Music Festival Makes Hedgehogs Move: How Individuals Cope Behaviorally in Response to Human-Induced Stressors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Semi-Experimental Design
2.3. Study Animals and Logger Attachment
2.4. Logger Setup
2.5. Data Analysis
2.5.1. Spatial Data Analysis
2.5.2. Acceleration Data Analyses
3. Results
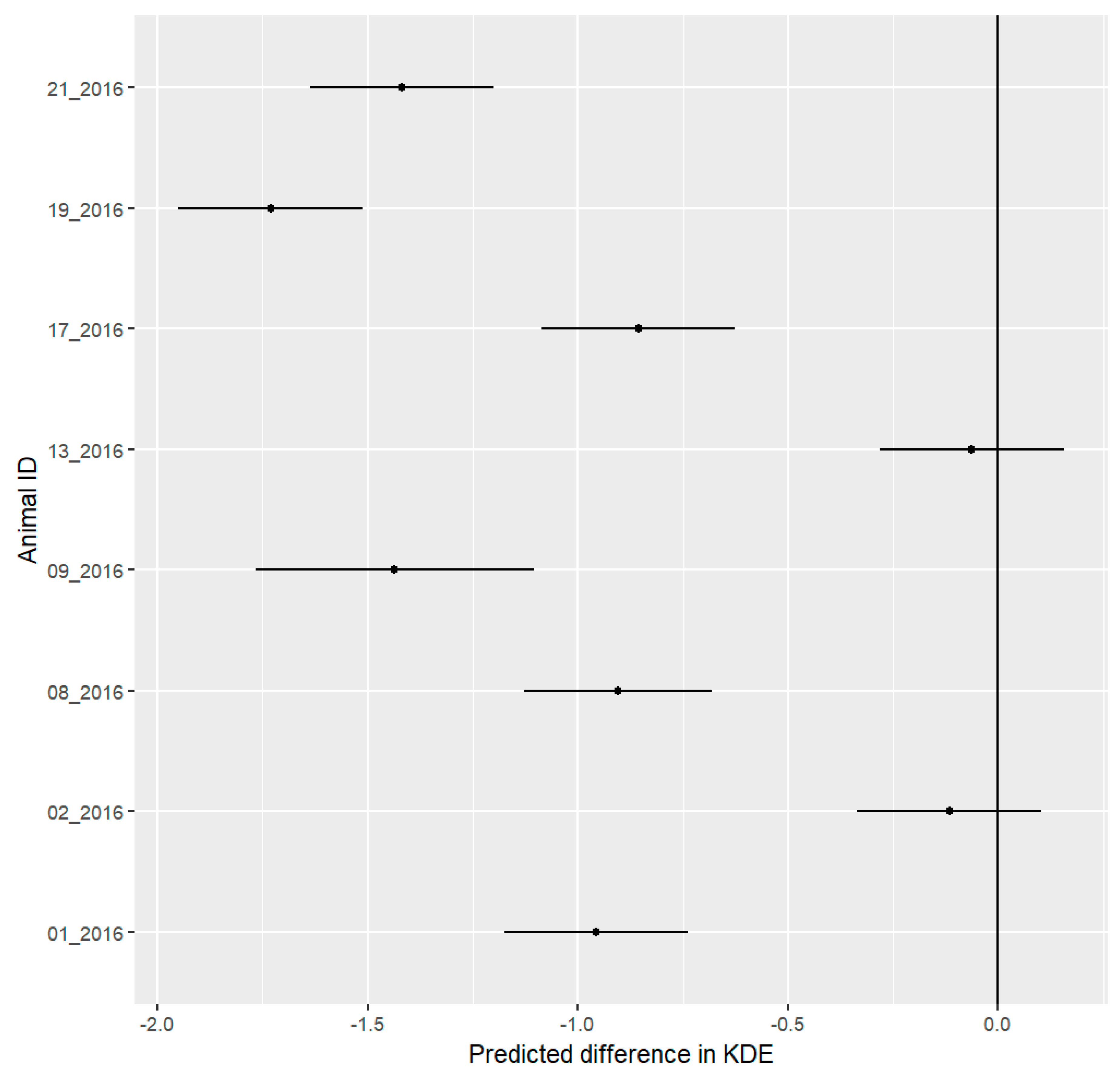
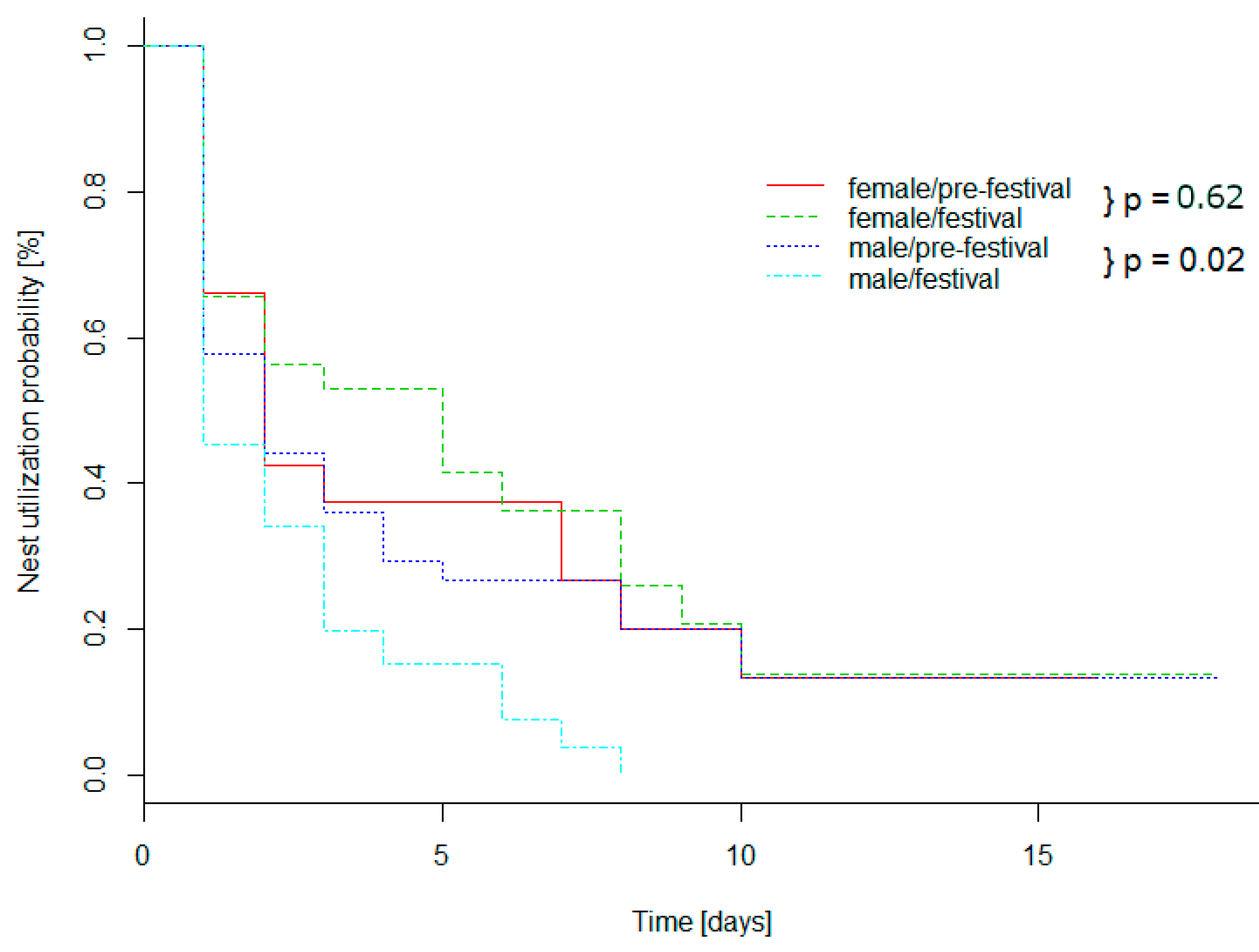
3.1. Spatial Results
3.2. Model Evaluation for Behavior Prediction
3.3. Behavioral Prediction
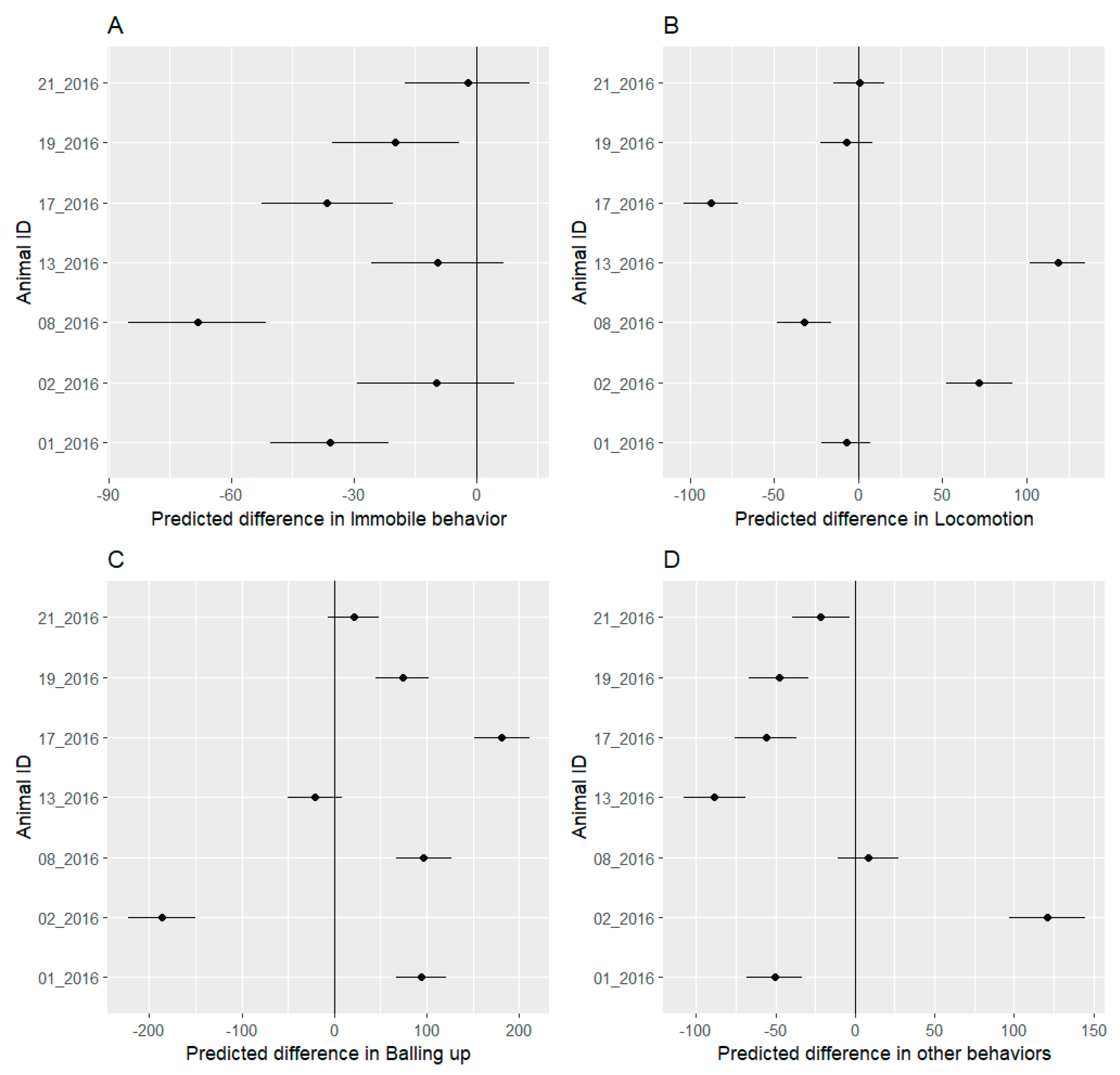
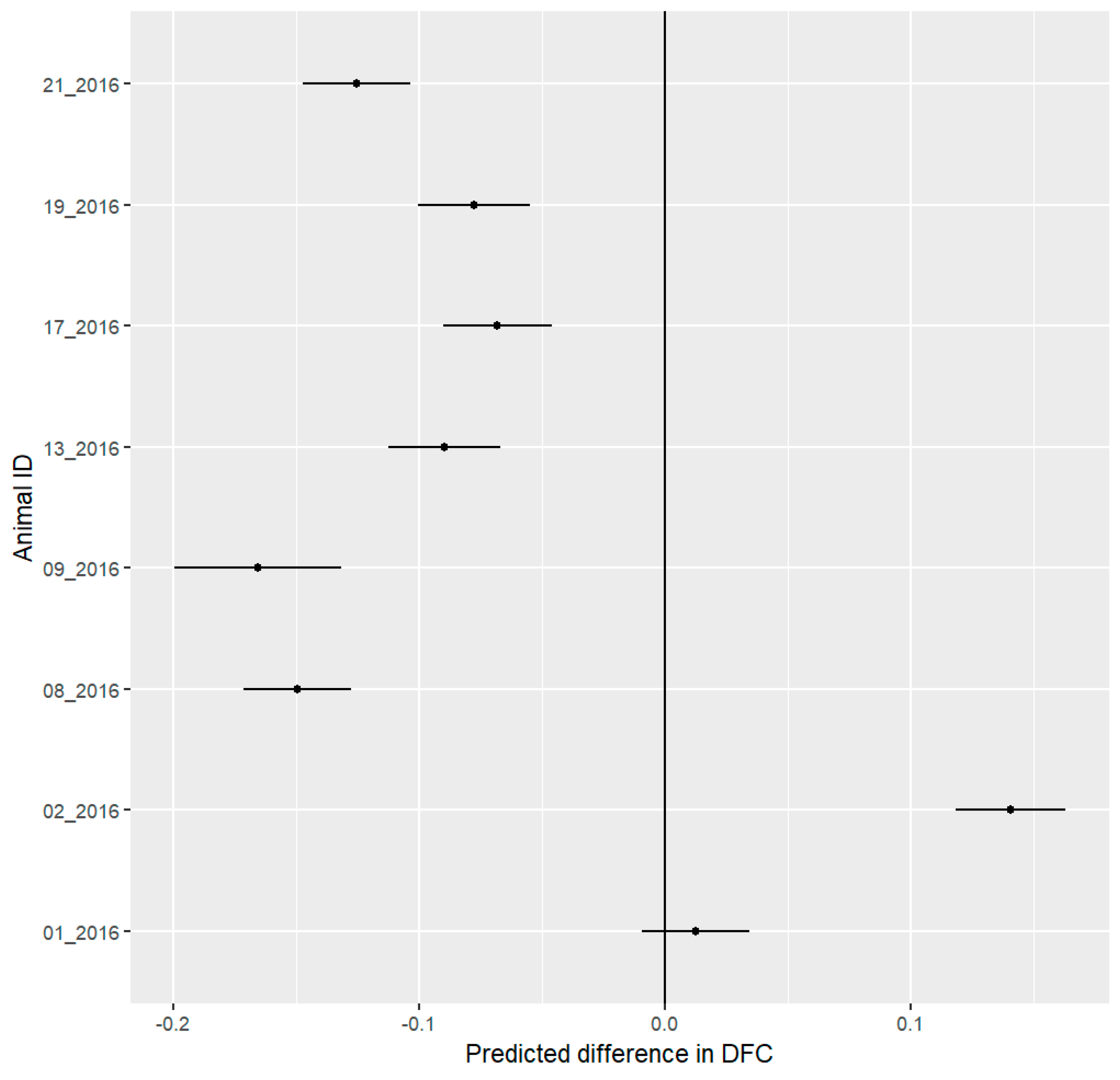
3.4. Behavioral Budget
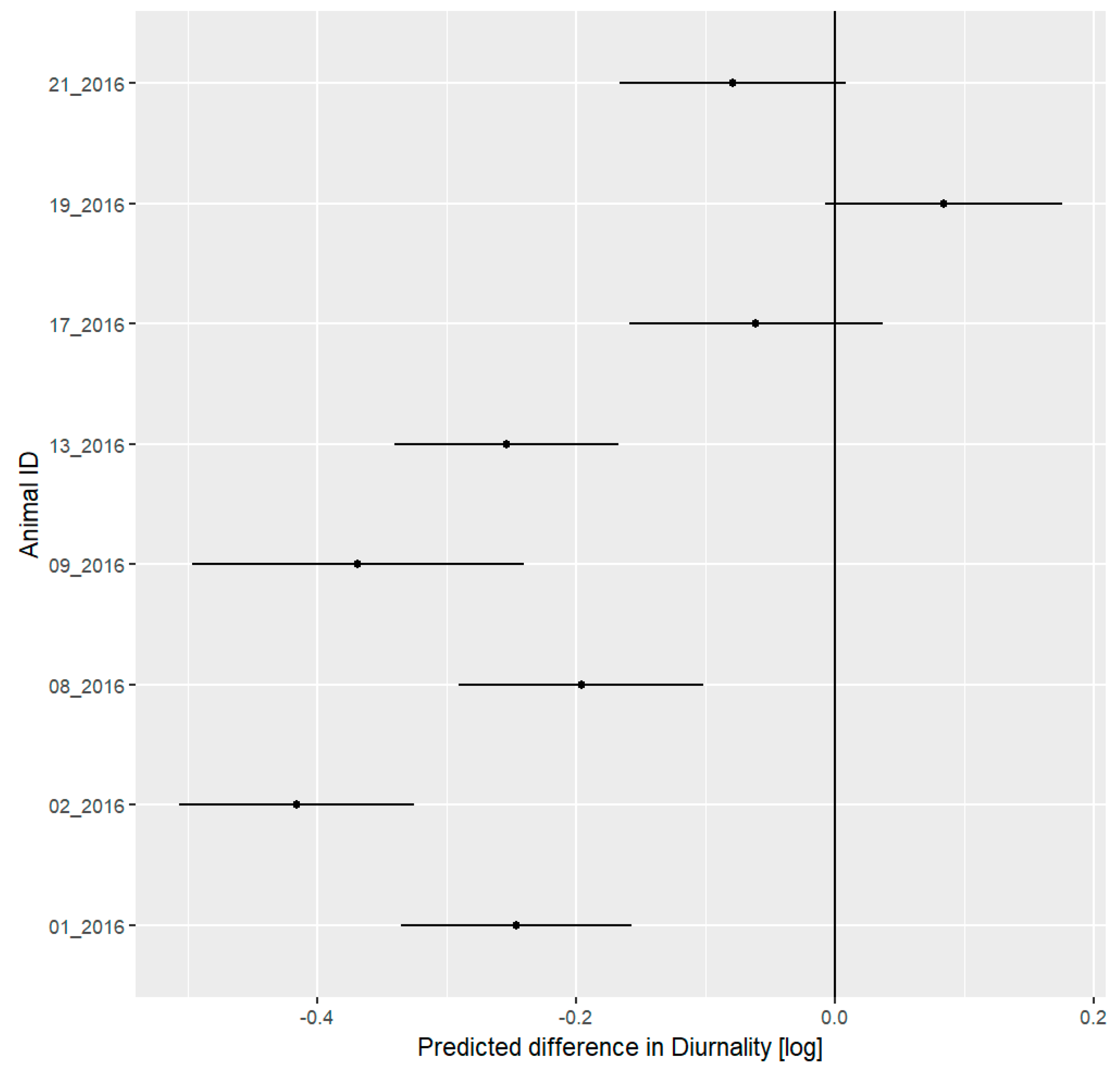
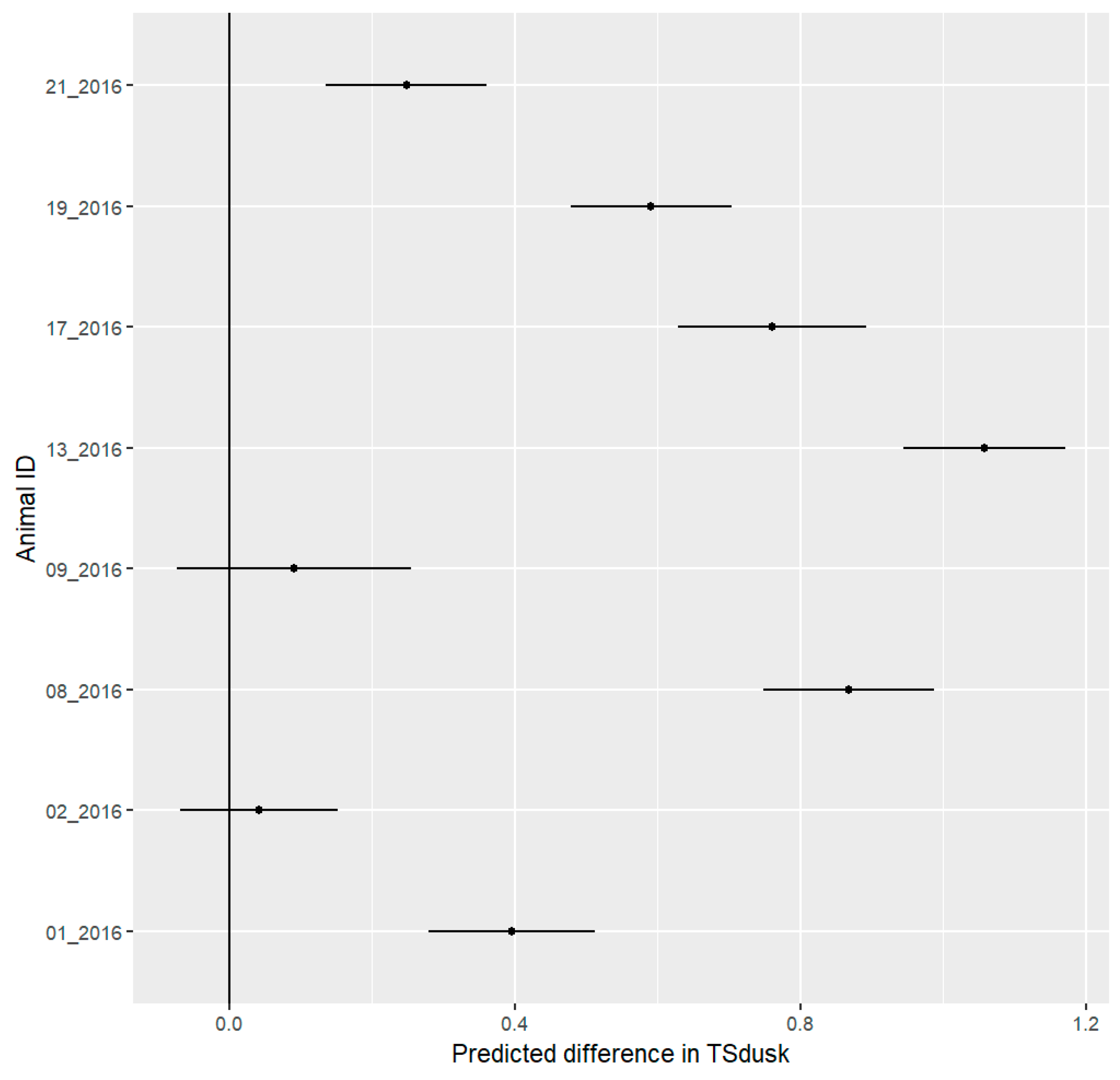
3.5. Daily Activity Pattern
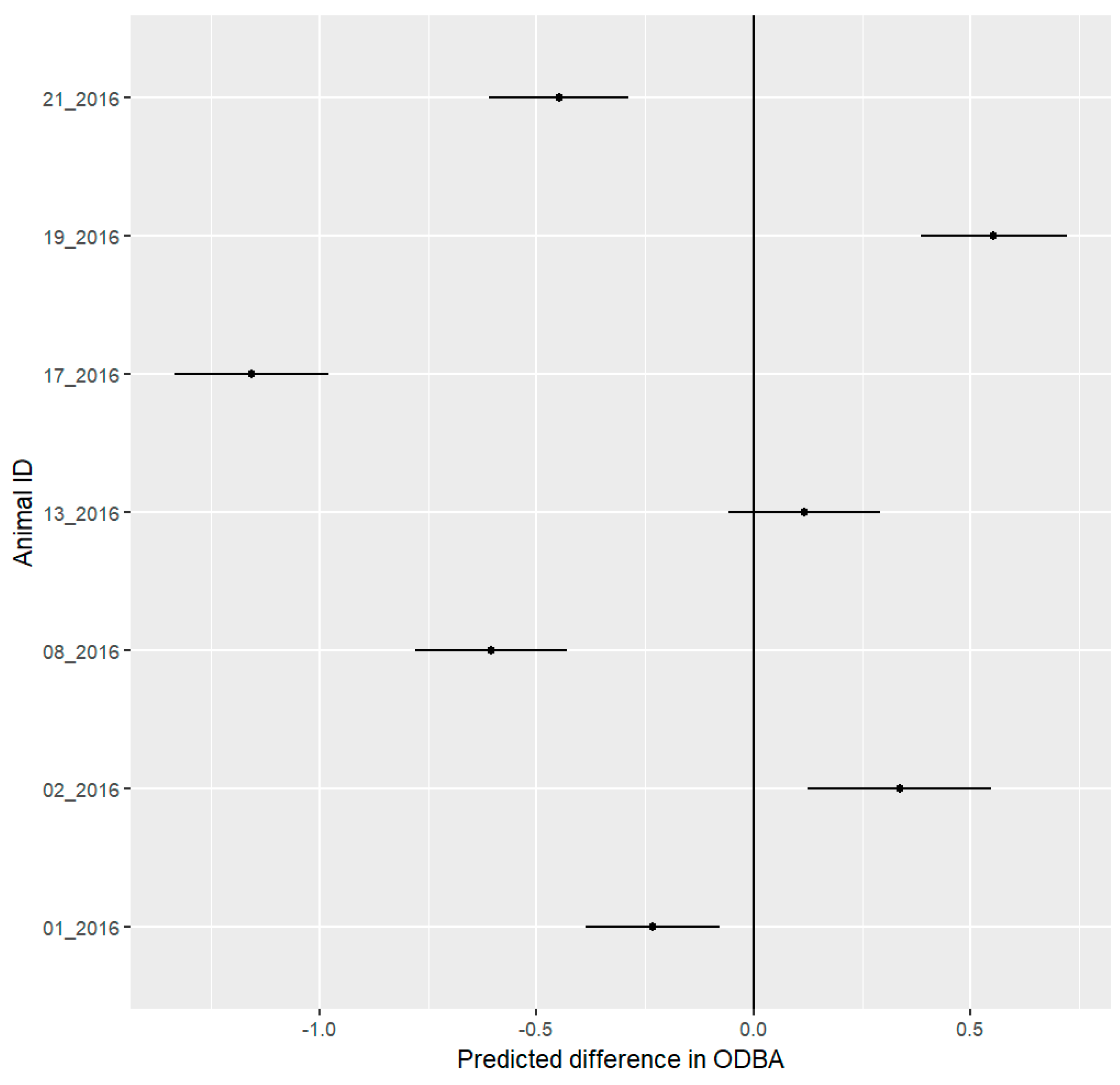
3.6. ODBA Analysis
3.7. Stress Detection
4. Discussion
4.1. Spatial Behavior
4.2. Behavioral Analysis
4.3. Daily Activity Pattern
4.4. ODBA Analysis
