Lower Eocene Footprints from Northwest Washington, USA. Part 1: Reptile Tracks
Abstract
1. Introduction
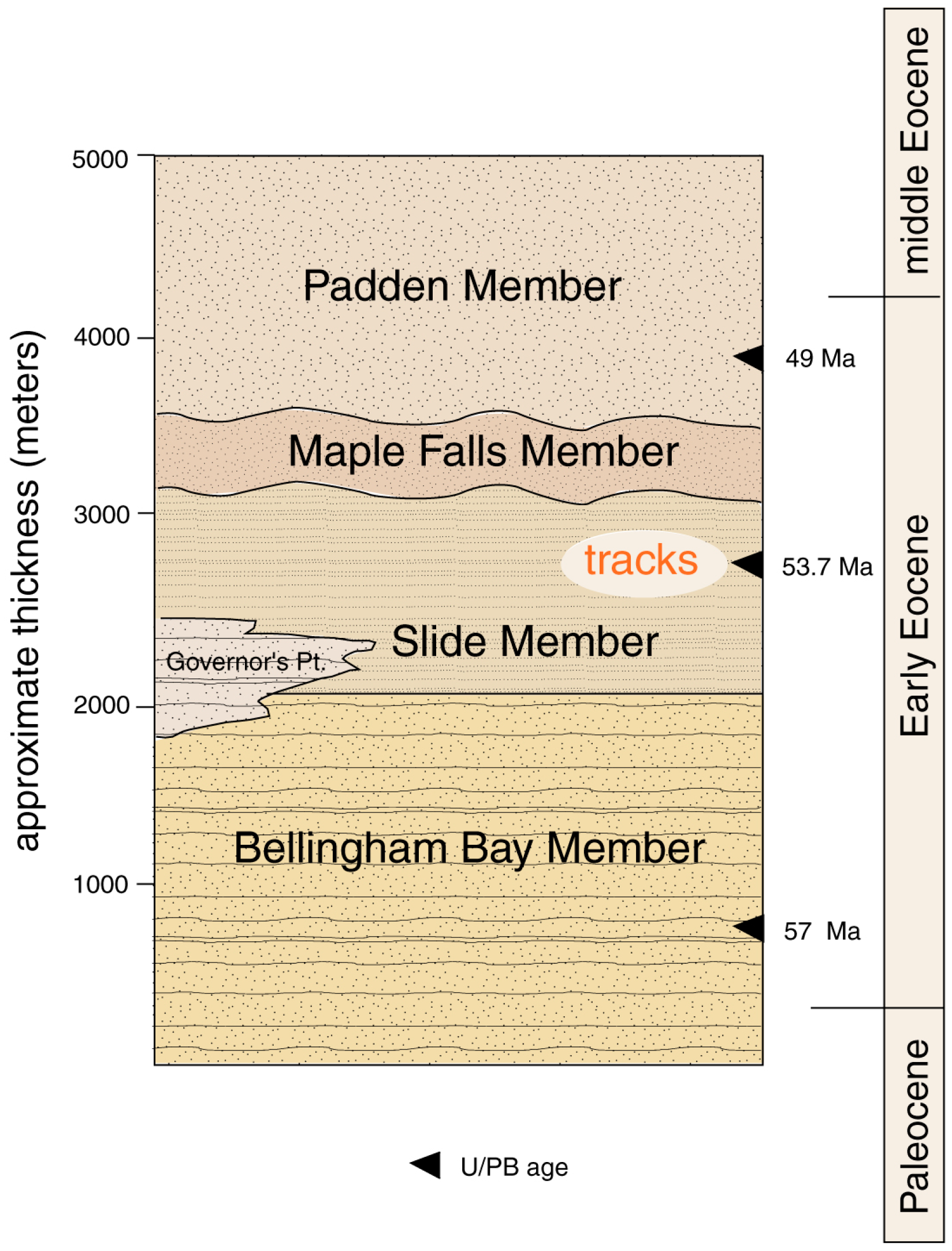
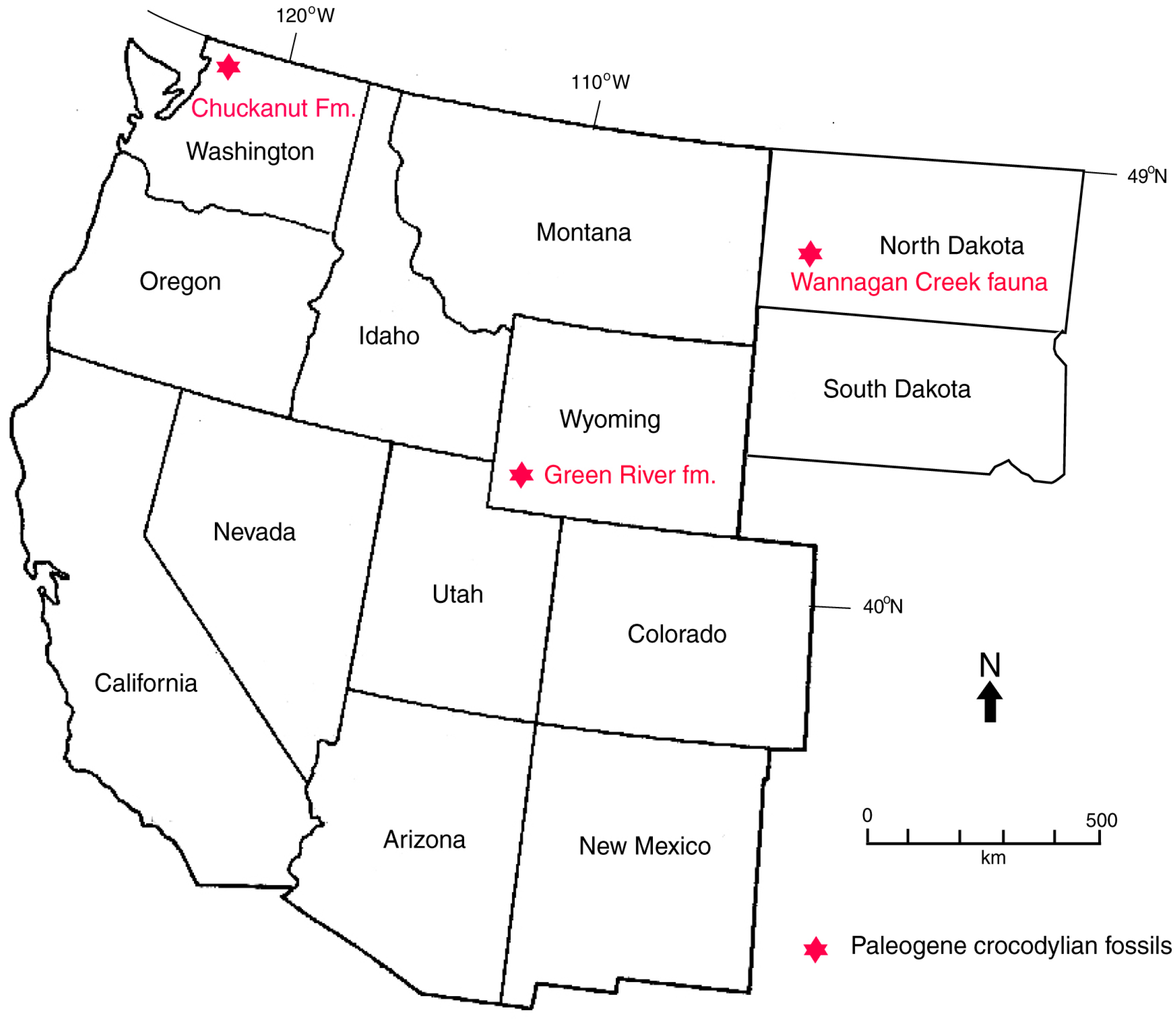
1.1. Geologic Setting
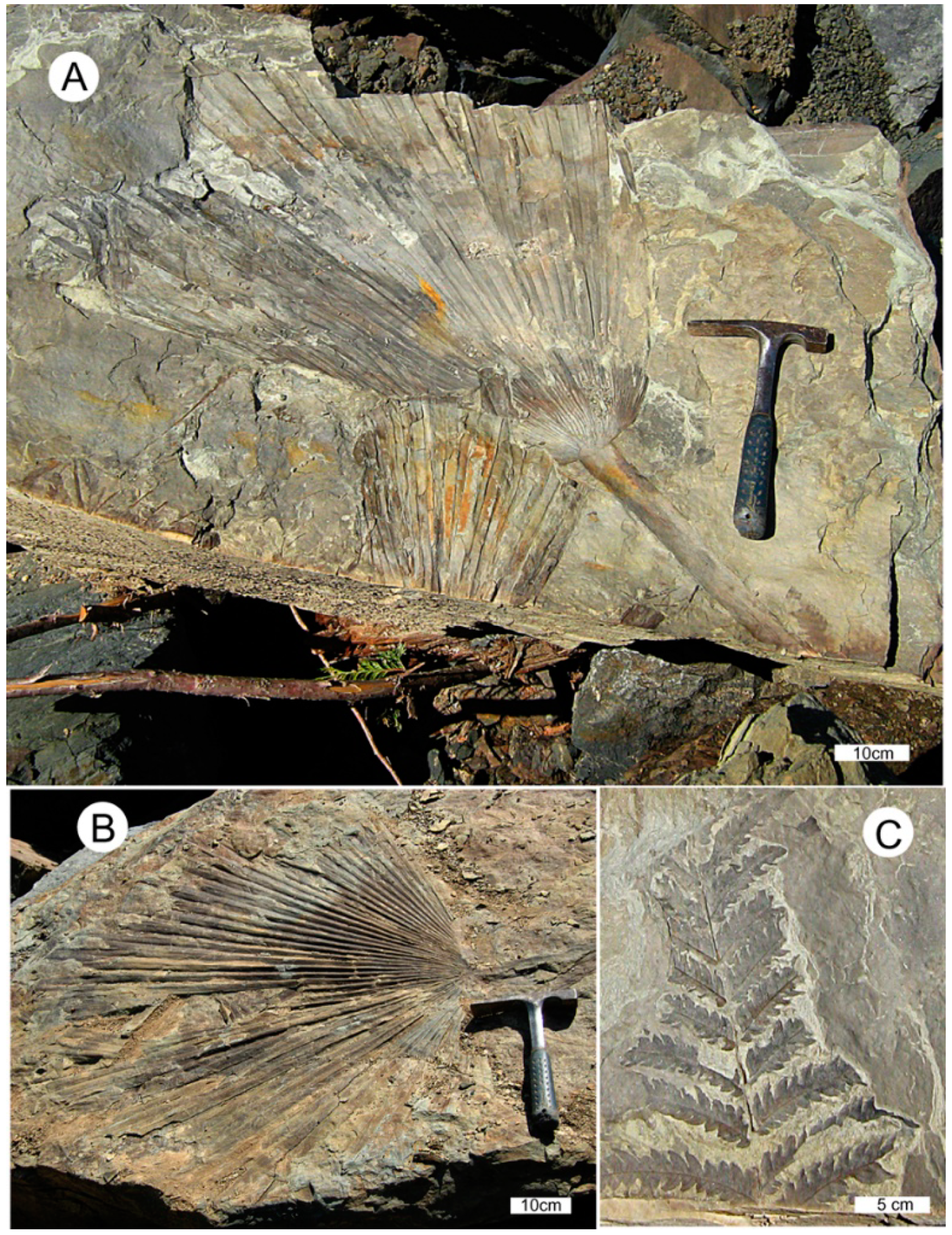
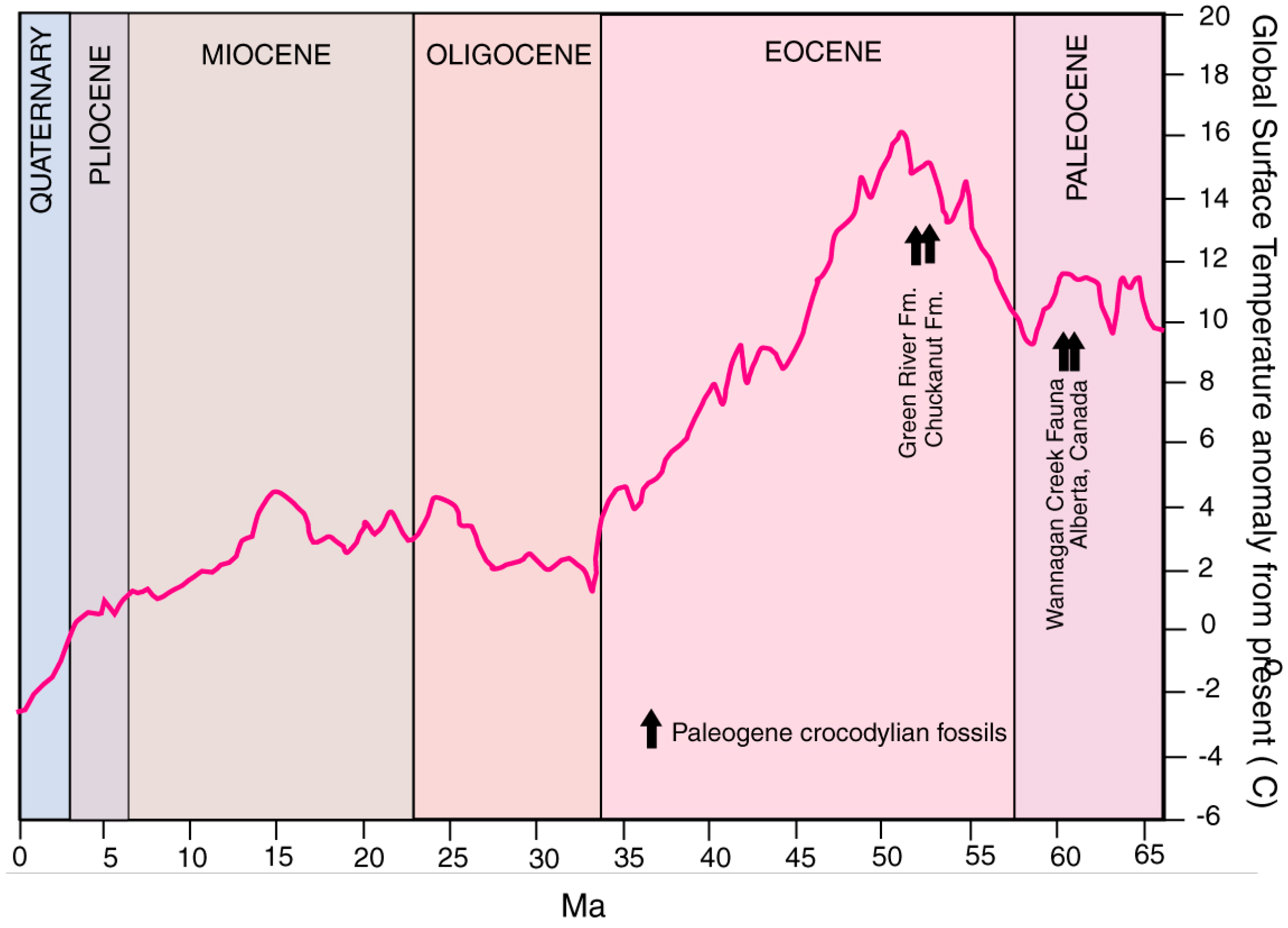
1.2. Paleoenvironment and Paleoclimate
1.3. Previous Work
2. Methods
3. Ichonotaxonomy
4. Chuckanut Formation Turtle Tracks
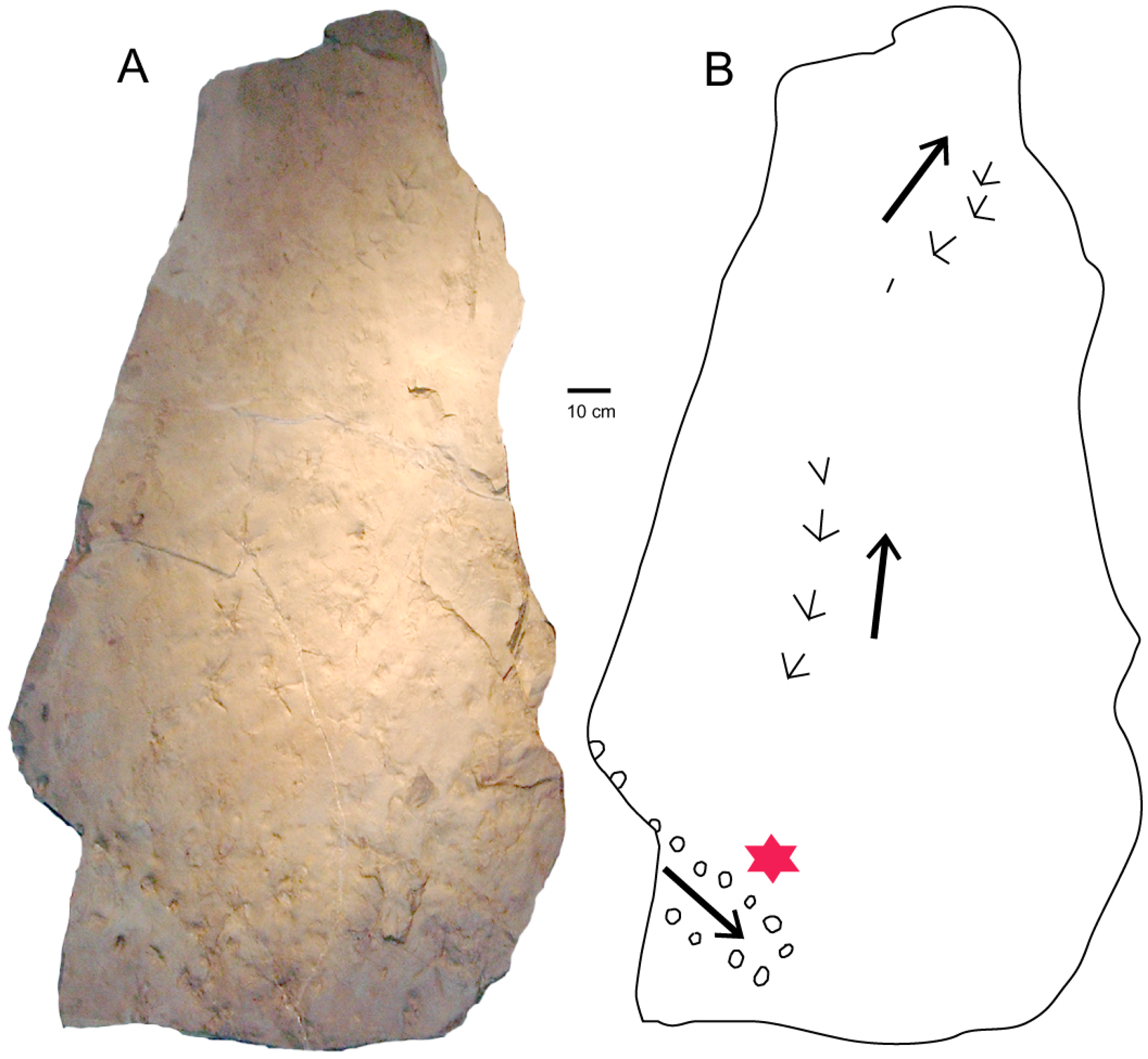
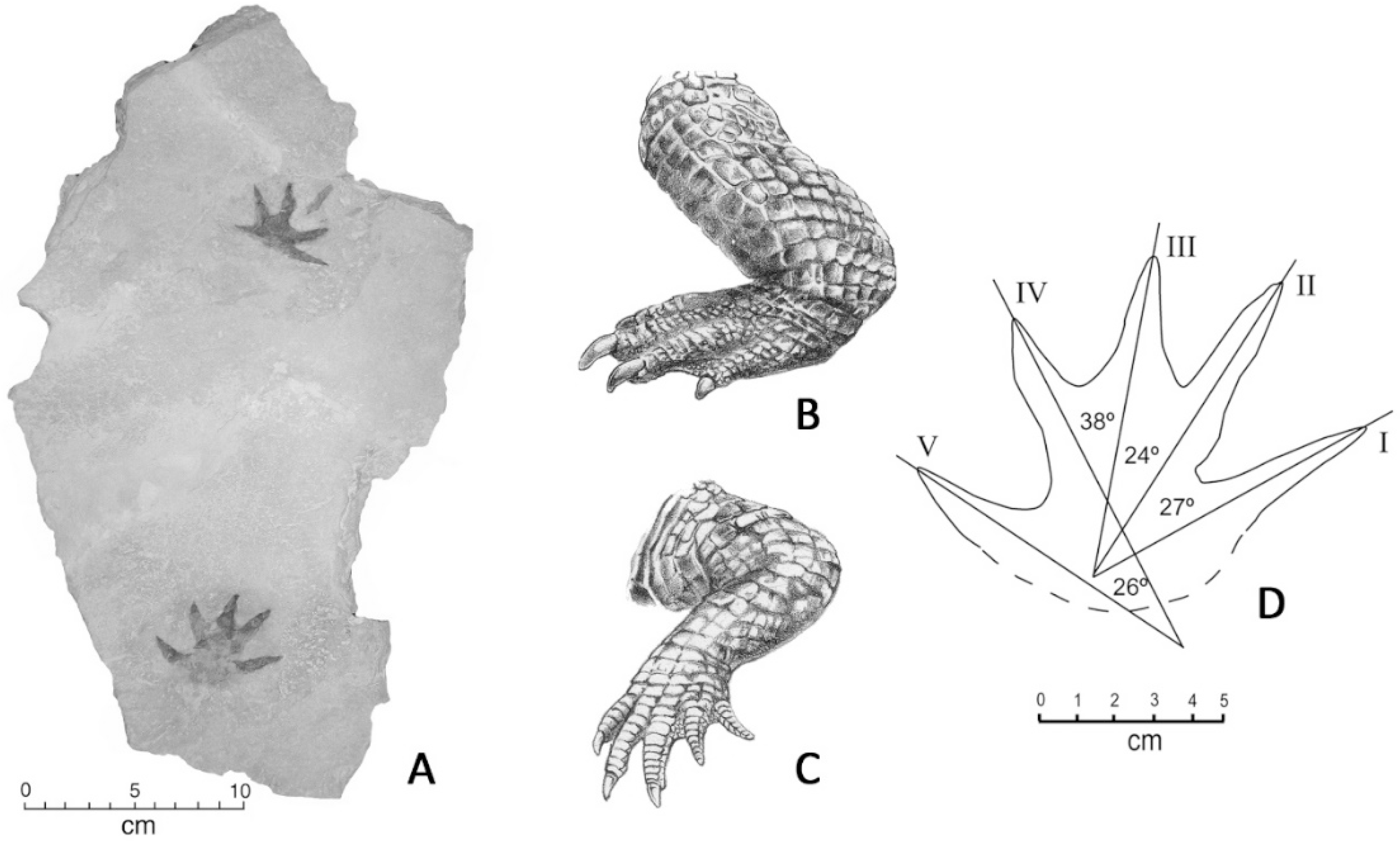
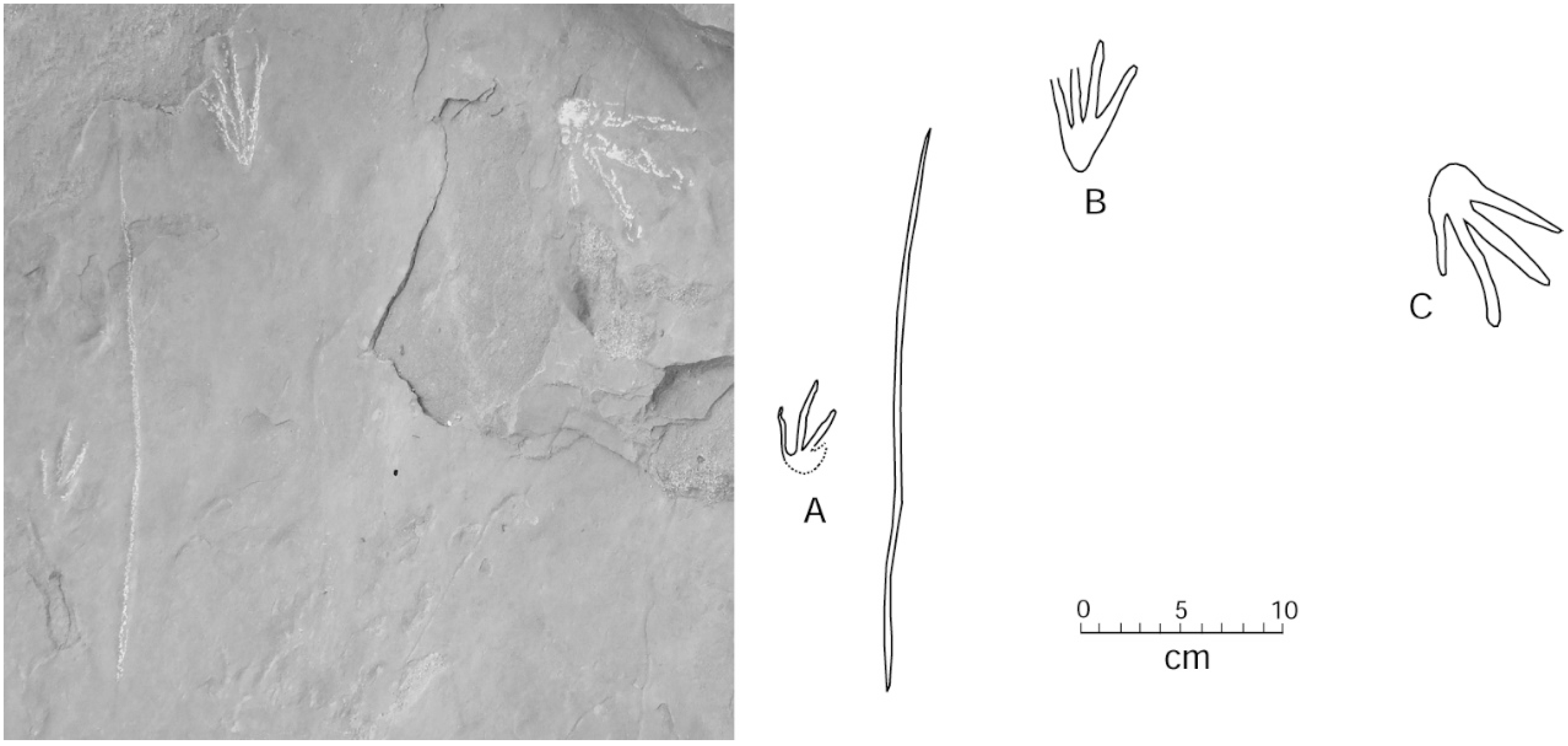
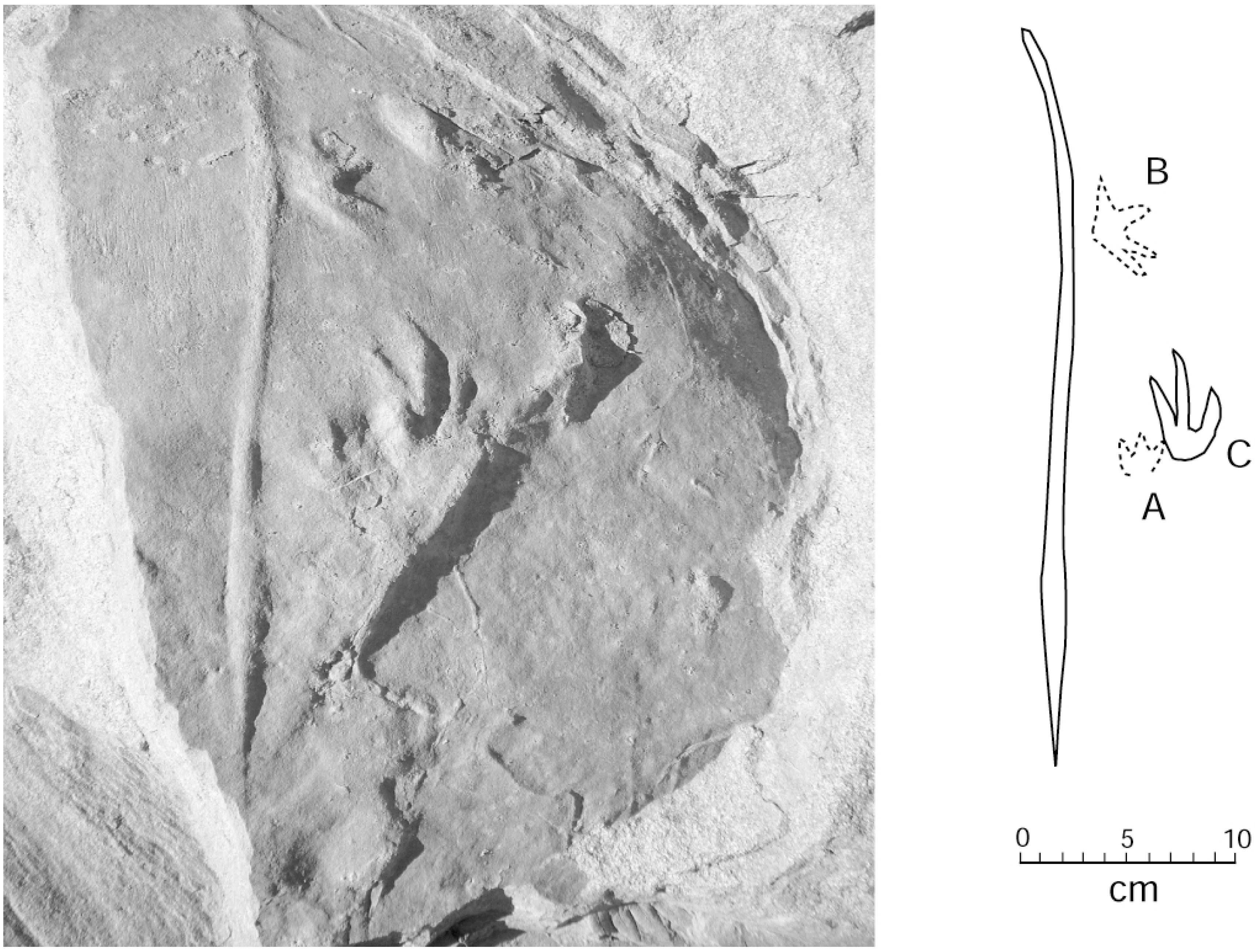
4.1. Systematic Ichnology
4.2. Comments
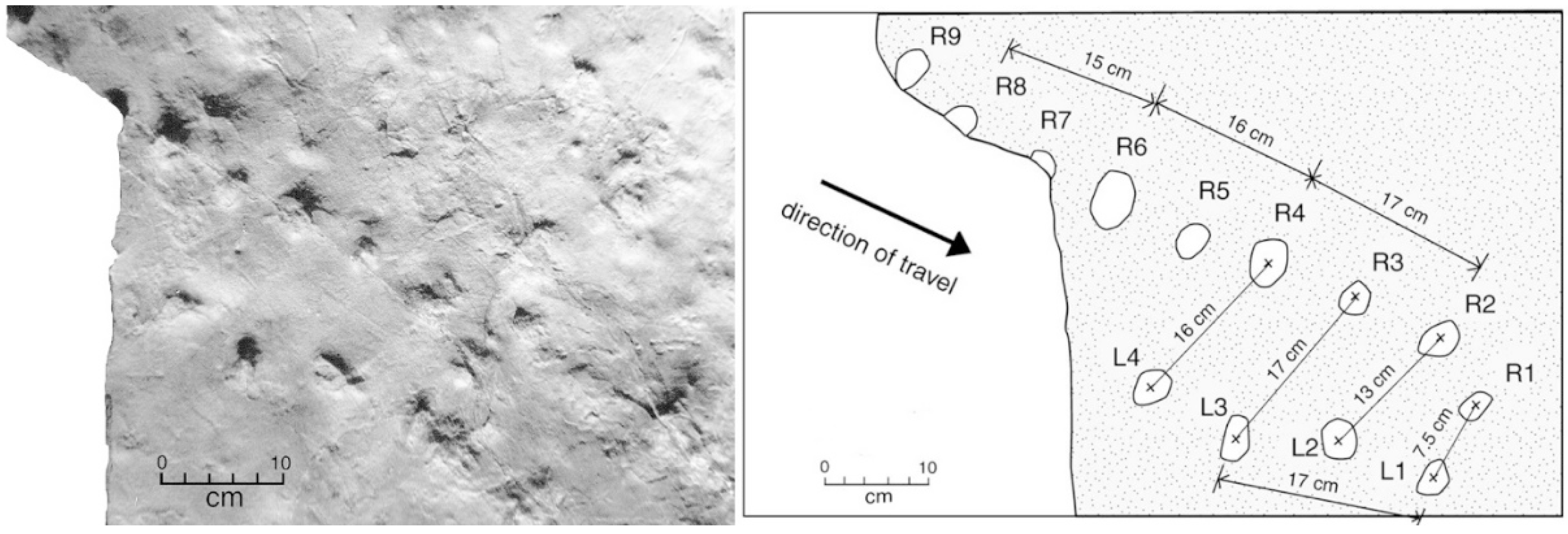
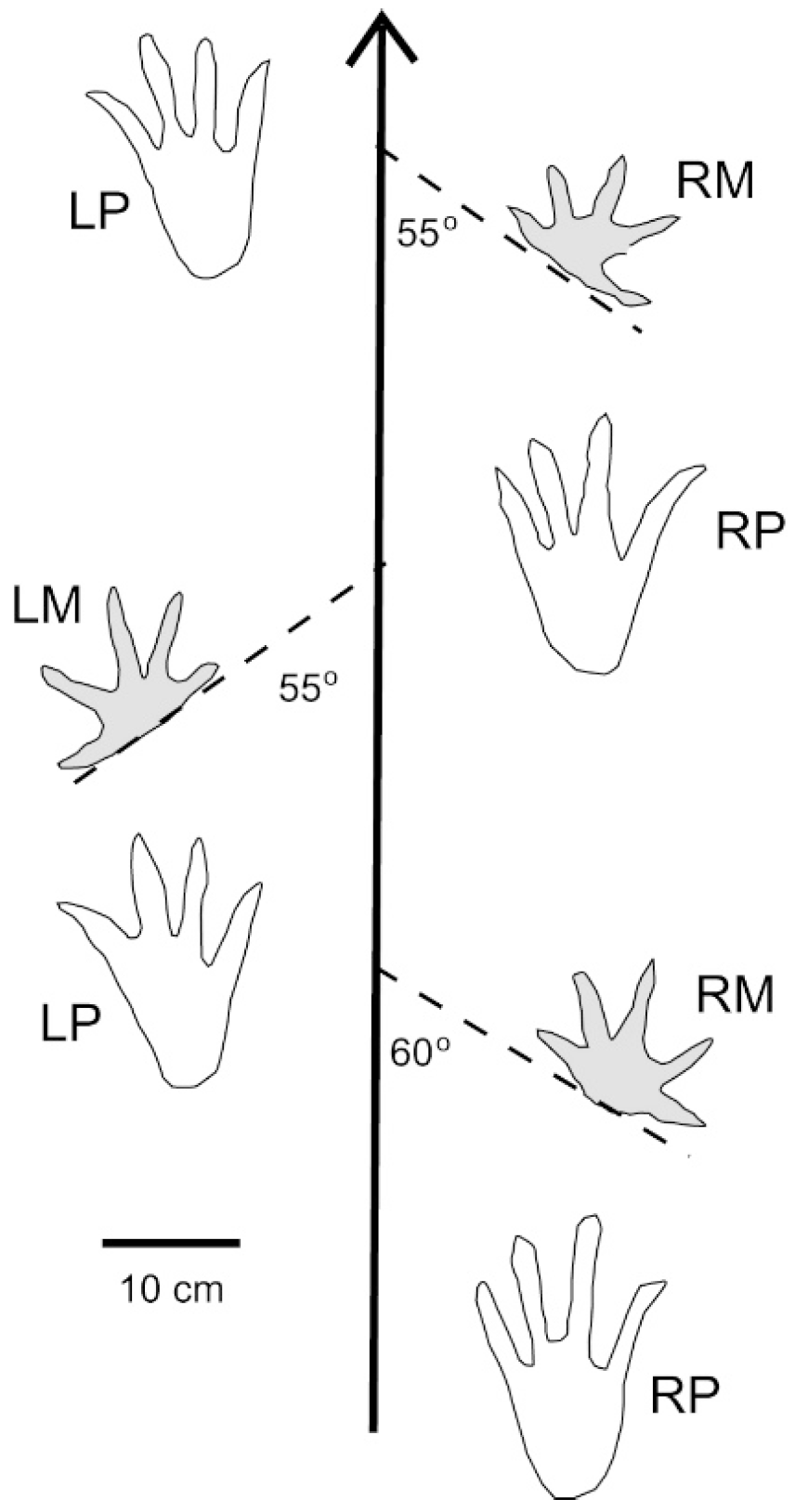
