Relationship Between Cardiac Troponin I Concentration and Myocardial Function in Hypertrophic Cardiomyopathy Cats With or Without Left Ventricular Outflow Tract Obstruction
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Standard Echocardiography
2.3. Two-Dimensional Speckle Tracking Echocardiography
2.4. cTnI Measurement
2.5. Statistical Analysis
3. Results
3.1. Clinical Prifiles and Standard Echocardiography
3.2. 2D-STE Variables
3.3. Correlation Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferasin, L.; Sturgess, C.P.; Cannon, M.J.; Caney, S.M.A.; Gruffydd-Jones, T.J.; Wotton, P.R. Feline Idiopathic Cardiomyopathy: A Retrospective Study of 106 Cats (1994–2001). J. Feline Med. Surg. 2003, 5, 151–159. [Google Scholar] [CrossRef]
- Fox, P.R.; Keene, B.W.; Lamb, K.; Schober, K.A.; Chetboul, V.; Luis Fuentes, V.; Wess, G.; Payne, J.R.; Hogan, D.F.; Motsinger-Reif, A.; et al. International Collaborative Study to Assess Cardiovascular Risk and Evaluate Long-Term Health in Cats with Preclinical Hypertrophic Cardiomyopathy and Apparently Healthy Cats: The REVEAL Study. J. Vet. Intern Med. 2018, 32, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Suzuki, R.; Yuchi, Y.; Fukuoka, H.; Satomi, S.; Teshima, T.; Matsumoto, H. Comparative Study of Myocardial Function in Cases of Feline Hypertrophic Cardiomyopathy with and without Dynamic Left-Ventricular Outflow-Tract Obstruction. Front. Vet. Sci. 2023, 10, 1191211. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Saito, T.; Yuchi, Y.; Kanno, H.; Teshima, T.; Matsumoto, H.; Koyama, H. Detection of Congestive Heart Failure and Myocardial Dysfunction in Cats With Cardiomyopathy by Using Two-Dimensional Speckle-Tracking Echocardiography. Front. Vet. Sci. 2021, 8, 771244. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Mochizuki, Y.; Yoshimatsu, H.; Niina, A.; Teshima, T.; Matsumoto, H.; Koyama, H. Layer-Specific Myocardial Function in Asymptomatic Cats with Obstructive Hypertrophic Cardiomyopathy Assessed Using 2-Dimensional Speckle-Tracking Echocardiography. J. Vet. Intern. Med. 2019, 33, 37–45. [Google Scholar] [CrossRef]