4.5. Stress Detection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Munguía, M.; Trejo, I.; González-Salazar, C.; Pérez-Maqueo, O. Human impact gradient on mammalian biodiversity. Glob. Ecol. Conserv. 2016, 6, 79–92. [Google Scholar] [CrossRef]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global Change and the Ecology of Cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Seto, K.C.; Guneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef] [PubMed]
- Lowry, H.; Lill, A.; Wong, B.B.M. Behavioural responses of wildlife to urban environments: Behavioural responses to urban environments. Biol. Rev. 2013, 88, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.D.; Schneider, S.C.; Ahlers, A.A.; Miller, J.R. Categorizing wildlife responses to urbanization and conservation implications of terminology: Terminology and urban conservation. Conserv. Biol. 2015, 29, 1246–1248. [Google Scholar] [CrossRef] [PubMed]
- Sih, A.; Ferrari, M.C.O.; Harris, D.J. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 2011, 4, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Gattermann, R.; Johnston, R.E.; Yigit, N.; Fritzsche, P.; Larimer, S.; Özkurt, S.; Neumann, K.; Song, Z.; Colak, E.; Johnston, J.; et al. Golden hamsters are nocturnal in captivity but diurnal in nature. Biol. Lett. 2008, 4, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Scheibe, K.-M.; Michaelis, S.; Streich, W.J. Evaluation of living conditions of free-ranging animals by automated chronobiological analysis of behavior. Behav. Res. Methods Instrum. Comput. 2003, 35, 458–466. [Google Scholar] [CrossRef]
- Berger, A. Activity patterns, chronobiology and the assessment of stress and welfare in zoo and wild animals: Stress Assessment by Applied Chronobiology. Int. Zoo Yearb. 2011, 45, 80–90. [Google Scholar] [CrossRef]
