Simple Summary
Sheep husbandry has played a crucial role in supporting rural livelihoods. However, climate change associated heat stress in sheep has a detrimental impact on growth performance. In order to address the negative effects of heat stress on animal growth, scientists have been exploring nutritional solutions like herbal supplements as they have significant effects on feed intake, water intake, and hormonal and molecular mechanisms governing growth in major livestock. However, negligible studies have delved into these aspects in heat-stressed sheep. Thus, the study targeted addressing these concerns focusing on the native Indian sheep breed, Kenguri. The 60-day study conducted using 24 ewes was divided into four groups under different treatments in climate-controlled chambers, including herbal supplementation. The results indicated that herbal supplements improved feed intake and growth-related hormones in heat-stressed ewes. Further, the herbal supplement had a significant role in increasing the expression pattern of genes governing growth. The study established that herbal supplements support the growth performance of heat-stressed sheep. The conclusions show promise for improving climate resilience in Kenguri ewes and open the way for navigating future strategies that sustain sheep production through innovative nutrition and breeding strategies.
Abstract
A study was designed to explore the possibility of using herbal supplementation to sustain growth performance during heat stress exposure in Kenguri sheep. This 60-day study was conducted on 24 Kenguri ewes (1–2 years old), randomly assigned to four treatment groups (n = 6 per group) as follows: KC (n = 6; Kenguri Control), KHS (n = 6; Kenguri Heat Stress), KCS (n = 6; Kenguri Control and herbal supplement), and KHSS (n = 6; Kenguri Heat Stress and herbal supplement). The herbal mixture of Ocimum sanctum (Tulsi), Emblica officinalis (Amla), Morinda citrifolia (Noni), Withania somnifera (Ashwagandha), and Phyllostachys edulis (Bamboo) was used in this study. The herbal supplement used in the present study was given to the KCS and KHSS groups’ animals in dry powder form at a dose of 0.8 g/Kg BW/Day. All variables were recorded fortnightly, and gene expression analysis was performed at the end of the experiment. The results indicated that the recorded temperature–humidity index (THI) provided thermal comfort for KC and KCS while inducing extremely severe heat stress to the KHS and KHSS groups. Heat stress did not alter the feed intake, while the herbal supplement during heat stress increased the feed intake from day 30 onwards. Furthermore, heat stress significantly (p < 0.001) increased the water intake, while the herbal supplement did not alter the heat stress-induced water intake. In addition, neither heat stress nor herbal supplements influenced the body weight and allometric measurements studied. Furthermore, heat stress significantly (p < 0.01) decreased the level of plasma tri-iodo-thyronine (T3) and thyroxin (T4) and had a non-significant effect on plasma growth hormone (GH), insulin-like growth factor-1 (IGF-1), while the herbal supplements significantly (p < 0.01) increased the levels of all these hormones studied. Likewise, in peripheral blood mononuclear cells (PBMCs) the expression patterns of growth hormone receptor (GHR), Insulin-like growth factor 1 (IGF1) and prolactin receptor (PRLR) were significantly (p < 0.001) downregulated during heat stress (0.25, 0.3, and 0.48-fold change, respectively). However, the herbal supplement significantly (p < 0.01) increased the heat stress-induced reduction in the expression pattern of these three genes (0.65, 0.61, and 0.61-fold change, respectively). Therefore, from this study, it could be concluded that although the herbal supplements did not bring positive changes in body weight and allometric measurements, it still had a beneficial impact on the endocrinology and genes governing growth performance in Kenguri ewes. Thus, the herbal feed additive used in the study shows promise for relieving heat stress in Kenguri ewes.
1. Introduction
Sheep farming has long been a sustainable source of income for rural communities [1]. The global sheep population has increased, reaching 1.266 billion in 2021, with China being the country with the largest sheep numbers [2]. Recently, there has been a greater understanding of the detrimental impacts of heat stress associated with climate change on livestock production [3,4,5,6]. As the climate crisis intensifies, urgent conservation efforts are needed for livestock species [7]. The conservation and production of indigenous, thermo-tolerant breeds can mitigate the climate change impact on the availability of animal produce globally [7,8], demanding significant research efforts for screening the climate resilience potential of native sheep breeds.
As a voluntary adaptive response to increased ambient temperature conditions, sheep decrease feed intake and increase water intake. Further, the reduced feed intake associated with heat stress affects animals particularly at various productive stages, reflecting reduced growth performance [7]. Elevated temperature also causes changes in their biological functions, like altered endocrine dynamics, affecting the growth performance of sheep. Heat stress in sheep exhibits a discernible effect on growth performance by causing decreased anabolic activity due to reduced feed intake and increased tissue catabolism [9,10,11,12]. The restriction of growth and other production variables’ performance during heat stress is attributed to stress hormones, particularly corticosteroids [13]. Heat stress also reduces the secretion of thyroid hormones (triiodothyronine and thyroxine), resulting in altered thermogenesis and basal metabolism, which can negatively influence the productive traits like growth and fattening in animals [13,14]. The expression patterns of genes that encode growth hormone (GH), growth hormone receptor (GHR), insulin-like growth factor-1 (IGF-1), leptin (LEP), leptin receptor (LEPR), and thyroid hormone receptor (THR) are linked to the impacts of heat stress on growth performance in small ruminants [15]. Apart from hormonal changes reflecting growth potential, the expression patterns of genes have also been used as potential indicators to evaluate the growth performance of heat-stressed sheep. However, there is a paucity of validated growth production-related biomarkers for assessing heat stress impacts in sheep.
Herbal supplements have been widely used to prevent and treat diseases in both human and veterinary medicine due to their no-residue nature, low toxicity, and lack of adverse effects [16]. In addition, it has been established that herbal mixtures improve feed intake, immunity, and performance and decrease disease occurrence during heat stress in cattle [17,18], buffaloes [19,20], sheep [16,21,22], goats [23,24], pigs [25], poultry [26,27,28,29], donkeys [30], and mice [31,32]. The herbal mixtures have anti-inflammatory, antioxidant, and anti-microbial effects [33,34], making them an ideal nutritional supplement for heat-stressed animals. The complex pathogenesis of heat stress in animals often means a single herbal remedy has an insignificant effect and frequently does not have the potential effect to ameliorate heat stress; instead, a combination of herbs would help to improve the overall impact of herbal components on heat-stressed cells [35]. Herbs such as Tulsi, Amla, Noni, Ashwagandha, and Bamboo have established roles in improving feed intake, growth performance, and immunity in addition to their antioxidant, analgesic, hepatoprotective, cardioprotective, and adaptogenic properties [16,19,20,23,26].
