Evaluation of Performance, Nitrogen Metabolism and Tissue Composition in Barrows Fed an n-3 PUFA-Rich Diet
Abstract
Simple Summary
Abstract
1. Introduction
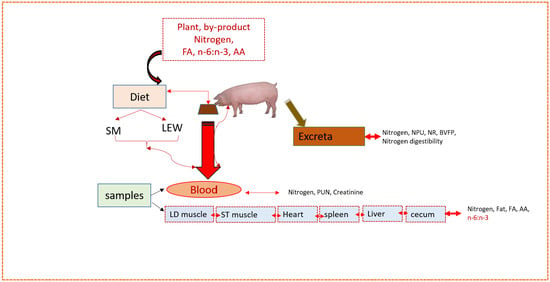
2. Materials and Methods
2.1. Experimental Design and Diets
2.2. Trial 1: Nitrogen Balance, Plasma Creatinine and Urea Concentration
2.3. Trial 2: Performance and Tissue Chemical Composition
2.4. Calculation and Statistical Analysis
3. Results
3.1. Trial 1: Nitrogen Balance, Plasma Creatinine and Urea Concentration
3.2. Trial 2: Performance and Tissue Composition
3.2.1. Growth Performance
3.2.2. Tissue Chemical Composition
3.2.3. Distribution of Fatty Acids and Amino Acids
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nielsen, K.L.; Hartvigsen, M.L.; Hedemann, M.S.; Lærke, H.N.; Hermansen, K.; Knudsen, K.E.B. Similar metabolic responses in pigs and humans to breads with different contents and compositions of dietary fibers: A metabolomics study. Am. J. Clin. Nutr. 2014, 99, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Vigors, S.; Sweeney, T.; O’Shea, C.J.; Kelly, A.K.; O’Doherty, J.V. Pigs that are divergent in feed efficiency, differ in intestinal enzyme and nutrient transporter gene expression, nutrient digestibility and microbial activity. Animal 2016, 10, 1848–1855. [Google Scholar] [CrossRef]
- Patience, J.F.; Rossoni-Serão, M.C.; Gutiérrez, N.A. A review of feed efficiency in swine: Biology and application. J. Anim. Sci. Biotechnol. 2015, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.J.; Patience, J.F.; Lonergan, S.M.; Dekkers, J.M.; Gabler, N.K. Improved nutrient digestibility and retention partially explains feed efficiency gains in pigs selected for low residual feed intake. J. Anim. Sci. 2012, 90, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Kohn, R.A.; Dinneen, M.M.; Russek-Cohen, E. Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs, and rats. J. Anim. Sci. 2005, 83, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Dugan, M.E.R.; Vahmani, P.; Turner, T.D.; Mapiye, C.; Juárez, M.; Prieto, N.; Beaulieu, A.D.; Zijlstra, R.T.; Patience, J.F.; Aalhus, J.L. Pork as a source of omega-3 (n-3) fatty acids. J. Clin. Med. 2015, 4, 1999–2011. [Google Scholar] [CrossRef]
- CVB Feed Table 2016. Chemical Composition and Nutritional Values of Feedstuffs. Available online: www.cvbdiervoeding.nl (accessed on 3 May 2019).