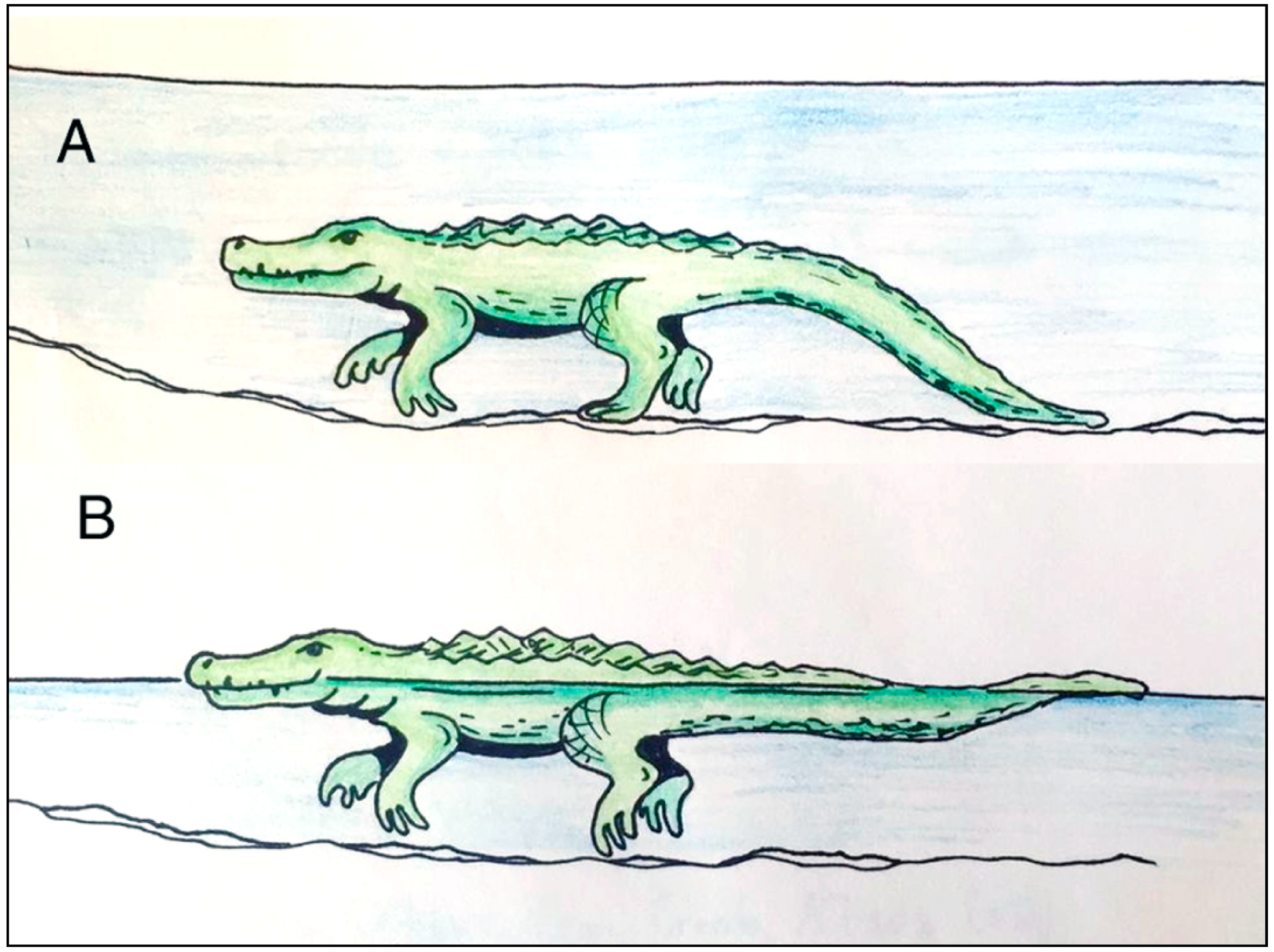
5. Crocodylian Trace Fossils
6. Discussion
Funding
Acknowledgments
Conflicts of Interest
References
- McDonald, H.G.; White, R.S.; Lockley, M.G.; Mustoe, G.E. An indexed bibliography of Cenezoic vertebrate tracks. In Cenozoic Vertebrate Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Lockkley, M.G., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2007; Volume 42, pp. 275–302. [Google Scholar]
- Sarjeant, W.A.S.; Langston, W., Jr. Vertebrate footprints and invertebrate traces from the Chardronian (Late Eocene) of Trans-Pecos, Texas. In Texas Memorial Museum Bulletin 36; University of Texas: Austin, TX, USA, 1994; p. 86. [Google Scholar]