Based on the available literature, it is evident that there is no detailed study on the influence of herbal additives on the growth performance, endocrinology, and molecular mechanisms governing growth in sheep during heat stress exposure. Thus, a hypothesis was framed based on a published study unravelling the significance of a herbal mixture in promoting growth performance in heat-stressed sheep [36]. Kenguri sheep, being a popular breed with high potential for meat production, was the breed of choice for the study. The objective of the present work was to study the behavioural responses (feed intake and water intake) of heat-stressed and herbal-supplemented Kenguri sheep. Further, the study attempted to understand the influence of the herbal supplement on the growth performance of heat-stressed indigenous Kenguri sheep using endocrine- and genetic growth performance-related biomarkers.
The present study was the first to evaluate the beneficial effects of herbal supplements on growth performance in heat-stressed Kenguri sheep through integration of behavioural, hormonal, and genetic variables, providing valuable insights on the use of herbal supplements. This study’s novel findings can contribute to the development of nutritional strategies and refinement of breeding programs for improving sheep resilience in challenging climatic conditions.
2. Materials and Methods
2.1. Experimental Animals
Kenguri, or Tenguri, is a well-known indigenous sheep breed popular for mutton production in the north-eastern region of Karnataka state, which features a tropical climate. The current work used 24 adult, one-to-two-year-old female Kenguri sheep with a body weight ranging between 15 and 23 kg. The grouping of animals was done randomly so that the average body weights of all four groups’ ewes were not statistically different at the start of the study. The animals were acclimatised to the study location for one month. During this period, the animals were exposed to the experimental diet and the local environmental conditions to acclimatise them before the start of the study. The study animals were housed in well-ventilated barns with asbestos roofing and were provided with a high-quality diet and drinking water. The feed and water residues were collected at fortnightly intervals to calculate the feed intake and water intake, respectively. Prophylactic measures (vaccination and deworming) against common infectious diseases and endo- and ectoparasitic infestations were performed according to the health management plan for maintaining animal health throughout the experimental period.
2.2. Experimental Location, Climate, and Design
The experiment was conducted at the Centre for Climate Resilient Animal Adaptation Studies (CCRAAS), ICAR-National Institute of Animal Nutrition and Physiology (ICAR-NIANP), Bangalore, India. The experimental location lies in the country’s southern Deccan plateau at longitude 12°57′04.3′′ N, latitude 77°36′25.3′′ E, and an elevation of 920 m above mean sea level (MSL). The study was carried out between the months of June and August. The experiment was conducted in the year 2024. Table 1 presents the average weather variables and temperature–humidity index (THI) recorded at 8:00 and 14:00 in the thermoneutral and heat stress chambers. The average THIs in the thermoneutral zone (TNZ) chamber from 8:00 h to 14:00 h were 71.25 and 68.93, respectively. Additionally, for the heating chamber, the average THIs from 8:00 h to 14:00 h were 72.27 and 93.34, respectively. Further, the average THIs from 16.00 h to 10.00 h were 74.38 and 74.45, respectively, in the TNZ and heating chamber, with negligible difference between the chambers. The animals in the thermoneutral zone chamber are exposed to a range of temperatures wherein they can maintain a stable body temperature without expending extra energy.

Table 1.
Weather variables recorded during the entire study period.
The THI was calculated using McDowell, ref. [37]’s formula, THI = 0.72 (Tdb + Twb) + 40.6, incorporating DBT and WBT. The THI obtained during the morning and afternoon clearly indicated the significantly (p < 0.01) higher THI obtained at 14.00 in the heat stress chamber as compared to the thermoneutral chamber. As per McDowell [37]’s model, THI values of 72 and less are considered comfortable; THI values between 75 and 78 are considered stressful; and THI values above 78 are considered extreme distress. The average THI value of 93.34 obtained at 14.00 clearly demonstrated the extremely severe heat stress experienced by both the KHS and KHSS groups. Further, the THI of 68.93 obtained in the thermoneutral chamber clearly indicated the comfortable condition experienced by the KC and KCS groups.
This 60-day study in a climate-controlled facility was conducted on 24 Kenguri ewes (1–2 years old). The body weight was taken as a criterion for grouping these animals. The animals were randomly allocated into four groups so that the average body weight across these groups was not statistically significant at the start of the study. The groupings were done based on a 2 × 2 factorial design that took into account temperature and diet as independent factors. Accordingly, two groups were placed in a thermoneutral chamber with different nutritional regimes, while the other two groups were kept in the heating chamber with two different dietary regimes. Thus, the groups were named as follows: KC (n = 6; Kenguri Control; standard diet), KHS (n = 6; Kenguri Heat Stress; standard diet), KCS (n = 6; Kenguri Control Supplement; standard diet + herbal powder), and KHSS (n = 6; Kenguri Heat Stress Supplement; standard diet + herbal powder). This sample size of six animals in each group is a standard practice for maintaining a minimum number of animals in each group for this study design. Following the same criteria, four animals with similar body weights were chosen and distributed, with one animal to each group. Likewise, the same process was repeated to allocate the same five animals to different groups, accordingly. Thus, the researchers were aware of the animals allocated to each group. All of the 24 animals chosen for this study were healthy, without ill-health. Those animals that were not healthy or had sicknesses were excluded from the study. The best 24 animals in the flock were chosen for this study.
In both chambers, the animals were individually housed in a pen with individual feeding and watering facilities and floor space to ensure free movement. The individual pens were kept on an elevated platform, and the floor material was plastic. The individual animals were housed in the particular pen throughout the study period. The diet comprised a 60% roughage (Hybrid Napier) and a 40% concentrate mixture. The experimental animals were fed in their respective enclosures. Table 1 lists the ingredients and chemical composition of the concentrate and roughage feed provided for the experimental animals. Table 2 details the composition of the dietary herbal supplement, and Table 3 lists the composition of herbal supplements used in the study. The herbal supplement was composed of a mixture of Ocimum sanctum (Tulsi), Emblica officinalis (Amla), Morinda citrifolia (Noni), Withania somnifera (Ashwagandha), and Phyllostachys edulis (Bamboo). The herbal supplement used in the present study was given to the KCS and KHSS groups’ animals in dry powder form at a dose of 0.8 g/Kg BW/Day along with the concentrate ration.