- Suzuki, R.; Mochizuki, Y.; Yuchi, Y.; Yasumura, Y.; Saito, T.; Teshima, T.; Matsumoto, H.; Koyama, H. Assessment of Myocardial Function in Obstructive Hypertrophic Cardiomyopathy Cats with and without Response to Medical Treatment by Carvedilol. BMC Vet. Res. 2019, 15, 1–8. [Google Scholar] [CrossRef]
- Yang, H.; Carasso, S.; Woo, A.; Jamorski, M.; Nikonova, A.; Wigle, E.D.; Rakowski, H. Hypertrophy Pattern and Regional Myocardial Mechanics Are Related in Septal and Apical Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2010, 23, 1081–1089. [Google Scholar] [CrossRef]
- Carasso, S.; Yang, H.; Woo, A.; Vannan, M.A.; Jamorski, M.; Wigle, E.D.; Rakowski, H. Systolic Myocardial Mechanics in Hypertrophic Cardiomyopathy: Novel Concepts and Implications for Clinical Status. J. Am. Soc. Echocardiogr. 2008, 21, 675–683. [Google Scholar] [CrossRef]
- Suzuki, R.; Mochizuki, Y.; Yoshimatsu, H.; Niina, A.; Teshima, T.; Matsumoto, H.; Koyama, H. Early Detection of Myocardial Dysfunction Using Two-Dimensional Speckle Tracking Echocardiography in a Young Cat with Hypertrophic Cardiomyopathy. JFMS Open Rep. 2018, 4, 2055116918756219. [Google Scholar] [CrossRef]
- Adams, J.E.; Bodor, G.S.; Dávila-Román, V.G.; Delmez, J.A.; Apple, F.S.; Ladenson, J.H.; Jaffe, A.S. Cardiac Troponin I. A Marker with High Specificity for Cardiac Injury. Circulation 1993, 88, 101–106. [Google Scholar] [CrossRef]
- Chapelle, J.P. Cardiac Troponin I and Troponin T: Recent Players in the Field of Myocardial Markers. Clin. Chem. Lab. Med. 1999, 37, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Rifai, N.; Sallan, S.E.; Lipsitz, S.R.; Dalton, V.; Sacks, D.B.; Ottlinger, M.E. Predictive Value of Cardiac Troponin T in Pediatric Patients at Risk for Myocardial Injury. Circulation 1997, 96, 2641–2648. [Google Scholar] [CrossRef] [PubMed]
- Coats, C.J.; Masri, A.; Barriales-Villa, R.; Abraham, T.P.; Brinkley, D.M.; Claggett, B.L.; Hagege, A.; Hegde, S.M.; Ho, C.Y.; Kulac, I.J.; et al. Cardiac Biomarkers and Effects of Aficamten in Obstructive Hypertrophic Cardiomyopathy: The SEQUOIA-HCM Trial. Eur. Heart. J. 2024, 45, 4464–4478. [Google Scholar] [CrossRef]