- Williams, B.M.; Baker, P.J.; Thomas, E.; Wilson, G.; Judge, J.; Yarnell, R.W. Reduced occupancy of hedgehogs (Erinaceus europaeus) in rural England and Wales: The influence of habitat and an asymmetric intra-guild predator. Sci. Rep. 2018, 8, 12156. [Google Scholar] [CrossRef]
- Van de Poel, J.L.; Dekker, J.; van Langevelde, F. Dutch hedgehogs Erinaceus europaeus are nowadays mainly found in urban areas, possibly due to the negative Effects of badgers Meles meles. Wildl. Biol. 2015, 21, 51–55. [Google Scholar] [CrossRef]
- Hubert, P.; Julliard, R.; Biagianti, S.; Poulle, M.-L. Ecological factors driving the higher hedgehog (Erinaceus europeaus) density in an urban area compared to the adjacent rural area. Landsc. Urban Plan. 2011, 103, 34–43. [Google Scholar] [CrossRef]
- Morris, P. Hedgehog; William Collins: Sydney, Austrilia, 2018; ISBN 978-0-00-823573-4. [Google Scholar]
- Stache, A.; Heller, E.; Hothorn, T.; Heurich, M. Activity patterns of European roe deer (Capreolus capreolus) are strongly influenced by individual behaviour. Folia Zool. 2013, 62, 67–75. [Google Scholar] [CrossRef]
- Lesmerises, R.; St-Laurent, M.-H. Not accounting for interindividual variability can mask habitat selection patterns: A case study on black bears. Oecologia 2017, 185, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Mori, E.; Menchetti, M.; Bertolino, S.; Mazza, G.; Ancillotto, L. Reappraisal of an old cheap method for marking the European hedgehog. Mammal Res. 2015, 60, 189–193. [Google Scholar] [CrossRef]
- Reeve, N.; Bowen, C.; Gurnell, J. An Improved Identification Marking Method for Hedgehogs; The Mammal Society: Southampton, UK, 2019. [Google Scholar]
- Barthel, L.M.F.; Hofer, H.; Berger, A. An easy, flexible solution to attach devices to hedgehogs (Erinaceus europaeus) enables long-term high-resolution studies. Ecol. Evol. 2019, 9, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Sikes, R.S.; Gannon, W.L. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2011, 92, 235–253. [Google Scholar] [CrossRef]
- Calenge, C. The package “adehabitat” for the R software: A tool for the analysis of space and habitat use by animals. Ecol. Model. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, Inc.: Boston, MA, USA, 2016. [Google Scholar]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000; ISBN 0-387-98784-3. [Google Scholar]
- Cibis, J. Automatic Behaviour Classification from Three-Axial Acceleration Data for the European Hedgehog (Erinaceus europaeus L., 1758). Master’s Thesis, Humbold-Universität zu Berlin, Berlin, Germany, 2017. [Google Scholar]
- Rast, W. Accelerater: Process Acceleration Data. Available online: https://github.com/wanjarast/accelerateR (accessed on 18 July 2019).