Table 2.
Feed ingredients and chemical composition of concentrate and roughage fed to the experimental animals.

Table 3.
Composition of the herbal supplement.
The entire herbal composition, their proportions, and the dose rate were calculated based on a review of the published literature. Further, all five ingredients were procured in powder form and the appropriate proportions of each ingredient were uniformly mixed, as described in Table 3, to prepare 100 g of the herbal mixture. The final dose rate of 0.8 g/Kg BW/Day herbal supplements has the same proportion of the extracts of these herbal plants, as indicated in Table 3. In addition, we would like to clarify that we did not measure the active ingredients. First, we wanted to try the crude extract of these plants to establish their heat stress-ameliorating effects. In subsequent studies, the active ingredients will be established.
The climate chamber measures 12.8 m long, 3.41 m wide, and 2.87 m high, with a built area of 143.00 m2, and is made of laminated steel sheets with anti-corrosion protection and filled with polystyrene foam. Each chamber has a holding capacity of 12 animals with individual animal pen areas of 1.95 m2. Figure 1 describes the outside view of the climate chambers. The pens were partitioned with stainless steel rods to prevent the entry of sheep to adjacent pens. At the same time, this ensured that the animals were free of socialising stress since they could see other animals. The floor of each individual pen was 0.6 m above the floor of the climate chamber. Each pen had an individual feeder, waterer, and urine and faecal collection trays. The animals were maintained in individual pens throughout the study duration. Figure 2 describes the Kenguri sheep kept inside the climate chambers. Both the thermal comfort zone climate chamber and heating climate chamber are designed to provide the appropriate environmental conditions required for any environmental stress-related studies. It is an established fact that the thermal comfort zone for sheep ranges between 20 and 28 °C [38]. Therefore, the thermal comfort zone chamber was maintained at 24 °C throughout the study period for a six hour duration daily, between 10:00 h and 16:00 h, to provide the ideal thermal comfort zone to the KC and KCS groups’ ewes. In the heating climate chamber, the KHS and KHSS groups’ ewes were exposed to a simulated heat stress model, as described [39]. The simulated heat stress model was developed based on thirty years of ambient temperature and relative humidity data for the summer season (April, May, and June) in the study locality [39]. The model was developed with the intention of mimicking the natural environmental stress condition in the climate chamber. The time duration of six hours, between 10:00 h and 16:00 h, was chosen since only during this period were the animals exposed to heat stress while grazing in an extensive system of rearing. Moreover, from an animal ethical point of view, the simulated heat stress model was more practical and less stressful than constant heat stress models. The KC and KCS animals were exposed to 24 °C in the thermoneutral zone chamber to maintain thermal comfort, while the KHS and KHSS groups’ animals were exposed to heat stress in the heating chamber under a simulated heat stress model [39].

Figure 1.
Climate chambers: the thermoneutral zone chamber (left) and the heating/cooling chamber (right)’s outside views.

Figure 2.
Kenguri sheep kept inside the climate chamber.
The study animals in the thermoneutral zone chamber and heating chamber were exposed to the respective temperatures for a duration of six hours a day (between 10:00 to 16:00) during the experimental period. Feeding and watering of the experimental animals were carried out in their individual cabins. All the procedures involved in the study, including the subjection of experimental animals to heat stress, were carried out with the approval of the institute’s animal ethics committee, letter number V-11011(13)/14/2021-CPCSEA-DADF.
2.3. Blood Collection for Hormonal Analysis
Blood sampling for hormonal analysis was performed at fortnightly intervals at 11:00 h. Blood samples (4 mL) were collected from the external jugular vein of the animals in all four groups using heparin-coated vacutainers. The plasma was harvested immediately following the blood collection by subjecting the blood samples to centrifugation at 3500 rpm for 20 min. The separated plasma samples were stored at −20 °C until hormonal analysis.
The enzyme-linked immunosorbent assay (ELISA) method was used to estimate the level of the target hormones in the sample (Puregene kit, Genetix, New Delhi). The reagents, standard solution, and samples were prepared as instructed and were thawed before using them in the reaction. Additionally, all the reagents were mixed well without foaming. Once the procedure started, all steps were completed without interruption. To the standard well, 50 μL of standard solution were added. Further, to the sample wells, 40 μL of the sample and then 10 μL of anti-GH antibody were added. Following this, 50 μL of streptavidin–HRP were added to the sample and standard wells (except the blank control well). The plates were mixed gently and sealed to incubate at 37 °C. The sealer was removed, and the plates were washed approximately 5–6 times using the prepared wash buffer (400 μL/well for each wash). Then, the plates were blotted by sharply striking against the absorbent paper to remove residual droplets (done by hand 6 times). Then, 50 μL of substrate solution A, followed by an equal amount of substrate solution B, were pipetted into each well at timed intervals. The plates were covered with a sealer and incubated for 10 min at 37 °C in the dark. Following this, upon the addition of 50 μL of stop solution to all the wells, the colour change from blue to yellow was observed, and the optical density (OD) value was determined using a microplate reader set to 450 nm within 10 min after the stop solution was added. The ODs of unknown samples were read against the standard curve drawn using immunoassay software and analysed.
2.4. Blood Collection for PBMC Isolation
On the last day of the study, blood sampling for PBMC isolation was carried out at 11.00 h simultaneously from all the study animals. Five ml of blood samples were collected from the external jugular vein in EDTA-containing vacutainers and were stored in ice. The blood samples were processed immediately for PBMC isolation. To the samples, ice-cold 1× RBC lysis buffer was added, and this was incubated at room temperature for 10 min, followed by centrifugation at 2500 rpm at 4 °C for 5 min. The total RNA was isolated from the PBMCs using the GeneJET Whole Blood RNA Purification Mini Kit (Thermo Scientific, Vilnius, Lithuania) in accordance with the manufacturer’s instructions. Fresh nuclease-free micro-centrifuge tubes were used to collect the eluted RNA.