- Hori, Y.; Iguchi, M.; Heishima, Y.; Yamashita, Y.; Nakamura, K.; Hirakawa, A.; Kitade, A.; Ibaragi, T.; Katagi, M.; Sawada, T.; et al. Diagnostic Utility of Cardiac Troponin I in Cats with Hypertrophic Cardiomyopathy. J. Vet. Intern. Med. 2018, 32, 922–929. [Google Scholar] [CrossRef]
- Borgeat, K.; Sherwood, K.; Payne, J.R.; Luis Fuentes, V.; Connolly, D.J. Plasma Cardiac Troponin I Concentration and Cardiac Death in Cats with Hypertrophic Cardiomyopathy. J. Vet. Intern. Med. 2014, 28, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Payne, J.R.; Novo Matos, J.; Fong, W.W.; Connolly, D.J.; Luis Fuentes, V. Biomarker Changes with Systolic Anterior Motion of the Mitral Valve in Cats with Hypertrophic Cardiomyopathy. J. Vet. Intern. Med. 2020, 34, 1718–1727. [Google Scholar] [CrossRef]
- Williams, L.K.; Chan, R.H.; Carasso, S.; Durand, M.; Misurka, J.; Crean, A.M.; Ralph-Edwards, A.; Gruner, C.; Woo, A.; Lesser, J.R.; et al. Effect of Left Ventricular Outflow Tract Obstruction on Left Atrial Mechanics in Hypertrophic Cardiomyopathy. Biomed. Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Novo Matos, J.; Sargent, J.; Silva, J.; Payne, J.R.; Seo, J.; Spalla, I.; Borgeat, K.; Loureiro, J.; Pereira, N.; Simcock, I.C.; et al. Thin and Hypokinetic Myocardial Segments in Cats with Cardiomyopathy. J. Vet. Cardiol. 2023, 46, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Luis Fuentes, V.; Abbott, J.; Chetboul, V.; Côté, E.; Fox, P.R.; Häggström, J.; Kittleson, M.D.; Schober, K.; Stern, J.A. ACVIM Consensus Statement Guidelines for the Classification, Diagnosis, and Management of Cardiomyopathies in Cats. J. Vet. Intern. Med. 2020, 34, 1062–1077. [Google Scholar] [CrossRef]
- Fox, P.R.; Liu, S.K.; Maron, B.J. Echocardiographic Assessment of Spontaneously Occurring Feline Hypertrophic Cardiomyopathy. An Animal Model of Human Disease. Circulation 1995, 92, 2645–2651. [Google Scholar] [CrossRef]
- Jackson, B.L.; Adin, D.B.; Lehmkuhl, L.B. Effect of Atenolol on Heart Rate, Arrhythmias, Blood Pressure, and Dynamic Left Ventricular Outflow Tract Obstruction in Cats with Subclinical Hypertrophic Cardiomyopathy. J. Vet. Cardiol. 2015, 17 (Suppl. 1), S296–S305. [Google Scholar] [CrossRef]