- McCrea, R.T.; Pemberton, S.G.; Currie, P.J. New ichnotaxa of mammal and reptile tracks from the upper Paleocene of Alberta. Ichnos 2004, 11, 323–339. [Google Scholar] [CrossRef]
- Erickson, B.R. Crocodile and arthropod tracks from the Late Paleocene Wannagan Creek fauna of North Dakota, USA. Ichnos 2005, 12, 303–308. [Google Scholar] [CrossRef]
- Johnson, S.Y. Stratigraphy, age, and paleogeography of the Eocene Chuckanut Formation, northwest Washington. Can. J. Earth Sci. 1984, 21, 92–106. [Google Scholar] [CrossRef]
- Johnson, S.Y. Cyclic fluvial sedimentation in a rapidly subsiding basin. Sediment. Geol. 1984, 38, 361–391. [Google Scholar] [CrossRef]
- Mustard, P.S.; Rouse, G.E. Stratigraphy and evolution of Tertiary Georgia Basin and subadjacent Upper Cretaceous sedimentary rocks, southwestern British Columbia and northwestern Washington. In Geology and Geological Hazards of the Vancouver Region, Southwestern British Columbia; Monger, J.W.H., Ed.; Geological Survey of Canada Bulletin: Ottawa, ON, Canada, 1994; Volume 481, pp. 97–169. [Google Scholar]
- Mustoe, G.E.; Gannaway, W.L. Paleogeography and paleontology of the early Tertiary Chuckanut Formation, northwest Washington. Wash. Geol. 1997, 25, 1–18. [Google Scholar]
- Haugerud, R. Preliminary report on significant thrusting and extension of the early Tertiary Chuckanut Formation, NW