The Revert Aid First Strand cDNA Synthesis Kit (Thermo Scientific, Vilnius, Lithuania) was used to reverse-transcribe the total RNA into complementary DNA (cDNA), following the manufacturer’s protocol, for use in real-time quantitative polymerase chain reaction (RT-qPCR). The product of the first-strand cDNA synthesis was diluted to a final concentration of 25 ng/μL with nuclease free water (NFW), and 2 μL of diluted cDNA were used for each reaction in qPCR. An online NCBI primer design software was used to create a gene-specific primer, and its specificity was verified using BLAST and Primer3 (http://www.ncbi.nlm.nih.gov/tools/primer-blast/ accessed on 15 March 2024). Table 4 describes the primer sequences of the housekeeping gene and target genes.

Table 4.
The primers used for studying the relative expression of the targeted genes.
The relative expressions of the targeted genes were studied using SYBR Green chemistry (Maxima SYBR Green qPCR master mix, Fermentas, Waltham, MA, USA). The 20 μL reaction was carried out in triplicate using 50 ng of template and 0.5 μM primer concentrations. The reaction condition was enzyme activation at 95 °C for 10 min and an amplification cycle (40 cycles; initial denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s). By examining the melt curve analysis towards the end of the reaction, non-specific amplification was ruled out. As an internal control, the housekeeping gene GAPDH (glyceraldehyde 3-phosphate dehydrogenase) was used to compare the relative expression of target genes using the 2−ΔΔCT method.
2.5. Variables Studied
The targeted weather variables in the study were as follows: maximum temperature, minimum temperature, and relative humidity, which were recorded using a thermo-hygrometer, and the dry-bulb temperature and wet-bulb temperature, which were recorded using a dry-bulb and wet-bulb thermometer, respectively. The weather variables were recorded twice daily at 8:00 h and 14:00 h. The THI was calculated using McDowell’s 1972 model [37]. Feed intake (FI) (g/w0.75/day) and water intake (WI) (L/KgDMI/day) were calculated fortnightly based on the feed and water residue data. The feed and water residue data were recorded daily at 7.00 h, and their fortnightly average was taken to maintain similarity with other parameters recorded in the study. The individual feed and water troughs provided for all 24 ewes facilitated the recording of the feed and water residue data, respectively. The feed and water residue data were measured using a digital weighing balance and measuring cylinder, respectively. The FI/WI was calculated using the following formula: feed/water offered on the previous day-feed/water left in the trough. The body weight of the animals was recorded at 7.00 h using a digital weighing balance, and allometric measurements were recorded at 7.00 h using a measuring tape at fortnightly intervals. Blood sampling for hormonal analysis was carried out at 11.00 h at fortnightly intervals, and hormones, including growth hormone (GH), insulin-like growth factor-1 (IGF-1), thyroxine (T4), and triiodothyronine (T3), were estimated by employing a Puregene enzyme-linked immunoassay (ELISA) kit and using a microplate reader. The collection of blood samples for the gene expression study was carried out at the end of the trial. The targeted genes were IGF1, growth hormone receptor (GHR), prolactin receptor (PRLR), and thyroid hormone receptor (THR).
2.6. Data Analysis
The feed intake, water intake, body weight, allometric measurements, and endocrine variables in the study were subjected to 2 × 2 factorial analysis with temperature, diet, and experimental days as fixed effects and the animal as a random effect. Furthermore, the interaction effects between diet × temperature, diet × experimental day, temperature × experimental day, and diet × temperature × experimental day were all considered. The significance of each effect was assessed by least square analysis (LSD). Similarly, the changes in relative expression of the PBMC target genes with GAPDH as the housekeeping gene were analysed by 2 × 2 factorial analysis with temperature, diet, and temperature × diet as fixed factors and the animal as a random effect. The significant difference between the main effects was compared using Bonferroni values. The significance level was set at p < 0.05. The data obtained were statistically analysed using the SPSS software (Version 29.0), considering the Restricted Maximum Likelihood (REML) approach.
3. Results
3.1. Effect of Heat Stress and Herbal Supplementation on Feed Intake and Water Intake in Kenguri Sheep
The feed and water intake were observed to be significantly influenced by several experimental factors, as depicted in Table 5.

Table 5.
Effect of heat stress and herbal supplementation on the behavioural responses in Kenguri ewes.
3.1.1. Results on Feed Intake
Feed intake increased under heat stress (49.93 vs. 51.30 g/w0.75/day for thermoneutral vs. heat stress; p < 0.01) and was further enhanced by herbal supplementation (50.03 vs. 51.20 g/w0.75/day for control vs. herbal diet; p < 0.05) (Table 5).
In addition, the feed intake was different between the days, being lowest on day 15 and highest on day 30 (50.61, 48.95, 51.55, 50.54, and 51.43 g/w0.75/day for day 0, day 15, day 30, day 45, and day 60, respectively, p < 0.01). Among the various interactions, temperature × day (p < 0.001), diet x day (p < 0.01), and temperature x diet x day (p < 0.01) influenced the feed intake.
3.1.2. Results on Water Intake
The water intake significantly increased during heat stress (4.45 vs. 6.51 L/Kg DMI/day for thermoneutral and heat stress conditions, respectively, p < 0.001). However, the diet did not influence the water intake (5.32 vs. 5.63 L/Kg DMI/day for the control and herbal diet, respectively, p = 0.481) (Table 5). In addition, the water intake was different between the days, being lowest on day 0 and highest on day 45 (3.44, 6.13, 5.95, 6.17, and 5.70 L/Kg DMI/day for day 0, day 15, day 30, day 45, and day 60, respectively, p < 0.001). Among the various interactions studied, only temperature × day significantly increased the water intake (p < 0.001). However, temperature × diet (p = 0.55), diet × day (p = 0.14), and temperature × diet × day (p = 0.69) did not influence the water intake.
3.2. Effect of Heat Stress and Herbal Supplementation on Body Weight in Kenguri Sheep
Both temperature treatment (20.83 vs. 20.93 kg for thermoneutral and heat stress conditions, respectively, p = 0.89) and diet (20.64 vs. 21.12 kg for control and herbal diet, respectively, p = 0.56) did not influence body weight (Table 6).

Table 6.
Effect of heat stress and herbal supplementation on the body weight in Kenguri ewes.