- Dini, F.L.; Capozza, P.; Donati, F.; Simioniuc, A.; Corciu, A.I.; Fontanive, P.; Pieroni, A.; Di Bello, V.; Marzilli, M. Patterns of Left Ventricular Remodeling in Chronic Heart Failure: Prevalence and Prognostic Implications. Am. Heart J. 2011, 161, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Yamada, S.; Iwano, H.; Nishino, H.; Nakabachi, M.; Yokoyama, S.; Abe, A.; Ichikawa, A.; Kaga, S.; Nishida, M.; et al. Myocardial Shortening in 3 Orthogonal Directions and Its Transmural Variation in Patients with Nonobstructive Hypertrophic Cardiomyopathy. Circ. J. 2015, 79, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, K.; Funabashi, N.; Takaoka, H.; Kamata, T.; Kanaeda, A.; Saito, M.; Nomura, F.; Kobayashi, Y. Characteristic Myocardial Strain Identified in Hypertrophic Cardiomyopathy Subjects with Preserved Left Ventricular Ejection Fraction Using a Novel Multi-Layer Transthoracic Echocardiography Technique. Int. J. Cardiol. 2015, 184, 237–243. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software “EZR” for Medical Statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; Holmes, D.R. Clinical Practice. Hypertrophic Obstructive Cardiomyopathy. N. Engl. J. Med. 2004, 350, 1320–1327. [Google Scholar] [CrossRef]
- Ferasin, L.; Kilkenny, E.; Ferasin, H. Evaluation of N-Terminal Prohormone of Brain Natriuretic Peptide and Cardiac Troponin-I Levels in Cats with Systolic Anterior Motion of the Mitral Valve in the Absence of Left Ventricular Hypertrophy. J. Vet. Cardiol. 2020, 30, 23–31. [Google Scholar] [CrossRef]
- Chang, S.A.; Lee, S.C.; Choe, Y.H.; Hahn, H.J.; Jang, S.Y.; Park, S.J.; Choi, J.O.; Park, S.W.; Oh, J.K. Effects of Hypertrophy and Fibrosis on Regional and Global Functional Heterogeneity in Hypertrophic Cardiomyopathy. Int. J. Cardiovasc. Imaging 2012, 28 (Suppl. 2), 133–140. [Google Scholar] [CrossRef]
- Katrukha, A.G.; Bereznikova, A.V.; Esakova, T.V.; Pettersson, K.; Lövgren, T.; Severina, M.E.; Pulkki, K.; Vuopio-Pulkki, L.M.; Gusev, N.B. Troponin I Is Released in Bloodstream of Patients with Acute Myocardial Infarction Not in Free Form but as Complex. Clin. Chem. 1997, 43, 1379–1385. [Google Scholar] [CrossRef]