Washington. In Slave-Northern Cordillera Lithospheric Evolution (SNORCLE) and Cordilleran Tectonics Workshop; Cook, F., Erdmer, P., Eds.; Lithoprobe Report; University of British Columbia: Vancouver, BC, Canada, 1998; p. 203. [Google Scholar]
- Mustoe, G.E.; Dillhoff, R.M.; Dillhoff, T.A. Geology and paleontology of the early Tertiary Chuckanut Formation. In Geological Society of America Field Guide 9: Floods, Faults, and Fire. Geological Field Trips in Washington State and Southwest British Columbia; Stelling, P., Tucker, D.S., Eds.; Geological Society of America: Boulder, CO, USA, 2007; pp. 121–135. [Google Scholar]
- Breedlovesrout, R.L. Paleofloristic Studies in the Paleogene Chuckanut Basin, Western Washington, USA. Unpublished Ph.D. dissertation, University of Idaho, Moscow, Russia, 2011; p. 953. [Google Scholar]
- Mustoe, G.E. Eocene bird tracks from the Chuckanut Formation, northwest Washington. Can. J. Earth Sci. 1993, 30, 987–990. [Google Scholar] [CrossRef]
- Crider, J.G.; Tucker, D.S.; Clark, D.H.; Linneman, S.R. The 2009 Racehorse Creek Landslide: Forensic Dynamics of a Large, Complex Catastrophic Mass Movement. Geol. Soc. Am. Abstr. Programs 2009, 41, 498. [Google Scholar]
- Wolfe, J.A. A Method of Obtaining Climatic Parameters from Tertiary Leaf Assemblages; 5 plates; US Geological Survey Bulletin: Reston, VA, USA, 1993; Volume 2040, p. 71.
