rivers and streams of two river basins in northern Greece. Sci. Total Environ. 2018, 624, 732–743. [Google Scholar] [CrossRef]
- Stamatis, N.; Hela, D.; Triantafyllidis, V.; Konstantinou, I. Spatiotemporal variation and risk assessment of pesticides in water of the lower catchment basin of Acheloos River, Western Greece. Sci. World J. 2013, 2013, 231610. [Google Scholar] [CrossRef]
| Fungicide | Chemical Group | Molecular Weight (g/mol) | Soil Degradation DT50 (Field) | Dissociation Constant (pKa) at 25 °C | Water Solubility at 20 °C (mg/L) | Octanol–Water Partition Coefficient at 20 °C (LogKow) | Vapor Pressure at 20 °C (mPa) | Henry’s Law Constant at 25 °C (Pa m3/mol) |
|---|---|---|---|---|---|---|---|---|
| Azoxystrobin | strobilurin | 403.4 | 180.7 | - | 6.7 | 2.5 | 1.10 × 10−7 | 7.4 × 10−9 |
| Boscalid | carboxamide | 343.21 | 254 | - | 4.6 | 2.96 | 7.2 × 10−4 | 5.2 × 10−5 |
| Captan | phthalimide | 300.61 | 3.7 | - | 5.2 | 2.5 | 0.41 | 3.0 × 10−4 |
| Cyproconazole | triazole | 291.78 | 129 | - | 93 | 3.09 | 0.026 | 5.0 × 10−5 |
| Cyprodinil | anilinopyrimidine | 225.29 | 45 | 4.44 | 13 | 4 | 0.51 | 6.6 × 10−3 |
| Hexaconazole | triazole | 314.21 | 225 | 2.3 | 18 | 3.9 | 0.018 | 3.3 × 10−4 |
| Metalaxyl | anilide | 279.33 | 14.1 | 0 | 8400 | 1.75 | 0.75 | 1.6 × 10−5 |
| Myclobutanil | triazole | 288.78 | 35.0 | 2.3 | 132 | 2.89 | 0.198 | 4.3 × 10−4 |
| Paclobutrazol | triazole | 293.8 | 29.5 | - | 22.9 | 3.11 | 1.9 × 10−3 | 2.4 × 10−5 |
| Prochloraz | conazole | 376.7 | 68.8 | 3.8 | 26.5 | 3.5 | 0.15 | 1.6 × 10−3 |
| Fungicides | Precursor Ions | Product Ions | Quantification Transitions (1) | Qualification Transitions (2) | Retention Time | CE1 (V) | CE2 (V) |
|---|---|---|---|---|---|---|---|
| Azozystrobin | 344.1 | 329 182.9 171.9 | 344.1 → 329 | 344.1 → 182.9 | 37.4 | 15 | 25 |
| Boscalid | 140 111.9 | 112 76 76 | 140 → 112 | 140 → 76 | 33.33 | 10 | 25 |
| Captan | 151 149 | 80 79 79.1 | 151 → 79 | 151 → 80 | 23 | 15 | 5 |
| Cyproconazole | 222 138.9 | 124.9 111 75 | 222 → 124.9 | 138.9 → 75 | 26.06 | 25 | 35 |
| Cyprodinil | 226.2 225.2 224.2 | 225.3 224.3 208.2 | 225.2 → 224.3 | 226.2 → 225.3 | 22.36 | 10 | 10 |
| Hexaconazole | 214 175 | 159 147 111 | 175 → 111 | 175 → 147 | 24.86 | 20 | 10 |
| Metalaxyl | 234 220 | 174.1 146.1 192.1 | 234 → 146.1 | 234 → 174.1 | 19.12 | 20 | 10 |
| Myclobutanil | 179 150 | 125.1 90 123 | 179 → 125.1 | 179 → 90 | 25.63 | 10 | 30 |
| Paclobutrazol | 236 125.1 214 | 167.1 125.1 89 | 236 → 125.1 | 236 → 167.1 | 24.04 | 10 | 10 |