- Nathan, R.; Spiegel, O.; Fortmann-Roe, S.; Harel, R.; Wikelski, M.; Getz, W.M. Using tri-axial acceleration data to identify behavioral modes of free-ranging animals: General concepts and tools illustrated for griffon vultures. J. Exp. Biol. 2012, 215, 986–996. [Google Scholar] [CrossRef]
- Collins, P.M.; Green, J.A.; Warwick-Evans, V.; Dodd, S.; Shaw, P.J.A.; Arnould, J.P.Y.; Halsey, L.G. Interpreting behaviors from accelerometry: A method combining simplicity and objectivity. Ecol. Evol. 2015, 5, 4642–4654. [Google Scholar] [CrossRef]
- Wilson, R.P.; White, C.R.; Quintana, F.; Halsey, L.G.; Liebsch, N.; Martin, G.R.; Butler, P.J. Moving towards acceleration for estimates of activity-specific metabolic rate in free-living animals: The case of the cormorant: Activity-specific metabolic rate in free-living animals. J. Anim. Ecol. 2006, 75, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Martiskainen, P.; Järvinen, M.; Skön, J.-P.; Tiirikainen, J.; Kolehmainen, M.; Mononen, J. Cow behaviour pattern recognition using a three-dimensional accelerometer and support vector machines. Appl. Anim. Behav. Sci. 2009, 119, 32–38. [Google Scholar] [CrossRef]
- Bidder, O.R.; Campbell, H.A.; Gómez-Laich, A.; Urgé, P.; Walker, J.; Cai, Y.; Gao, L.; Quintana, F.; Wilson, R.P. Love Thy Neighbour: Automatic Animal Behavioural Classification of Acceleration Data Using the K-Nearest Neighbour Algorithm. PLoS ONE 2014, 9, e88609. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Dimitriadou, E.; Hornik, K.; Weingessel, A.; Leisch, F. e1071: Misc Functions of the Department of Statistics, Probability Theory Group (Formerly: E1071), TU Wien. R Package version 3.5.1. 2017. Available online: https://cran.r-project.org/web/packages/e1071/index.html (accessed on 18 July 2019).
- Hoogenboom, I.; Daan, S.; Dallinga, J.H.; Schoenmakers, M. Seasonal change in the daily timing of behaviour of the common vole, Microtus arvalis. Oecologia 1984, 61, 18–31. [Google Scholar] [CrossRef]
- Halsey, L.G.; Shepard, E.L.C.; Quintana, F.; Gomez Laich, A.; Green, J.A.; Wilson, R.P. The relationship between oxygen consumption and body acceleration in a range of species. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 152, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Qasem, L.; Cardew, A.; Wilson, A.; Griffiths, I.; Halsey, L.G.; Shepard, E.L.C.; Gleiss, A.C.; Wilson, R. Tri-Axial Dynamic Acceleration as a Proxy for Animal Energy Expenditure; Should We Be Summing Values or Calculating the Vector? PLoS ONE 2012, 7, e31187. [Google Scholar] [CrossRef]
- Rousset, F.; Ferdy, J.-B. Testing environmental and genetic effects in the presence of spatial autocorrelation. Ecography 2014, 37, 781–790. [Google Scholar] [CrossRef]
- Tucker, M.A.; Böhning-Gaese, K.; Fagan, W.F.; Fryxell, J.M.; Moorter, B.V.; Alberts, S.C.; Ali, A.H.; Allen, A.M.; Attias, N.; Avgar, T.; et al. Moving in the Anthropocene: Global reductions in terrestrial mammalian movements. Science 2018, 359, 466–469. [Google Scholar] [CrossRef]
- Reeve, N.J.; Morris, P.A. Construction and use of summer nests by the hedgehog (Erinaceus europaeus). Mammalia 1985, 49, 187–194. [Google Scholar] [CrossRef]
- Schneirla, T.C. The relationship between observation and experimentation in the field study of behavior. Ann. N. Y. Acad. Sci. 1950, 51, 1022–1044. [Google Scholar] [CrossRef]
- Lima, S.L. Stress and Decision Making under the Risk of Predation: Recent Developments from Behavioral, Reproductive, and Ecological Perspectives. In Advances in the Study of Behavior; Elsevier: Amsterdam, The Netherlands, 1998; Volume 27, pp. 215–290. ISBN 978-0-12-004527-3. [Google Scholar]
- Speakman, J.R. The physiological costs of reproduction in small mammals. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 375–398. [Google Scholar] [CrossRef]
- Ordiz, A.; Kindberg, J.; Sæbø, S.; Swenson, J.E.; Støen, O.-G. Brown bear circadian behavior reveals human environmental encroachment. Biol. Conserv. 2014, 173, 1–9. [Google Scholar] [CrossRef]
- Gaynor, K.M.; Hojnowski, C.E.; Carter, N.H.; Brashares, J.S. The influence of human disturbance on wildlife nocturnality. Science 2018, 360, 1232–1235. [Google Scholar] [CrossRef]