However, the experimental days highly significantly (p < 0.001) influenced the body weight, with the lowest value recorded on day 0 and the highest on day 60 (18.97, 20.00, 20.80, 21.75, and 22.90 kg for day 0, day 30, day 45, and day 60, respectively, p < 0.001). Among the various interactions, only diet × day significantly (p < 0.001) influenced the body weight. However, temperature × diet (p = 0.48), temperature × day (p = 0.69), and temperature × diet × day (p = 0.29) interactions did not alter the body weight in the study.
3.3. Effect of Heat Stress and Herbal Supplementation on Allometric Measurements in Kenguri Sheep
The study revealed both temperature and diet to have a comparable impact (p > 0.05) on the allometric measurements body length, body height, and chest girth (Table 7). However, the experimental days highly influenced all the allometric variables, with the highest recording observed on day 60. Among the various interactions, only diet × day significantly (p < 0.05) influenced body length. However, no other significant interactions were observed for the other allometric variables.

Table 7.
Effect of heat stress and herbal supplementation on the allometric measurements in Kenguri ewes.
3.4. Effect of Heat Stress and Herbal Supplementation on Endocrine Variables in Kenguri Sheep
The experimental factors (diet, temperature, day, and the interaction effects) were observed to significantly influence the endocrine profile in Kenguri sheep (Table 8).

Table 8.
Effect of heat stress and herbal supplementation on the endocrine profile in Kenguri ewes.
3.4.1. Growth Hormone (GH)
Heat stress did not influence the plasma growth hormone (GH) concentration (3.28 vs. 2.85 ng/mL for thermoneutral and heat stress conditions, respectively, p = 0.33), however, the herbal supplement significantly (p < 0.01) increased the GH concentration (2.32 vs. 3.80 ng/mL, for control and herbal diet, respectively, p < 0.01) (Table 8).
Further, the GH concentration was different between the days, with the lowest value recorded on day 0 and the highest value recorded on day 60 (2.79, 2.67, 3.13, 3.22, and 3.50 ng/mL for day 0, day 30, day 45, and day 60, respectively, p < 0.001). Among the various interactions, only diet x day significantly (p < 0.001) influenced the GH levels. However, temperature x diet (p = 0.29), temperature × day (p = 0.49), and temperature × diet × day (p = 0.76) interactions did not influence the GH concentration in the study.
3.4.2. Insulin-like Growth Factor (IGF-1)
Heat stress did not influence the plasma insulin-like growth factor (IGF-1) concentration (55.77 vs. 50.25 ng/mL for thermoneutral and heat stress conditions, respectively, p = 0.30), however, the herbal supplement significantly increased the IGF-1 concentration (45.63 vs. 60.38 ng/mL, for control and herbal diet, respectively, p < 0.01) (Table 8). Further, the IGF-1 concentration was different between the days, with the lowest value recorded on day 0 and the highest value recorded on day 60 (48.63, 48.71, 54.76, 56.45, and 56.48 ng/mL for day 0, day 30, day 45, and day 60, respectively, p < 0.01). Among the various interactions, only diet × day significantly (p < 0.001) influenced the IGF-1 levels. However, temperature × diet (p = 0.45), temperature × day (p = 0.07), and temperature × diet × day (p = 0.42) interactions did not influence the IGF-1 concentration in the study.
3.4.3. Tri-Iodo-Thyronine (T3)
The plasma T3 concentration significantly decreased during heat stress (28.77 vs. 24.79 nmol/L for thermoneutral and heat stress conditions, respectively, p < 0.001) and was significantly increased by the herbal supplement (22.74 vs. 30.82 nmol/L for control and herbal diet, respectively, p < 0.001) (Table 8). However, the experimental day did not influence the T3 concentration. Further, temperature × diet, diet × day, temperature × day, and temperature × diet × day interactions highly significantly (p < 0.001) influenced the plasma T3 concentration.
3.4.4. Thyroxine (T4)
The plasma T4 concentration significantly decreased during heat stress (118.78 vs. 101.70 nmol/L for thermoneutral and heat stress conditions, respectively, p < 0.05) and was significantly increased by the herbal supplement (92.76 vs. 127.71 for control and herbal diet, respectively, p < 0.001) (Table 8). Further, the plasma T4 concentration was different between the days, with the lowest value on day 0 and the highest value on day 60 (98.0, 105.86, 108.42, 117.62, and 121.28 for day 0, day 15, day 30, day 45, and day 60, respectively, p < 0.001). In addition, the temperature × diet (p < 0.05), temperature × day (p < 0.001), diet × day (p < 0.001), and temperature × diet × day interactions influenced the plasma T4 concentration.
3.5. Effect of Heat Stress and Herbal Supplementation on Expression Pattern of Targeted Genes in Kenguri Sheep
3.5.1. Expression Pattern of GHR
The effect of heat stress and herbal supplementation on the growth hormone receptor (GHR) expression pattern in Kenguri sheep is described in Figure 3.
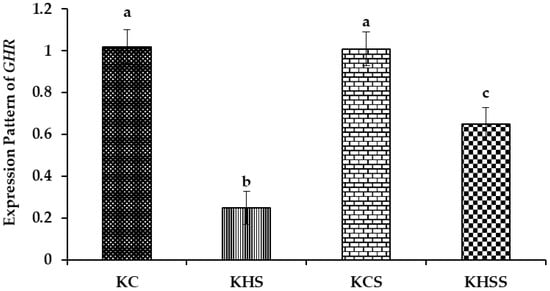
Figure 3.
Effect of heat stress and herbal supplementation on the GHR mRNA expression pattern in Kenguri sheep. The expression patterns of GHR in the KC, KHS, KCS, and KHSS groups were 1.02 ± 0.08, 0.25 ± 0.08, 1.01 ± 0.08, and 0.65 ± 0.08, respectively. KC-Kenguri Control; KHS-Kenguri Heat Stress; KCS-Kenguri Control Supplement; KHSS- Kenguri Heat Stress Supplement. Different superscript letters (a, b and c) indicated extremely significant statistical differences among the treatment groups (p < 0.001).