- Dunn, M.E.; Coluccio, D.; Hirkaler, G.; Mikaelian, I.; Nicklaus, R.; Lipshultz, S.E.; Doessegger, L.; Reddy, M.; Singer, T.; Geng, W. The Complete Pharmacokinetic Profile of Serum Cardiac Troponin I in the Rat and the Dog. Toxicol. Sci. 2011, 123, 368–373. [Google Scholar] [CrossRef]
- Dybro, A.M.; Rasmussen, T.B.; Nielsen, R.R.; Andersen, M.J.; Jensen, M.K.; Poulsen, S.H. Randomized Trial of Metoprolol in Patients With Obstructive Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2021, 78, 2505–2517. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Maron, M.S.; Wigle, E.D.; Braunwald, E. The 50-Year History, Controversy, and Clinical Implications of Left Ventricular Outflow Tract Obstruction in Hypertrophic Cardiomyopathy from Idiopathic Hypertrophic Subaortic Stenosis to Hypertrophic Cardiomyopathy: From Idiopathic Hypertrophic Subaortic Stenosis to Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2009, 54, 191–200. [Google Scholar] [CrossRef]
- Coppini, R.; Ferrantini, C.; Pioner, J.M.; Santini, L.; Wang, Z.J.; Palandri, C.; Scardigli, M.; Vitale, G.; Sacconi, L.; Stefàno, P.; et al. Electrophysiological and Contractile Effects of Disopyramide in Patients with Obstructive Hypertrophic Cardiomyopathy: A Translational Study. JACC Basic Transl. Sci. 2019, 4, 795–813. [Google Scholar] [CrossRef] [PubMed]
- Teichman, S. The Anticholinergic Side Effects of Disopyramide and Controlled-Release Disopyramide. Angiology 1985, 36, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Masri, A.; Choudhury, L.; Barriales-Villa, R.; Elliott, P.; Maron, M.S.; Nassif, M.E.; Oreziak, A.; Owens, A.T.; Saberi, S.; Tower-Rader, A.; et al. Standard-of-Care Medication Withdrawal in Patients with Obstructive Hypertrophic Cardiomyopathy Receiving Aficamten in FOREST-HCM. J. Am. Coll. Cardiol. 2024, 84, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Wang, S.; Liu, Z.; Han, E.; Wu, C.; Luo, C.; Chen, W.; Mei, F.; Lu, X.; Yan, M.; et al. An Innovative Minimally Invasive Approach for Hypertrophic Obstructive Cardiomyopathy: Transaortic Septal Myectomy via Right Infra-Axillary Incision. JTCVS Tech. 2024, 28, 50–58. [Google Scholar] [CrossRef]
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Dearani, J.A.; Epps, K.C.; Evanovich, L.; Ferrari, V.A.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1239–e1311. [Google Scholar] [CrossRef]