- Wilf, P. When are leaves good thermometers? A new case for leaf margin Analysis. Paleobiology 1997, 23, 213–215. [Google Scholar] [CrossRef]
- Mustoe, G.E.; Pevear, D.R. Vertebrate fossils from the Chuckanut Formation of northwest Washington. Northwest Sci. 1983, 5, 3–18. [Google Scholar]
- Mustoe, G.E.; Girouard, S.P., Jr. A fossil trionychid turtle from the early Tertiary Chuckanut Formation of northwestern Washington. Northwest Sci. 2001, 75, 211–218. [Google Scholar]
- Simpson, S. The morphological classification of trace fossils. In The Study of Trace Fossils; Frey, R.W., Ed.; Springer-Verlag: New York, NY, USA, 1975; pp. 39–54. [Google Scholar]
- Ekdale, A.A.; Bromley, R.G.; Pemberton, S.G. Ichnology: The Use of Trace Fossils in Sedimentology and Stratigraphy; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 1984. [Google Scholar]
- Bertling, M.; Braddy, S.J.; Bromley, R.G.; Demathieu, G.R.; Genise, J.; Mikuláš, R.; Nielsen, J.K.; Nielsen, K.S.; Rindsberg, A.K.; Schlirf, M.; et al. Names for trace fossils: A uniform approach. Lethaia 2006, 39, 265–286. [Google Scholar] [CrossRef]
- Bertling, M. What’s in a name? Nomenclature, systematic, ichnotaxonomy. In Trace Fossils; Miller, W., III, Ed.; Chapter 5; Elsevier: Amsterdam, The Netherlands, 2007; pp. 81–91. [Google Scholar]
- Rindsberg, A.K. Ichnological consequences of the 1985 International Code of Zoological Nomenclature. Ichnos 1990, 1, 59–63. [Google Scholar] [CrossRef]
- Rindsberg, A.K. Construction of ichnogeneric names. Ann. Sociatis Geol. Pol. 2015, 85, 529–549. [Google Scholar] [CrossRef]
- Vialov, O.S. Stratigrafiya Neogeonovykh molass Predkarpatskovo progroba (Stratigraphy of the Neogne molasse of the PreCarpathian basin). In Akadimya Nauk Urainskoy SSR Institut Geologii I Geokhimii Goryuchikh Iskopaymykh; Naukkova Dumka: Kiev, Ukraine, 1965; p. 165. [Google Scholar]
- Vialov, O.S. Sledy zxhinedeyatelnosti organizmov I ikh paleoentologischeskoe znacheniye (Traces of living organisms and their paleontological significance). In Akadimya Nauk Urainskoy SSR Institut Geologii I Geokhimii Goryuchikh Iskopaymykh; Naukova Dumka: Kiev, Ukraine, 1966; p. 219. [Google Scholar]
- Lucas, S.G. Cenozoic vertebrate footprint ichnotaxa named by O.S. Vyalov in 1965 and 1966. In Cenozoic Vertebrate Tracks and Traces; Lucas, S.G., Spielmann, J.H., Lockley, M.G., Eds.; 34 plates; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2007; Volume 42, pp. 113–117. [Google Scholar]
- Vialov, O.S. The classification of fossil traces of life. In Proceedings of the 24th International Congress, Montreal, QC, Canada, 21–26 August 1972; pp. 71–78. [Google Scholar]
- Palii, V.M. The contribution of O.S. Vialov to the development of ichnological classification and nomenclature. Stratigr. Geol. Correl. 2013, 21, 249–251. [Google Scholar] [CrossRef]
- Scrivner, P.J.; Bottjer, D.J. Neogne avian and mammalian tracks from Death Valley National Monument, California: Their context, classification, and preservation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1986, 57, 285–331. [Google Scholar] [CrossRef]
- Haubold, H. Ichnia Amphibiorum et Reptiliorum Fossilium. Handbuch der Paläoherpatologie; part 18; Gustav Fischer: Stuttgart, Germany, 1971; p. 123. [Google Scholar]
- Rühle von Lilienstern, H. Fährten und Spüren im Chirotherium−Sandstein von Südthüringen. Fortschr. Geol. Paläontol. 1939, 12, 93–387. [Google Scholar]
- Lichtig, A.J.; Lucas, S.G.; Klein, H.; Lovelace, D.M. Triassic turtle tracks and the origin of turtles. Hist. Biol. 2018, 30, 1112–1122. [Google Scholar] [CrossRef]
- Ernst, C.H.; Lovich, J.E. Turtles of the United States and Canada; Johns Hopkins University Press: Baltimore, MD, USA, 2009; ISBN 9780801891212. [Google Scholar]
- Milán, J.; Hedegaard, R. Interspecific variation in tracks and trackways from extant Crocodylians. In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 15–30. [Google Scholar]
- Kumagai, C.J.; Farlow, J.O. Observations on traces of the American crocodile (Crocodylus acutus) from northwestern Costa Rica. In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 41–50. [Google Scholar]
- Farlow, J.O.; Elsey, R.M. Footprints and trackways of the American alligator. Rockefeller Wildlife Refuge, Louisiana. In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 31–40. [Google Scholar]
- Grigg, G.; Kirshner, D. Biology and Evolution of Crocodylians; Cornell University Press: Ithaca, NY, USA, 2015; p. 649. [Google Scholar]
- Farlow, J.O.; Robinson, N.J.; Turner, M.L.; Black, J.; Gatesy, S.M. Footfall pattern of a bottom-walking crocodile (Crocodylus acutus). Palaios 2018, 33, 406–413. [Google Scholar] [CrossRef]
- Martinez, M.M. Issues for aquatic pedestrian locomotion. Am. Zool. 1996, 36, 619–627. [Google Scholar] [CrossRef]
- Martinez, M.M.; Full, R.J.; Koehl, M.A.R. Underwater punting by an intertidal crab: A novel gait revealed by the kinematics of pedestrian locomotion in air versus water. J. Exp. Biol. 1998, 201, 2609–2623. [Google Scholar]
- Koester, D.M.; Spiritu, C.P. Punting: An usual mode of locomotion in the little skate, Leucoraja erinaceae (Chondricthes: Rajidae). Copea 2003, 2003, 553–561. [Google Scholar] [CrossRef]
- Mikuláš, R.; Dvořák, Z. Charachichnos isp., probable Crocodylian swim traces from the Miocene of Ceseké basin, Czech Republic. In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 121–136. [Google Scholar]
- Farlow, J.O.; Robinson, N.J.; Kumagai, C.J.; Paladino, F.V.; Falkingham, P.L.; Esey, R.M.; Martin, A.J. Trackways of the American Crocodile (Crocodylus acutus) in northwestern Costa Rica: Implications for Crocodylian ichnology. Ichnos 2017, 9, 1–36. [Google Scholar] [CrossRef]
- Weymouth, H. Systematik der Rezenten Krokodile. Mitt. Mus. Berl. 1953, 29, 275–514. [Google Scholar]
- Owen, R. Report on British fossil reptiles. Rep. Br. Assoc. Adv. Sci. 1842, 9, 60–204. [Google Scholar]
- Avanzini, M.; Piñuela, L.; Garcia-Ramos, J.C. Preservational morphotypes of Crocodylopus from the Late Jurassic of Asgturia (Spain). In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 239–244. [Google Scholar]
- Olsen, K.; Padian, P.E. Earliest records of Batrachopus from the southwestern United States, and a revision of some early Mesozoic crocodylomorph inchnogenera. In The Beginning of the Age of Dinosaurs; Padian, K., Ed.; Cambridge University Press: Cambridge, UK, 1986; pp. 259–273. [Google Scholar]
- Lockley, M.G. A solution to the Mehiella mystery: Tracking, naming, identifying, and measuring the first Crocodylian trackway reported from the Cretaceous (Dakota Group, Colorado). In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 157–174. [Google Scholar]
- Lockley, M.G.; Foster, J.R. An assemblage of probably Crocodylian traces and associated dinosaur tracks from the lower Morrison Formation (Upper Jurassic) of eastern Utah. In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 93–98. [Google Scholar]
- Avanzini, M.A.; Piñuela, L.A.; Ruiz-Omeñaca, J.I.; Garcia-Ramos, J.C. The crocodile track Hatcherichnus from the Upper Jurassic of Aturia (Spain). In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2009; Volume 51, pp. 89–92. [Google Scholar]
- Lockley, M.G.; Li, R.; Matxkawa, M.; Li, J. Tracking Chinese Crocodylians: Kuangyanpus, Laiyangpus, and implications for naming Crocodylian and Crocodylian-like tracks and associated ichnofacies. In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 99–108. [Google Scholar]
- Mateus, O.; Milán, J. First record of Crocodyle and Pterosaur tracks in the upper Jurassic of Portugal. In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 83–88. [Google Scholar]
- Le Leuf, J.; Lauprasert, K.; Suteethorn, S.; Souillat, V.; Suteethorn, V.; Buffetaut, E. Late Early Cretaceous crocodyliform trackways from northeastern Thailand. In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 175–178. [Google Scholar]
- Rajkumar, H.S.; Mustoe, G.E.; Khaidem, K.S.; Soibam, I. Crocodylian tracks from Lower Eocene flysch deposits of the Barail Group, Manipur, India. Ichnos 2015, 22, 122–131. [Google Scholar] [CrossRef]
- Brandt, M.E. Coral Disease Epizootiology in the Florida Keys (U.S.A.) and Cayman Islands (British West Indies), and the Development of the Simulation of Infected Corals Model. Ph.D. Thesis, University of Miami, Coral Gables, FL, USA, December 2017. [Google Scholar]
- Lockley, M.G.; Lucas, S.G.; Milàn, J.; Harris, J.D.; Avenzini, M.; Foster, J.R.; Spielmann, J. The fossil record of Crocodylian tracks and traces: An overview. In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 1–14. [Google Scholar]
- Brochu, C.A. Phylogenetic approaches toward Crocodylian history. Annu. Rev. Earth Planet. Sci. 2003, 31, 357–397. [Google Scholar] [CrossRef]
- Scheyer, T.M.; Aguilera, O.A.; Delfino, M.; Fortier, D.C.; Carlini, A.A.; Sánchez, R.; Carillo-Briceño, J.D.; Quiroz, L.; Sánchez-Villagra, M.R. Crocodylian diversity peak and extinction in the late Cenozoic of the northern Neotropics. Nat. Commun. 2013, 4, 1–9. [Google Scholar] [CrossRef]
- Brochu, C.A.; Storrs, G.W. A giant crocodile from the Plio-Pleistocene of Kenya, the phylogenetic relationship of Neogene African crocodylines, and the antiquity of Crocodylus in Africa. J. Vertebr. Paleontol. 2012, 32, 587–602. [Google Scholar] [CrossRef]
- Grande, L. Paleontology of the Green River Formation, with a Review of Fish Fauna; Geological Survey of Wyoming Bulletin 63: Laramie, WY, USA, 1984; Volume 63, pp. 198–203.