- Untea, A.E.; Criste, R.D.; Vladescu, L. Development and validation of a liver samples preparation method for FAAS trace elements content determination. Rev. Chim. 2012, 63, 341–346. [Google Scholar]
- Moreira, I.; Fraga, A.L.; Paiano, D.; Oliveira, G.C.; Scapinello, C.; Martins, E.N. Nitrogen balance of starting barrow pigs fed on increasing lysine levels. Braz. Arch. Biol. Techn. 2004, 47, 85–91. [Google Scholar] [CrossRef]
- White, G.A.; Smith, L.A.; Haudijk, J.G.M.; Homer, D.; Kyriazakis, I.; Wiseman, J. Replacement of soya bean meal with peas and faba beans in growing/finishing pig diets: Effect on performance, carcass composition and nutrient excretion. Anim. Feed Sci. Tech. 2015, 209, 202–210. [Google Scholar] [CrossRef]
- Varzaru, I.; Untea, A.E.; Martura, T.; Olteanu, M.; Panaite, T.D.; Schitea, M.; Van, I. Development and validation of an RP-HPLC method for methionine, cystine and lysine separation and determination in corn samples. Rev. Chim. 2013, 64, 673–679. [Google Scholar]
- Hăbeanu, M.; Thomas, A.; Bispo, E.; Gobert, M.; Gruffat, D.; Durand, D.; Bauchart, D. Extruded linseed and rapeseed both influenced fatty acid composition of total lipids and their polar and neutral fractions in longissimus thoracis and semitendinosus muscles of finishing Normand cows. Meat Sci. 2014, 96, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Adeola, O. Digestion and balance techniques in pigs. In Swine Nutrition; Lewis, A.J., Southern, L.L., Eds.; CRC Press: Boca Raton, FL, USA, 2001; Available online: https://www.crcpress.com/Swine-Nutrition-Second-Edition/Lewis-Southern/p/book/9780849306969 (accessed on 3 May 2019).
- Seong, P.N.; Park, K.M.; Cho, S.H.; Kang, S.M.; Kang, G.H.; Park, B.Y.; Moon, S.S.; Ba, H.V. Characterization of Edible Pork By-products by Means of Yield and Nutritional Composition. Korean J. Food Sci. 2014, 34, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Mariscal-Landin, G.; Souza, T.C.R.; Ramirez, E. Metabolizable energy, nitrogen balance, and ileal digetsibility of amino acids in quality protein maize for pigs. J. Anim. Sci. Biotechnol. 2014, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Lea, M.H.A.; Buchet, A.D.G.; Beltranena, E.; Gerrits, W.J.J.; Zijlstra, R.T. Digestibility and intestinal fermentability of canola meal from Brassica juncea and Brassica napus fed to ileal-cannulated grower pigs. Anim. Feed Sci. Tech. 2017, 234, 43–53. [Google Scholar] [CrossRef]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implication cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Leslie, M.A.; Cohen, D.J.; Liddle, D.M.; Robinson, L.E.; Ma, D.W. A review of the effect of omega-3 polyunsaturated fatty acids on blood triacylglycerol levels in normolipidemic and borderline hyperlipidemic individuals. Lipids Health Dis. 2015, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Kim, I.H. Effects of different dietary n-6:n-3 PUFA ratios on growth performance, blood lipid profiles, fatty acid composition of pork, carcass traits and meat quality in finishing pigs. Ann. Anim. Sci. 2018, 18, 143–154. [Google Scholar] [CrossRef]
- Bingham, S.A. Urine Nitrogen as a Biomarker for the Validation of Dietary Protein Intake. Supplement publication “Biomarkers of Nutritional Exposure and Nutritional Status”. J. Nutr. 2003, 133, 921S–924S. [Google Scholar] [CrossRef] [PubMed]
- Hlatini, V.A.; Zindove, T.J.; Chimonyo, M. The influence of polyethylene glycol inclusion in Vachellia tortilis leaf meal on nitrogen balance in growing pigs. S. Afr. J. Anim. Sci. 2017, 47, 298–306. [Google Scholar] [CrossRef][Green Version]
- Loh, T.C.; Wang, W.S.; Foo, H.L. Effects of dietary protein and inulin on growth and nitrogen balance in growing pigs. J. Appl. Anim. Res. 2010, 38, 55–59. [Google Scholar] [CrossRef]
- Whang, K.Y.; Easter, R.A. Blood urea nitrogen as an index of feed efficiency and lean growth potential in growing-finishing swine. Asian Austral. J. Anim. 2000, 13, 811–816. [Google Scholar] [CrossRef]
- Liao, S.F.; Wang, T.; Regmi, N. Lysine nutrition in swine and the related monogastric animals: Muscle protein biosynthesis and beyond. SpringerPlus 2015, 4, 147. [Google Scholar] [CrossRef] [PubMed]
- The Merck Veterinary Manual; Merck & Co. Inc.: Kenilworth, NJ, USA, 2015.