| Variables | HNCM (n = 13) | HOCM (n = 33) | p-Value |
|---|---|---|---|
| Sex (male, female) | 6, 7 | 23, 10 | 0.18 |
| Age (year) | 3.4 (1.7, 7.1) | 2.7 (1.4, 5.7) | 0.45 |
| Body weight (kg) | 4.2 (3.6, 4.9) | 4.3 (3.8, 5.4) | 0.35 |
| ACVIM (B1, B2, C/D) | 12, 1, 0 | 30, 3, 0 | 0.99 |
| Heart rate (bpm) | 180 (132, 202) | 192 (166, 229) | 0.79 |
| Systolic blood pressure (mmHg) | 131 (125, 140) | 135 (121, 142) | 0.26 |
| LVOTVrest (m/s) | 1.1 (0.9, 1.3) | 3.7 (2.8, 4.5) * | <0.01 |
| LVOTVexcited (m/s) | 1.4 (1.2, 1.9) | 4.3 (4.0, 4.9) * | <0.01 |
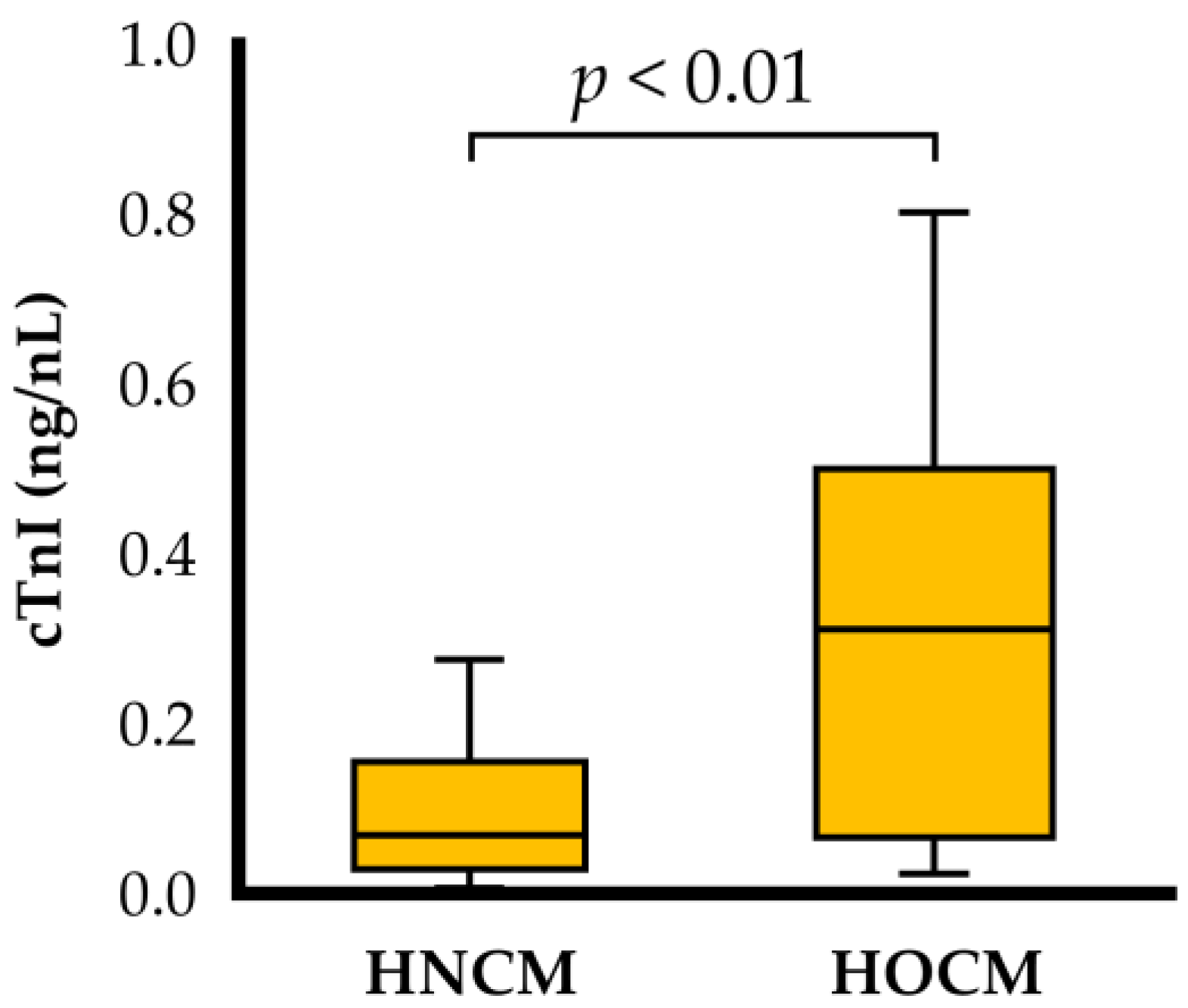
| cTnI (ng/mL) | 0.069 (0.029, 0.156) | 0.311 (0.066, 0.500) * | <0.01 |
| Number of cases with high cTnI levels (n) | 4 | 24 | 0.02 |
| Medication (yes, no) | 9, 4 | 13, 20 | 0.10 |
| Medical Drugs | HNCM (n = 13) | HOCM (n = 33) | p-Value |
|---|---|---|---|
| Beta blocker (n) | 7 | 10 | 0.18 |
| Angiotensin converting enzyme inhibitor (n) | 1 | 2 | 0.99 |
| Pimobendan (n) | 1 | 1 | 0.99 |
| Clopidogrel (n) | 2 | 0 | 0.36 |
| No medication (n) | 4 | 20 | 0.10 |
| Variables | HNCM | HOCM | p-Value |
|---|---|---|---|
| LA/Ao | 1.2 (1.1, 1.2) | 1.3 (1.2, 1.4) * | 0.049 |
| IVSd (mm) | 6.0 (5.0, 7.1) | 6.3 (5.3, 7.0) | 0.52 |
| LVPWd (mm) | 5.2 (4.6, 6.6) | 5.8 (5.4, 6.9) | 0.053 |
| LVIDd (mm) | 13.3 (11.4, 15.5) | 12.8 (11.9, 15.3) | 0.85 |
| RWT | 0.90 (0.81, 0.93) | 0.94 (0.76, 1.08) | 0.28 |
| FS (%) | 42.2 (37.4, 54.9) | 43.0 (37.9, 48.4) | 0.92 |
| E vel (m/s) | 0.6 (0.5, 0.7) | 0.8 (0.7, 0.9) * | 0.01 |
| E/A | 0.8 (0.8, 1.1) | 0.9 (0.8, 1.3) | 0.45 |
| E/A fusion | 1.0 (0.8, 1.0) (n = 5) | 1.0 (0.9, 1.1) (n = 9) | 0.62 |
| HNCM | HOCM | p-Value | |
|---|---|---|---|
| Longitudinal strain (%) | |||
| Whole layer | 13.1 (10.9, 14.2) | 13.0 (9.2, 16.9) | 0.89 |
| Endocardium | 15.0 (12.6, 16.2) | 14.0 (10.8, 20.1) | 0.94 |
| Epicardium | 11.6 (9.4, 12.9) | 10.7 (8.0, 13.9) * | 0.52 |
| End/Epi | 1.3 (1.2, 1.4) | 1.3 (1.2, 1.6) | 0.70 |
| Circumferential strains (%) | |||
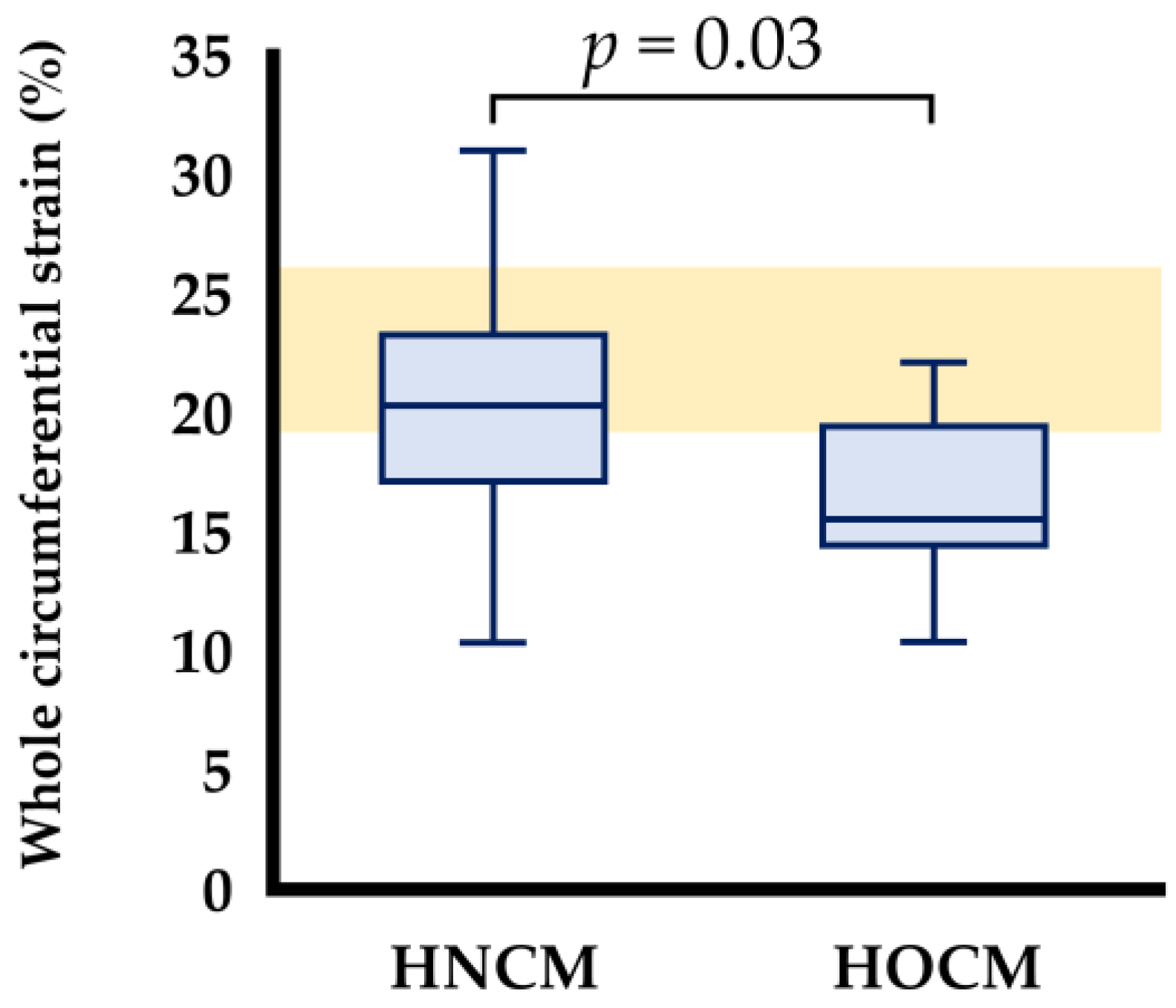
| Whole layer | 20.3 (16.0, 23.5) | 15.5 (14.3, 19.7) * | 0.028 |