| Prochloraz | 310 195.9 180 | 69.8 96.9 138 | 180 → 138 | 195.9 → 96.9 | 32.31 | 10 | 30 |
| Fungicides | Linearity (R2) |
LOD (μg/L) | Recovery (%) | Repeatability (%RSD) | Reproducibility (%RSD) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 | 0.1 | 1 | 0.01 | 0.1 | 1 | 0.01 | 0.1 | 1 | |||
| (μg/L) | (μg/L) | (μg/L) | |||||||||
| Azoxystrobin | 0.9999 | 0.0018 | 98 | 83 | 89 | 16 | 14 | 17 | 16 | 12 | 10 |
| Boscalid | 0.9996 | 0.0014 | 89 | 85 | 80 | 15 | 11 | 13 | 15 | 17 | 13 |
| Captan | 0.9991 | 0.01 | 60 | 71 | 64 | 9 | 10 | 8 | 17 | 12 | 13 |
| Cyproconazole | 0.9999 | 0.0011 | 89 | 107 | 85 | 7 | 10 | 9 | 8 | 16 | 16 |
| Cyprodinil | 0.9998 | 0.0015 | 87 | 92 | 100 | 13 | 18 | 17 | 12 | 14 | 9 |
| Hexaconazole | 0.9998 | 0.0017 | 95 | 109 | 100 | 11 | 15 | 14 | 17 | 11 | 18 |
| Metalaxyl | 0.9994 | 0.0016 | 87 | 95 | 89 | 15 | 16 | 12 | 10 | 13 | 14 |
| Myclobutanil | 0.9992 | 0.0013 | 98 | 89 | 110 | 12 | 17 | 14 | 9 | 13 | 12 |
| Paclobutrazol | 0.9993 | 0.0019 | 107 | 93 | 100 | 13 | 11 | 17.6 | 12 | 10 | 10 |
| Prochloraz | 0.9997 | 0.0012 | 92 | 97 | 106 | 9 | 16 | 14 | 15 | 11 | 14 |
| Fungicides | Neochori | Abelakia | Lepti | Inoi | Pimeniko | Orestiada | GUS |
|---|---|---|---|---|---|---|---|
| Azoxystrobin | 0.096 ± 0.08 | 0.097 ± 0.07 | 0.096 ± 0.08 | 0.096 ± 0.08 | 0.096 ± 0.08 | 0.097 ± 0.1 | 3.10 |
| Boscalid | 0.048 ± 0.06 | 0.050 ± 0.06 | 0.089 ± 0.08 | 0.047 ± 0.06 | 0.053 ± 0.04 | 0.051 ± 0.07 | 2.68 |
| Captan | nd | nd | nd | nd | nd | nd | 0.97 |
| Cyproconazole | 0.071 ± 0.04 | 0.069 ± 0.07 | 0.067 ± 0.05 | 0.067 ± 0.06 | 0.071 ± 0.09 | 0.068 ± 0.06 | 3.04 |
| Cyprodinil | 0.016 ± 0.01 | 0.024 ± 0.01 | 0.014 ± 0.01 | 0.014 ± 0.01 | 0.018 ± 0.01 | 0.016 ± 0.01 | 1.06 |
| Hexaconazole | 0.214 ± 0.04 | 0.063 ± 0.06 | 0.063 ± 0.03 | 0.063 ± 0.07 | 0.065 ± 0.03 | 0.090 ± 0.05 | 2.31 |
| Metalaxyl | 0.028 ± 0.02 | 0.029 ± 0.04 | 0.029 ±0.02 | 0.032 ± 0.02 | 0.030 ± 0.063 | 0.030 ± 0.02 | 2.06 |
| Myclobutanil | 1.617 ± 0.02 | 0.084 ± 0.02 | nd | 0.137 ± 0.04 | 0.963 ± 0.09 | 2.094 ± 0.16 | 1.99 |
| Paclobutrazol | 0.080 ± 0.05 | 0.082 ± 0.05 | 0.081 ± 0.05 | 0.081 ±0.05 | 0.081 ±0.08 | 0.081 ± 0.06 | 2.47 |
| Prochloraz | 0.125 ± 0.12 | 0.123 ± 0.09 | 0.124 ± 0.08 | 0.127 ± 0.1 | 0.124 ± 0.1 | 0.126 ± 0.11 | 1.55 |
| Temperature (°C) | 12.2 ± 0.01 | 12.5 ± 0.01 | 11.9 ± 0.01 | 12.6 ± 0.01 | 12.5 ± 0.01 | 11.8 ± 0.01 | |
| pH | 8.13 ± 0.01 | 8.92 ± 0.01 | 8.2 5 ± 0.01 | 8.54 ± 0.01 | 8.33 ± 0.01 | 8.61 ± 0.01 |