- Friendship, R.M.; Lumsden, J.H.; McMillan, I.; Wilson, M.R. Hematology and biochemistry reference values for Ontario swine. Can. J. Comp. Med. 1984, 48, 390–393. [Google Scholar] [PubMed]
- Kabalin, A.E.; Balenović, T.; Šperanda, M.; Milinković-Tur, S.; Štoković, I.; Menčik, S.; Maurić, M.; Pavičić, Ž. Serum biochemical parameters in suckling piglets with low and average birth mass. Vet. Arhiv. 2017, 87, 171–184. [Google Scholar]
- Turner, T.D.; Mapiye, C.; Aalhus, J.L.; Beaulieu, A.D.; Patience, J.F.; Zijlstra, R.T.; Dugan, M.E.R. Flaxseed fed pork: N−3 fatty acid enrichment and contribution to dietary recommendations. Meat Sci. 2014, 96, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, R.; Wang, W.; Wu, Z.; Dai, Z.; Wang, J.; Wu, G. Biochemical and physiological bases for utilization of dietary amino acids by young pigs. J. Anim. Sci. Biotechnol. 2013, 4, 7. [Google Scholar] [CrossRef] [PubMed]
| Ingredients g/kg (as-Fed Basis) | Control (SM Diet) a | Experimental (LEW Diet) b |
|---|---|---|
| Ground corn | 380.4 | 343.0 |
| Millet | 250 | 250 |
| Rice flour | 170 | 170 |
| Extruded linseed:walnut meal, 50:50 (wt/wt) mix | 0 | 60 |
| Soybean meal (44%) | 160 | 140 |
| Soybean oil | 5 | 2 |
| DL-methionine | 0.3 | 0.7 |
| L-Lysine HCl | 2.7 | 2.7 |
| Carbonate calcium | 14.5 | 14.5 |
| Phytase | 0.1 | 0.1 |
| Monocalcium phosphate | 5 | 5 |
| Salt | 1 | 1 |
| Premix choline | 1 | 1 |
| Vitamin/mineral premix c | 10 | 10 |
| Nutritional value g/kg (as-fed basis) | ||
| Metabolizable energy (EM, MJ/kg) | 12.81 | 12.80 |
| Crude protein | 160 | 164.2 |
| Lys d | 9.7 | 9.7 |
| SID Lys 4 | 8.0 | 8.1 |
| Met + Cys d | 6.0 | 6.2 |
| SID Met + Cys 4 | 5.0 | 5.3 |
| Calcium | 8.0 | 8.0 |
| Total Phosphorus | 5.9 | 5.9 |
| Fatty acids composition (% FAME) | ||
| ALA d | 1.88 | 15.25 |
| LA d | 50.73 | 39.68 |
| ⅀ n-3 | 2.28 | 15.58 |
| ⅀ n-6 | 51.4 | 40.0 |
| n-6:n-3 PUFA | 22.54 | 2.56 |
| ITEMS (g/day) ** | SM Diet | LEW Diet | SEM | p Value * |
|---|---|---|---|---|
| Feed intake | 2780 | 2770 | 0.05 | 0.95 |
| Dry matter (DM) intake | 2460 | 2450 | 0.04 | 0.95 |
| Nitrogen intake | 70.76 | 72.91 | 0.45 | 0.46 |
| Fecal DM | 251.12 | 238.32 | 7.24 | 0.50 |
| Nitrogen intake (DM basis) | 34.81 | 34.71 | 0.06 | 0.46 |
| Fecal nitrogen | 7.95 | 8.11 | 0.30 | 0.78 |
| Urinary nitrogen | 10.81 | 8.24 | 0.49 | 0.008* |
| TNO | 18.76 | 16.36 | 0.59 | 0.042* |