| Endocardium | 37.2 (31.8, 41.1) | 31.8 (28.6, 35.6) | 0.07 |
| Epicardium | 8.9 (7.3, 12.0) | 6.6 (5.0, 8.8) * | 0.011 |
| End/Epi | 4.2 (3.2, 5.3) | 4.7 (3.8, 5.8) | 0.23 |
| cTnI | LVOTVexcited | LVOTVrest | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| cTnI | ― | ― | 0.51 | <0.01 | 0.50 | <0.01 |
| LVOTVexcited | ― | ― | 0.72 | <0.01 | ||
| LVOTVrest | ― | ― | ||||
| Longitudinal strain (%) | ||||||
| Whole layer | −0.27 | 0.13 | −0.02 | 0.90 | −0.01 | 0.98 |
| Endocardium | −0.19 | 0.28 | −0.09 | 0.64 | −0.03 | 0.87 |
| Epicardium | −0.29 | 0.10 | −0.15 | 0.39 | −0.15 | 0.26 |
| Circumferential strains (%) | ||||||
| Whole layer | −0.20 | 0.26 | −0.08 | 0.67 | −0.18 | 0.30 |
| Endocardium | −0.13 | 0.46 | −0.12 | 0.51 | −0.07 | 0.71 |
| Epicardium | −0.12 | 0.10 | −0.03 | 0.85 | −0.20 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satomi, S.; Suzuki, R.; Yuchi, Y.; Yoshii, Y.; Kanno, H.; Teshima, T.; Matsumoto, H. Relationship Between Cardiac Troponin I Concentration and Myocardial Function in Hypertrophic Cardiomyopathy Cats With or Without Left Ventricular Outflow Tract Obstruction. Animals 2025, 15, 1313. https://doi.org/10.3390/ani15091313
Satomi S, Suzuki R, Yuchi Y, Yoshii Y, Kanno H, Teshima T, Matsumoto H. Relationship Between Cardiac Troponin I Concentration and Myocardial Function in Hypertrophic Cardiomyopathy Cats With or Without Left Ventricular Outflow Tract Obstruction. Animals. 2025; 15(9):1313. https://doi.org/10.3390/ani15091313
Chicago/Turabian StyleSatomi, Shuji, Ryohei Suzuki, Yunosuke Yuchi, Yayoi Yoshii, Haruka Kanno, Takahiro Teshima, and Hirotaka Matsumoto. 2025. "Relationship Between Cardiac Troponin I Concentration and Myocardial Function in Hypertrophic Cardiomyopathy Cats With or Without Left Ventricular Outflow Tract Obstruction" Animals 15, no. 9: 1313. https://doi.org/10.3390/ani15091313
APA StyleSatomi, S., Suzuki, R., Yuchi, Y., Yoshii, Y., Kanno, H., Teshima, T., & Matsumoto, H. (2025). Relationship Between Cardiac Troponin I Concentration and Myocardial Function in Hypertrophic Cardiomyopathy Cats With or Without Left Ventricular Outflow Tract Obstruction. Animals, 15(9), 1313. https://doi.org/10.3390/ani15091313