Among the different groups, significantly (p < 0.05) higher expression of GHR was recorded in the KC and KCS groups, followed by the KHS and KHSS groups. The expression of GHR was significantly reduced by heat stress (1.02 vs. 0.25; p < 0.001), but this significant reduction was increased by supplementation with herbs (0.25 vs. 0.65; p < 0.05). Further, there was a significant (p < 0.05) interaction between heat treatment and diet, such that the herbal supplementation increased the GHR expression during heat stress (0.25 vs. 0.65) but not under thermoneutral conditions (1.02 vs. 1.01).
3.5.2. Expression Pattern of IGF1
The effect of heat stress and herbal supplementation on the insulin-like growth factor (IGF1) expression pattern in Kenguri sheep is described in Figure 4.
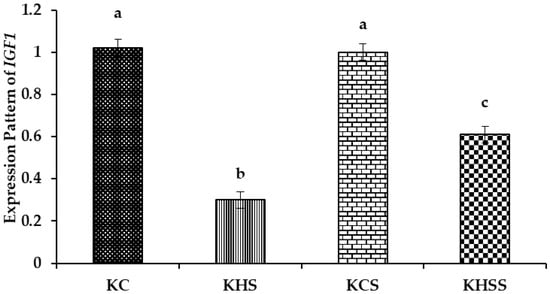
Figure 4.
Effect of heat stress and herbal supplementation on the IGF1 mRNA expression pattern in Kenguri sheep. The expression patterns of IGF1 in the KC, KHS, KCS, and KHSS groups were 1.02 ± 0.04, 0.3 ± 0.04, 1.0 ± 0.04, and 0.61 ± 0.04, respectively. KC-Kenguri Control; KHS-Kenguri Heat Stress; KCS-Kenguri Control Supplement; KHSS- Kenguri Heat Stress Supplement. Different superscript letters (a, b and c) indicated extremely significant statistical differences among the treatment groups (p < 0.001).
Among the different groups, significantly (p < 0.001) higher expression of IGF1 was recorded in the KC and KCS groups, followed by the KHS and KHSS groups. The expression of IGF1 was significantly (p < 0.001) reduced by heat stress (1.02 vs. 0.3), but this significant reduction was increased by supplementation with herbs (0.30 vs. 0.61; p < 0.001). Further, there was a significant (p < 0.01) interaction between heat treatment and diet, such that the herbal supplementation increased the IGF1 expression during heat stress (0.30 vs. 0.61) but not under thermoneutral conditions (1.02 vs. 1.00).
3.5.3. Expression Pattern of PRLR
The effect of heat stress and herbal supplementation on the prolactin receptor (PRLR) expression pattern in Kenguri sheep is described in Figure 5.
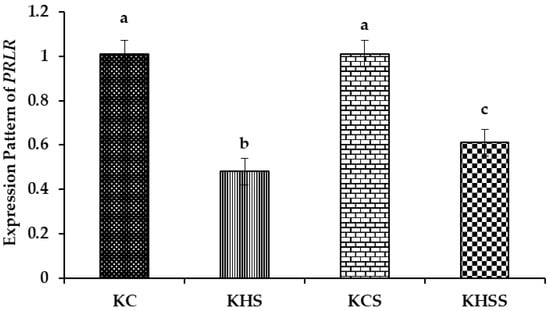
Figure 5.
Effect of heat stress and herbal supplementation on the PRLR mRNA expression pattern in Kenguri sheep. The expression patterns of PRLR in the KC, KHS, KCS, and KHSS groups were 1.01 ± 0.06, 0.48 ± 0.06, 1.01 ± 0.06, and 0.61 ± 0.06, respectively. KC-Kenguri Control; KHS-Kenguri Heat Stress; KCS-Kenguri Control Supplement; KHSS- Kenguri Heat Stress Supplement. Different superscript letters (a, b and c) indicated extremely significant statistical differences among the treatment groups (p < 0.001).
Among the different groups, significantly (p < 0.001) higher expression of PRLR was recorded in the KC and KCS groups, and the lowest was recorded in the KHS and KHSS groups. The expression of PRLR was significantly (p < 0.001) reduced by heat stress (1.01 vs. 0.48), but the reduced expression of PRLR was not altered by herbal supplementation (0.48 vs. 0.61). Further, the temperature x diet interaction did not significantly influence the expression pattern of PRLR.
3.5.4. Expression Pattern of THRA
The effect of heat stress and herbal supplementation on the thyroid hormone receptor alpha (THRA) expression pattern in Kenguri sheep is described in Figure 6.
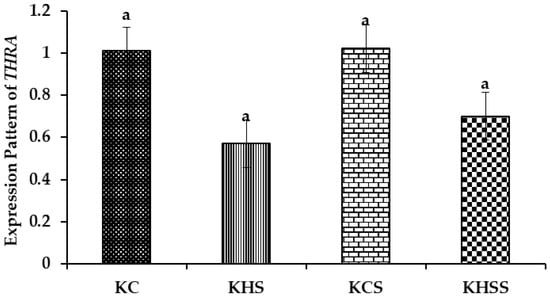
Figure 6.
Effect of heat stress and herbal supplementation on the THRA mRNA expression pattern in Kenguri sheep. The expression patterns of THRA in the KC, KHS, KCS, and KHSS groups were 1.01 ± 0.10, 0.57 ± 0.10, 1.02 ± 0.10, and 0.70 ± 0.10, respectively. KC-Kenguri Control; KHS-Kenguri Heat Stress; KCS-Kenguri Control Supplement; KHSS- Kenguri Heat Stress Supplement. Different superscript letters (a, b and c) indicated extremely significant statistical differences among the treatment groups (p < 0.001).
Among the different groups, on comparison, the expression pattern of THRA did not differ significantly. Further, the treatment, diet, and treatment x diet interaction also did not influence THRA, such that the expression values remained comparable across the groups.
4. Discussion
The results obtained from this study assessed the various phenotypic and genotypic traits governing growth performance in Kenguri sheep during heat stress exposure. This study is the need of the hour as global researchers try to find solutions for climate change associated with livestock production by identifying various climate-resilient livestock breeds. This approach may help the farming community to capitalise on such breeds by propagating them to poor and marginal farmers for sustainable livestock production. Although indigenous in nature, the available literature clearly suggests that, even among indigenous breeds, there are differences in their climate-resilient potential [40]. The present study is one such attempt to study in detail the various mechanisms that govern growth performance during heat stress exposure in Kenguri sheep. The results obtained from the study also assessed novel herbal supplements to improve the climate resilience in Kenguri sheep, which may revolutionise future breeding strategies in sheep production.