| NAB | 26.86 | 25.60 | 0.31 | 0.041* |
| NR | 16.05 | 17.35 | 0.58 | 0.26 |
| Nitrogen excretion of % intake | 53.90 | 48.52 | 1.70 | 0.11 |
| NPU % | 46.09 | 51.47 | 1.70 | 0.11 |
| BVFP % | 58.94 | 67.67 | 1.94 | 0.02* |
| Nitrogen digestibility % | 77.16 | 75.93 | 0.88 | 0.48 |
| CTTAD | 0.77 | 0.76 | 0.08 | 0.48 |
| CAM | 0.46 | 0.51 | 0.017 | 0.11 |
| Plasma parameter | ||||
| Nitrogen g·mL−1 | 89.16 | 89.00 | 0.82 | 0.92 |
| PUN mg·dL−1 | 10.3 | 10.9 | 0.27 | 0.27 |
| Creatinine mg·dL−1 ** | 1.11 | 1.00 | 0.02 | 0.03 * |
| PUN:creatinine | 7.62 | 8.88 | 0.23 | 0.006 ** |
| Specification * (kg) | SM Diet | LEW Diet | SEM | p Value |
|---|---|---|---|---|
| Initial weight | 32.4 | 30.6 | 0.60 | 0.14 |
| Final weight | 55.64 | 54.68 | 0.97 | 0.62 |
| BWG | 23.10 | 24.10 | 0.26 | 0.06 |
| ADG | 1.10 | 1.14 | 0.03 | 0.053 |
| Gain to feed | 0.40 | 0.42 | 0.009 | 0.29 |
| RFI | +1.08 | +1.07 | 0.05 | 0.30 |
| Specification (g × 100 g DM−1) * | SM Diet | LEW Diet | SEM | p Value ** | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LD | ST | Heart | Spleen | Liver | LD | ST | Heart | Spleen | Liver | Diet Effect | Tissue Effect | Diet × Tissue | ||
| DM % | 26.41 | 25.12 | 19.49 | 27.12 | 25.36 | 25.56 | 28.03 | 18.24 | 23.09 | 28.29 | 0.89 | 0.97 | 0.009 | 0.47 |
| EE | 3.82 | 5.06 | 2.25 | 5.08 | 2.19 | 3.67 | 5.62 | 1.96 | 5.74 | 2.64 | 0.36 | 0.74 | <0.0001 | 0.93 |
| N | 3.34 | 3.0 | 2.46 | 2.50 | 2.85 | 3.27 | 3.31 | 2.32 | 2.32 | 3.33 | 0.10 | 0.7 | <0.0001 | 0.37 |
| Fatty Acids(%)* | SM | LEW | SM Diet | LEW Diet | SEM | p Value ** | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DIET | LD | ST | Heart | Spleen | Liver | Cecum | LD | ST | Heart | Spleen | Liver | Cecum | Diet Effect | Tissue Effect | Diet × Tissue | |||
| LA | 16.42 | 1.6 | 13.05 | 13.9 | 23.19 | 11.49 | 20.19 | 16.71 | 13.18 | 14.7 | 22.47 | 10.84 | 21.31 | 13.84 | 0.67 | 0.82 | <0.0001 | 0.63 |
| ALA | 0.5 | 1.83 | 1.35 | 1.36 | 0.43 | 1.00 | 0.38 | 0.46 | 1.55 | 1.44 | 2.34 | 1.69 | 1.86 | 2.79 | 0.12 | <0.001 | 0.45 | 0.018 |
| DPA | 0.25 | 0.12 | 0.10 | 0.06 | 0.30 | 0.17 | 1.49 | 0 | 0.2 | 0.005 | 0 | 0.28 | 0.80 | 0.13 | 0.12 | 0.69 | <0.0001 | 0.45 |
| DHA | 0.10 | 0.80 | 0 | 0 | 0.08 | 0 | 0.55 | 0 | 0.08 | 0.07 | 0,56 | 0.43 | 2,87 | 0 | 0.15 | 0.09 | <0.0001 | 0.005 |
| ⅀n-6 | 21.92 | 21.25 | 15.35 | 16.36 | 30.22 | 14.37 | 36.59 | 20.10 | 15.21 | 16.81 | 27.34 | 14.24 | 36.21 | 16.69 | 1.37 | 0.85 | <0.0001 | 0.17 |