- Grande, L. The Lost World of Fossil Lake; University of Chicago Press: Chicago, IL, USA, 2013; pp. 210–213. ISBN 13: 978-0-226-92296-6. [Google Scholar]
- Erickson, B.R. Wannagonosuchus, a new alligator from the Paleocene of North America. J. Paleontol. 1982, 56, 492–506. [Google Scholar]
- Erickson, B.R. The Wannagan Creek Quarry and Its Reptilian Fauna (Bullion Creek Formation, Paleocene) in Billins County, North Dakota; Report of Investigations No. 72, 1982; North Dakota Geological Survey: Grand Forks, ND, USA, 1982.
- Erisckson, B.R. Fossil Lake Wannegan (Paleocene:Tiffanian), Billings County, North Dakota; Series No. 87; North Dakota Geological Survey Misc: Grand Forks, ND, USA, 1999.
- Lance, V.A. Alligator physiology and life history: The importance of temperature. Exp. Gerontol. 2003, 38, 801–805. [Google Scholar] [CrossRef]
- Washington Herp Alas, Washington Department of Natural Resources. 2017. Available online: http://www1.dnr.wa.gov/nhp/refdesk/herp/speciesmain.html (accessed on 16 June 2019).
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 282, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Zachos, J.C.; Dickens, G.R.; Zeebe, R.E.; Zeebe, R.E. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 2008, 451, 279–283. [Google Scholar] [CrossRef] [PubMed]
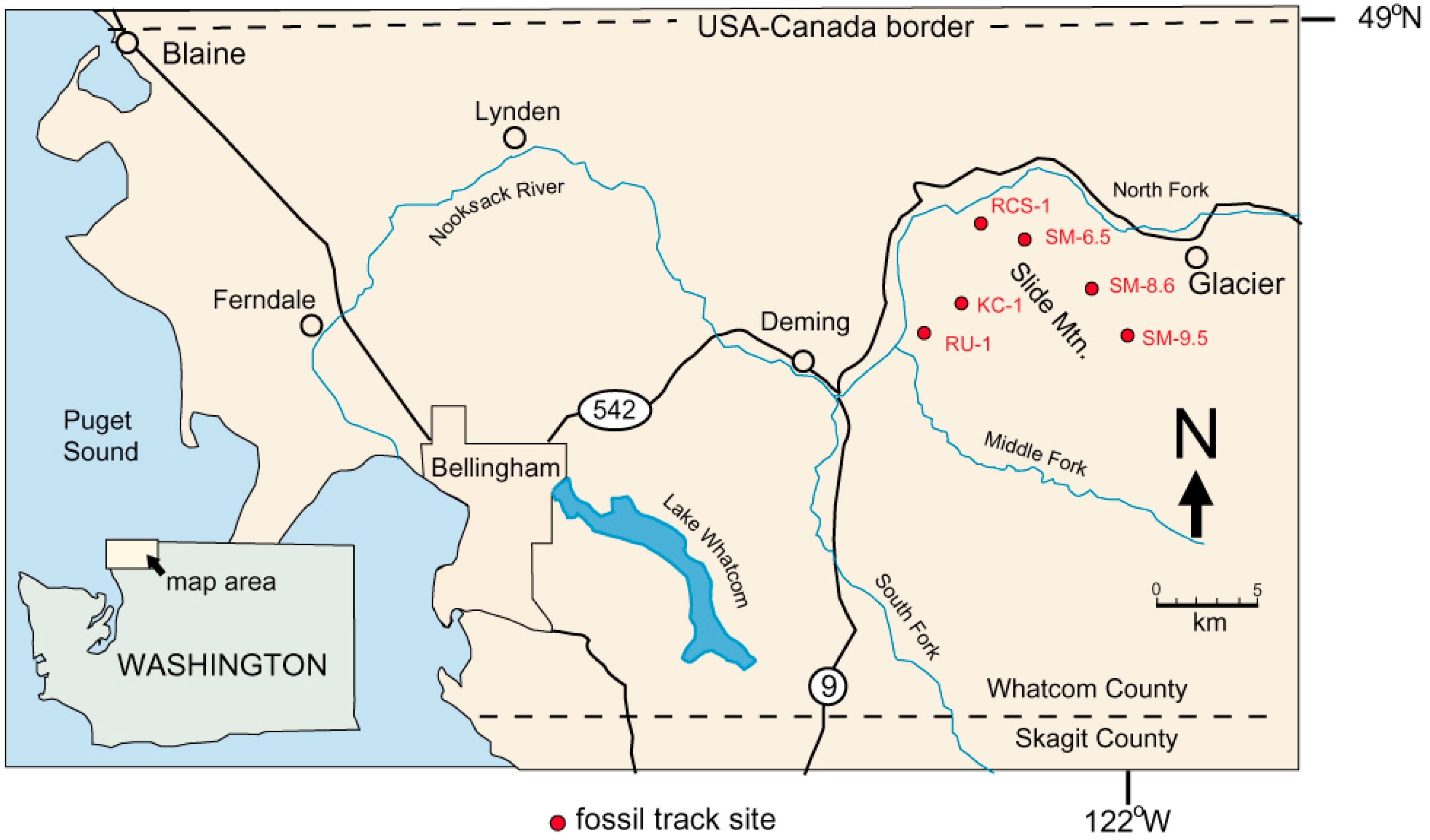







| Location | Locality Number | Latitude | Longitude | Elevation (Meters) | |
|---|---|---|---|---|---|
| WWU * | UWBM ** | ||||
| Kenney Creek | KC-1 | C0644 | 48.841° N | 122.107° W | 627 |
| Racehorse Creek | RCS-1 | C1481 | 48.871° N | 122.118° W | 541 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustoe, G.E. Lower Eocene Footprints from Northwest Washington, USA. Part 1: Reptile Tracks. Geosciences 2019, 9, 321. https://doi.org/10.3390/geosciences9070321
Mustoe GE. Lower Eocene Footprints from Northwest Washington, USA. Part 1: Reptile Tracks. Geosciences. 2019; 9(7):321. https://doi.org/10.3390/geosciences9070321
Chicago/Turabian StyleMustoe, George E. 2019. "Lower Eocene Footprints from Northwest Washington, USA. Part 1: Reptile Tracks" Geosciences 9, no. 7: 321. https://doi.org/10.3390/geosciences9070321
APA StyleMustoe, G. E. (2019). Lower Eocene Footprints from Northwest Washington, USA. Part 1: Reptile Tracks. Geosciences, 9(7), 321. https://doi.org/10.3390/geosciences9070321