4.1. Temperature–Humidity Index
The THI recorded during the study period clearly demonstrates that the KHS and KHSS groups were subjected to extremely severe heat stress, while the KC and KCS animals were subjected to comfort conditions. This was in accordance with the THI model developed by McDowell in 1972 [37]. This proved the hypothesis of providing a simulated condition to compare the animals in thermoneutral zone versus extreme heat stress conditions with and without herbal supplements, to explore the possibility of identifying potential phenotypic and genotypic biomarkers. The average THIs during the time duration between 10:00 h and 16:00 h in the TNZ and heating chambers were 74.38 and 74.45, respectively, which do not fall under the stress category, as per McDowell [37].
4.2. Feed Intake
Heat stress significantly increased the feed intake, which was not surprising given the fact that Kenguri sheep are extremely adapted and well-known for grazing during the summer months. This finding could be attributed to the indigenous nature of this particular breed. The indigenous breeds are well-known for grazing during the summer months without compromising their feed intake. Since Kenguri is an indigenous sheep breed, it could have possessed the ability to cope with heat stress without compromising feed intake. This could have been the reason for the increased feed intake, probably to supply regular energy for vital body organ functioning to dissipate body heat to cope with heat stress. However, a contrasting finding of a reduction in feed intake during extreme heat stress exposure was recorded, with the probable mechanism of reducing metabolic heat production in ruminants [41]. A study reported that heat stress reduced feed intake by stimulating the peripheral thermal receptor for transmitting a suppressive nerve impulse to the appetite centre located in the hypothalamus. The heat stress-associated reduction in feed intake could be an adaptive mechanism of the animal to generate low body heat [9]. There are also contrasting reports of recording reduced feed intake after heat stress exposure in Dorper × Pelibuey ewe lambs and Lacaune ewes [42,43]. A similar study in small-tailed Han sheep [44] reported reduced feed intake during an increased THI in ewes. Further, in the current study, the herbal supplement increased the feed intake, which could be attributed to its heat stress-protective effects to stimulate feed intake. A study conducted on Barbari kids [23] established increased voluntary feed intake after herbal mixture (Amla, Haldi, Arni, and Tulsi) supplementation. The already increased feed intake in this particular breed during heat stress exposure was further boosted by herb supplements, establishing the potential for its improved climate resilience. The significant temperature × day interaction indicates that the increased feed intake during heat stress exposure persisted throughout the study period. Further, the significant interaction between diet × day indicates that the herbal diet had a positive influence on feed intake throughout the study period. In addition, the significant interaction between temperature × diet × day clearly suggests that both heat stress and herbal feed supplements induced increased feed intake throughout the study period.
4.3. Water Intake
Heat stress significantly (p < 0.001) increased the water intake, while the herbal supplement did not alter the heat stress-induced increase in water intake. A similar study conducted in climate chambers in Malpura ewes reported significantly higher water intake in heat-stressed animals [45]. These authors attributed this to the animals meeting the increment of water requirements for heat dissipation through evaporation. In a similar study, ref. [43], it was reported that heat-stressed dairy ewes consumed more water than the control group ewes. Likewise, ref. [46] reported similar findings of heat stress associated with increased water intake in Sardinian ewes. However, ref. [47] conducted a study to compare Merino wethers and Awassi rams and reported that the water intake increased only in Awassi rams during heat stress exposure, but it remained unaltered in Merino wethers. A study conducted in Dorper × Katahdin male lambs reported a 49% higher water intake in heat-stressed lambs than their counterpart. These authors opined that increased water intake is an important thermoregulatory mechanism to avoid hyperthermia and dehydration in animals experiencing heat stress [48]. Further, it was expected that the evaporation of water content increases during heat stress and this should have influenced the water intake of the KHS and KHSS groups. Therefore, the accuracy of water intake would be determined by the rate of evaporation of water in the heat-stressed animals. The significant temperature × day interaction on water intake indicates that the severity of heat stress persisted, and Kenguri ewes increased their water intake to cope with heat stress throughout the study period.
4.4. Body Weight
In this study, the temperature treatment did not influence the body weight. Although exposed to such extreme heat stress, Kenguri sheep showed high resilience by maintaining their body weight. This could be attributed to the indigenous, adapted nature of this breed. The reason for no effect of increased feed intake on body weight gain during heat stress exposure could be due to the possibility of energy getting channelled only towards adaptive pathways and not towards productive pathways in these animals. However, in a study conducted on Comisanas and Sardinian sheep by [49], it was reported that their body weight reduced during heat stress exposure. Similarly, studies have noted that exposure of sheep to elevated temperatures results in a decrease in body weight [9,45]. A study conducted in Deccani and Nellore sheep also reported a significant reduction in body weight [50]. These authors opined that the combined effects of reduced feed consumption, increased energy cost required for heat dissipation, and altered metabolic and gut physiological processes could be the cause for the reduction in weight gain.
Comparing these findings, it is evident that Kenguri ewes were superior in terms of maintaining body weight during heat stress exposure. Although the feed intake increased during heat stress, it still could not reflect on body weight as the energy could have been diverted to adaptive pathways rather than production. However, as the experiment progressed, the herbal supplement was able to increase the body weight, as reflected by the significant diet x day interaction, indicating the beneficial role of herbal supplements. Likewise, in a similar study, the inclusion of herbal supplements as a mixture composed of different herbs may have had multiple effects, and it demonstrated the synergistic effect of a herbal formula in improving body weight gain in fattening lambs [8]. Similarly, a study investigated the effects of Morinda citrifolia (Noni) on growth and feed efficiency in large, domestic ruminants. The improvement in growth was attributed to improved immune function in Noni-supplemented calves. Further, it was suggested that the antioxidant activity could be responsible for the improved growth and feed efficiency of Noni-fed cattle [51]. The significant effect of diet x day interaction on body weight reflects the positive impact of the herbal feed additive on this particular variable throughout the study period. These findings highlight the effectiveness of herbal supplements in reducing the negative impact of heat stress on the growth performance of Kenguri sheep, thereby indicating its potential for sustainable sheep production in the changing climate scenario.