| ⅀ n-3 | 1.92 | 3.58 | 1.98 | 1.75 | 1.36 | 1.96 | 3.31 | 0.69 | 2.32 | 1.83 | 3.38 | 3.19 | 6.67 | 3.21 | 0.27 | 0.005 | <0.0001 | 0.004 |
| n-6:n:3 | 15.03 | 6.52 | 7.73 | 9.35 | 22.13 | 10.03 | 11.49 | 29.46 | 6.63 | 9.30 | 8.12 | 4.49 | 5.42 | 5.17 | 1.12 | 0.004 | 0.012 | 0.019 |
| Items | Tissue | |||
|---|---|---|---|---|
| n-6:n-3 | ALA | |||
| r | P | r | P | |
| Diet: | ||||
| n-6:n-3 ALA | 0.51 * ‒0.51* | 0.02 0.02 | ‒0.65 ** +0.65 | 0.002 0.002 |
| Amino Acids (g × 100 g DM−1) | SM Diet | LEW Diet | SEM | p Value * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LD | ST | Heart | Spleen | Liver | LD | ST | Heart | Spleen | Liver | Diet Effect | Tissue Effect | Diet × Tissue | ||
| Threonine | 3.42 | 3.26 | 3.31 | 2.44 | 2.73 | 3.38 | 3.31 | 3.33 | 2.66 | 2.74 | 0.07 | 0.18 | 0.35 | 0.58 |
| Lysine | 5.21 | 5.02 | 4.85 | 3.62 | 3.28 | 5.10 | 4.92 | 6.97 | 3.95 | 3.41 | 0.3 | 0.25 | 0.008 | 0.51 |
| Cysteine | 0.60 | 0.48 | 0.84 | 0.71 | 0.75 | 0.69 | 0.67 | 0.86 | 0.66 | 0.83 | 0.08 | 0.01 | 0.034 | 0.03 |
| Methionine | 1.73 | 1.47 | 1.75 | 1.43 | 1.51 | 1.96 | 1.73 | 1.75 | 1.81 | 1.66 | 0.1 | 0.002 | 0.21 | 0.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hăbeanu, M.; Lefter, N.A.; Gheorghe, A.; Untea, A.; Ropotă, M.; Grigore, D.-M.; Varzaru, I.; Toma, S.M. Evaluation of Performance, Nitrogen Metabolism and Tissue Composition in Barrows Fed an n-3 PUFA-Rich Diet. Animals 2019, 9, 234. https://doi.org/10.3390/ani9050234
Hăbeanu M, Lefter NA, Gheorghe A, Untea A, Ropotă M, Grigore D-M, Varzaru I, Toma SM. Evaluation of Performance, Nitrogen Metabolism and Tissue Composition in Barrows Fed an n-3 PUFA-Rich Diet. Animals. 2019; 9(5):234. https://doi.org/10.3390/ani9050234
Chicago/Turabian StyleHăbeanu, Mihaela, Nicoleta Aurelia Lefter, Anca Gheorghe, Arabela Untea, Mariana Ropotă, Daniela-Mihaela Grigore, Iulia Varzaru, and Smaranda Mariana Toma. 2019. "Evaluation of Performance, Nitrogen Metabolism and Tissue Composition in Barrows Fed an n-3 PUFA-Rich Diet" Animals 9, no. 5: 234. https://doi.org/10.3390/ani9050234
APA StyleHăbeanu, M., Lefter, N. A., Gheorghe, A., Untea, A., Ropotă, M., Grigore, D.-M., Varzaru, I., & Toma, S. M. (2019). Evaluation of Performance, Nitrogen Metabolism and Tissue Composition in Barrows Fed an n-3 PUFA-Rich Diet. Animals, 9(5), 234. https://doi.org/10.3390/ani9050234