- Shirley, M.D.F.; Armitage, V.L.; Barden, T.L.; Gough, M.; Lurz, P.W.W.; Oatway, D.E.; South, A.B.; Rushton, S.P. Assessing the impact of a music festival on the emergence behaviour of a breeding colony of Daubenton’s bats (Myotis daubentonii). J. Zool. 2001, 254, 367–373. [Google Scholar] [CrossRef]
- Krone, O.; Berger, A.; Schulte, R. Recording movement and activity pattern of a White-tailed Sea Eagle (Haliaeetus albicilla) by a GPS datalogger. J. Ornithol. 2009, 150, 273–280. [Google Scholar] [CrossRef]
- Langbein, J.; Scheibe, K.-M.; Eichhorn, K. Investigations on periparturient behaviour in free-ranging mouflon sheep (Ovis orientalis musimon). J. Zool. 1998, 244, 553–561. [Google Scholar] [CrossRef]
- Both, C.; Dingemanse, N.J.; Drent, P.J.; Tinbergen, J.M. Pairs of extreme avian personalities have highest reproductive success. J. Anim. Ecol. 2005, 74, 667–674. [Google Scholar] [CrossRef]
- Hof, A.R. A Study of the Current Status of the Hedgehog (Erinaceus europaeus), and Its Decline in Great Britain Since 1960. PhD Thesis, Royal Holloway, University of London, London, UK, 2009. [Google Scholar]
- Johnson, H.; Reeve, N.J.; Baker, P.; Yarnell, R.; Allen, S.; Petrovan, S. Conservation Strategy for West-European Hedgehog (Erinaceus europaeus) in the United Kingdom (2015–2025); People’s Trust for Endangered Species: London, UK, 2015. [Google Scholar]
- Müller, F. Langzeit-Monitoring der Stra\s senverkerhsopfer beim Igel (Erinaceus europaeus L.) zur Indikation von Populationsdichteveränderungen entlang zweier Teststrecken im Landkreis Fulda. Beitr. Naturkunde Osthess. 2018, 54, 21–26. [Google Scholar]
- Pettett, C.E.; Johnson, P.J.; Moorhouse, T.P.; Macdonald, D.W. National predictors of hedgehog Erinaceus europaeus distribution and decline in Britain. Mammal Rev. 2018, 48, 1–6. [Google Scholar] [CrossRef]
- Roos, S.; Johnston, A.; Noble, D. UK hedgehog datasets and their potential for long-term monitoring. BTO Res. Rep. 2012, 598, 1–63. [Google Scholar]
- Wilson, E.; Wembridge, D. The State of Britain’s Hedgehogs 2018; People’s Trust for Endangered Species: London, UK, 2018. [Google Scholar]
- Bottinelli, N.; Capowiez, Y.; Ranger, J. Slow recovery of earthworm populations after heavy traffic in two forest soils in northern France. Appl. Soil Ecol. 2014, 73, 130–133. [Google Scholar] [CrossRef]
- Geffroy, B.; Samia, D.S.M.; Bessa, E.; Blumstein, D.T. How Nature-Based Tourism Might Increase Prey Vulnerability to Predators. Trends Ecol. Evol. 2015, 30, 755–765. [Google Scholar] [CrossRef]
- Samia, D.S.M.; Nakagawa, S.; Nomura, F.; Rangel, T.F.; Blumstein, D.T. Increased tolerance to humans among disturbed wildlife. Nat. Commun. 2015, 6, 8877. [Google Scholar] [CrossRef]
- Réale, D.; Reader, S.M.; Sol, D.; McDougall, P.T.; Dingemanse, N.J. Integrating animal temperament within ecology and evolution. Biol. Rev. 2007, 82, 291–318. [Google Scholar] [CrossRef]
| Animal ID | Sex | Body Mass (g) |
|---|---|---|
| 01_2016 | m | 1060 |
| 02_2016 | f | 1090 |
| 08_2016 | f | 795 |
| 09_2016 | m | 830 |
| 13_2016 | f | 725 |
| 17_2016 | f | 1480 |
| 19_2016 | m | 890 |
| 21_2016 | m | 1015 |
| Behavior | Recall | Precision |
|---|---|---|
| Immobile | 0.77 | 0.88 |
| Balling up | 0.78 | 0.90 |
| Locomotion | 0.91 | 0.93 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rast, W.; Barthel, L.M.F.; Berger, A. Music Festival Makes Hedgehogs Move: How Individuals Cope Behaviorally in Response to Human-Induced Stressors. Animals 2019, 9, 455. https://doi.org/10.3390/ani9070455
Rast W, Barthel LMF, Berger A. Music Festival Makes Hedgehogs Move: How Individuals Cope Behaviorally in Response to Human-Induced Stressors. Animals. 2019; 9(7):455. https://doi.org/10.3390/ani9070455
Chicago/Turabian StyleRast, Wanja, Leon M.F. Barthel, and Anne Berger. 2019. "Music Festival Makes Hedgehogs Move: How Individuals Cope Behaviorally in Response to Human-Induced Stressors" Animals 9, no. 7: 455. https://doi.org/10.3390/ani9070455
APA StyleRast, W., Barthel, L. M. F., & Berger, A. (2019). Music Festival Makes Hedgehogs Move: How Individuals Cope Behaviorally in Response to Human-Induced Stressors. Animals, 9(7), 455. https://doi.org/10.3390/ani9070455