| Fungicides | Neochori | Abelakia | Lepti | Inoi | Pimeniko | Orestiada | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index | A | C | A | C | A | C | A | C | A | C | A | C | |
| Azoxystrobin | HQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Boscalid | HQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Captan | HQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| R | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Cyproconazole | HQ | 0.072 | 0.154 | 0.069 | 0.150 | 0.067 | 0.146 | 0.067 | 0.146 | 0.072 | 0.154 | 0.068 | 0.148 |
| Cyprodinil | HQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Hexaconazole | HQ | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Metalaxyl | HQ | 0.001 | 0.019 | 0.001 | 0.005 | 0.001 | 0.005 | 0.001 | 0.005 | 0.001 | 0.006 | 0.001 | 0.008 |
| Myclobutanil | HQ | 0.105 | 0.227 | 0.005 | 0.012 | n/a | 0.000 | 0.009 | 0.019 | 0.063 | 0.135 | 0.136 | 0.294 |
| Paclobutrazol | HQ | 0.016 | 0.035 | 0.017 | 0.036 | 0.016 | 0.035 | 0.016 | 0.035 | 0.016 | 0.035 | 0.016 | 0.035 |
| Prochloraz | HQ | 0.101 | 0.218 | 0.099 | 0.214 | 0.100 | 0.216 | 0.102 | 0.221 | 0.100 | 0.216 | 0.102 | 0.219 |
| R | 0.003 | 0.002 | 0.003 | 0.002 | 0.003 | 0.002 | 0.003 | 0.002 | 0.003 | 0.002 | 0.003 | 0.002 | |
| HQ Sum | 0.295 | 0.652 | 0.192 | 0.417 | 0.185 | 0.402 | 0.196 | 0.427 | 0.252 | 0.546 | 0.324 | 0.704 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arvanitidis, A.; Adamidis, G.S.; Parlakidis, P.; Gikas, G.D.; Alexoudis, C.; Vryzas, Z. A Dynamic Multiple Reaction Monitoring Analytical Method for the Determination of Fungicide Residues in Drinking Water. Environments 2024, 11, 5. https://doi.org/10.3390/environments11010005
Arvanitidis A, Adamidis GS, Parlakidis P, Gikas GD, Alexoudis C, Vryzas Z. A Dynamic Multiple Reaction Monitoring Analytical Method for the Determination of Fungicide Residues in Drinking Water. Environments. 2024; 11(1):5. https://doi.org/10.3390/environments11010005
Chicago/Turabian StyleArvanitidis, Aggelos, George S. Adamidis, Paraskevas Parlakidis, Georgios D. Gikas, Christos Alexoudis, and Zisis Vryzas. 2024. "A Dynamic Multiple Reaction Monitoring Analytical Method for the Determination of Fungicide Residues in Drinking Water" Environments 11, no. 1: 5. https://doi.org/10.3390/environments11010005
APA StyleArvanitidis, A., Adamidis, G. S., Parlakidis, P., Gikas, G. D., Alexoudis, C., & Vryzas, Z. (2024). A Dynamic Multiple Reaction Monitoring Analytical Method for the Determination of Fungicide Residues in Drinking Water. Environments, 11(1), 5. https://doi.org/10.3390/environments11010005