4.5. Allometric Measurements
Body measurements are used to evaluate the characteristics of animals that may vary due to the influence of breed evolution, environment, and nutrition. The body measurements are related to growth and can be used to predict growth [52,53]. However, neither temperature treatment nor the diet influenced any of the allometric measurements in Kenguri ewes in the present study. Similar to this finding, in another study conducted in native sheep of Bangladesh, heat exposure resulted in no significant differences in body weight, length, and heart girth [54]. However, ref. [55] reported reduced body measurements like body length, wither height, and chest girth in Naeemi sheep during the summer season, and it attributed this to the heat stress experienced by these animals. Further, the herbal supplement also did not influence the body measurements in the present study. The significant effect of diet x day interaction on body length reflects the positive impact of the herbal feed additive on this particular variable throughout the study period.
4.6. Endocrine Variables
4.6.1. Plasma GH and IGF-1 Concentration
In the present study, heat stress did not influence the plasma GH concentration. However, the herbal supplement significantly increased its concentration during the entire study period. GH is considered one of the prominent hormones that are altered under heat stress conditions, and its plasma levels can be a potential indicator of the physiological changes in heat-stressed animals [56]. Further, it has also been established that prolonged heat exposure may decrease the GH concentration [57,58]. The non-significant effect of heat stress in the current study could be due to the extreme adaptive nature of the particular breed. Further, the non-significant effect of heat stress on the body weight in the current study establishes the climate-resilient potential of this particular breed. Similar to our finding, ref. [59] also established a non-significant effect of prolonged heat stress on blood GH concentration in sheep. The non-significant effect of heat stress on the plasma GH concentration could be partially dependent on the plane of nutrition in these animals as these animals are ad libitum fed, which should have provided them with the appropriate nutrition to counter the heat stress. Supporting this notion, the KHSS group’s animals were subjected to herbal supplements, and the concentration of GH was markedly increased in this study. Further, ref. [60] also supported this explanation through their finding of chronic heat stress mediating reduced circulating GH levels in dairy cows, and they attributed this decrease partially to the poor plane of nutrition. Similar to the results obtained for the GH level, the concentration of IGF-1 also did not differ for the heat stress treatment. Thus, the unaltered levels of GH and IGF-1 in heat-stressed Kenguri ewes demonstrate the adaptive potential of this particular breed. Further, both IGF-1 and GH were established to work in coordination in hormone response pathways controlling energy metabolism during the thermoregulation process in cattle [61]. Further, there are reports that suggest that the adverse impact of heat stress could be alleviated by regulation of IGF-1 through its thermo-protective action [62,63]. However, a study in Malpura lambs by [64] reported that heat stress significantly increased both GH and IGF-1 concentrations. In addition, the progressive increase in the GH and IGF-1 concentrations in the KHSS group as the experimental days progressed clearly demonstrates the climate-resilient potential of this particular breed, as once they adapted to the heat stress, the herbal supplement had a beneficial role by improving the GH and IGF-1 concentrations. The non-significant interaction between temperature treatment and experimental days on plasma GH and IGF-1 concentrations indicates that the heat stress did not alter the levels of these two hormones throughout the study period. However, the significant interaction between diet and experimental days clearly indicates the positive effects of the herbal feed additives on both the GH and IGF-1 concentrations persisted throughout the study period. These results clearly demonstrate the significance of using herbal supplements in ameliorating the adverse heat stress impacts on growth performance in heat-stressed sheep.
4.6.2. Plasma Thyroid Hormone Concentration
Thyroid hormones have an essential role in thermoregulation and homothermy. There are also seasonal influences established on the activity of the thyroid gland and on blood thyroid hormone concentration [65]. In the present study, heat stress significantly reduced both T3 and T4 concentrations. This could be attributed to the metabolic adaptation of Kenguri ewes, reducing their levels to produce less metabolic heat to cope with the external harsh environmental condition. Similar to our findings, ref. [66] also reported reduced T3 and T4 concentrations in Iranian fat-tailed sheep. Further, ref. [67] also reported decreased blood T3 and T4 levels in indigenous sheep breeds during heat stress exposure. In addition, the herbal supplements during heat stress conditions also significantly increased the levels of both T3 and T4, reflecting the heat stress-protecting effects of these supplements. It is to be noted that there was no literature available, especially studying the influence of multiple herbal supplements on the endocrine profile of sheep. The significant temperature × day interaction on both the plasma T3 and T4 concentrations indicates that the heat stress-induced reduced plasma thyroid hormone concentration persisted throughout the study period in Kenguri ewes, probably to adapt to heat stress challenges by reducing metabolic heat production. Further, the diet × day interaction indicates that the herbal feed additives induced the increased plasma thyroid hormone concentration throughout the study period. In addition, the significant interaction between temperature × diet × day on plasma thyroid hormone concentration suggests the heat stress protection effects of herbal feed additives in heat-stressed Kenguri ewes persisted throughout the study period. These results clearly demonstrate the practical significance of herbal supplements in mitigating the adverse effects of heat stress on the growth performance of sheep.
4.7. Gene Expression Patterns
4.7.1. The GHR and IGF-1 Expression Patterns
The growth hormone receptor (GHR) and insulin-like growth factor (IGF1) expression patterns were significantly lower during heat stress exposure. Further, the herbal supplement significantly upregulated the expression patterns of GHR and IGF1. The supplementation in the thermoneutral animals did not bring any beneficial effect, as evident from the comparable levels of expression patterns between the KC and KCS groups. However, the significant upregulation of the supplemented group during heat stress clearly establishes the heat stress-protective effects of the supplement. A study in Osmanabadi goats observed lower expression of IGF1 during heat stress exposure and attributed this to the lower feed intake during heat stress exposure [68]. In the current study, although heat stress mediated an increase in the feed intake, the level of IGF1 was still lower at the mRNA level, indicating the severity of heat stress in this study, and thus the increase in the feed intake could not bring about any positive change in the body weight, allometric measurements, IGF-1, and IGF1 mRNA expression. The direct physiological effects of GH result from the activation of extracellular GHR [69]. Further, GHR mediated the effect of GH on IGF-1, as GH–GHR binding was necessary to stimulate IGF-1 synthesis and release [70]. These hypotheses have been proved by a significant decrease in the GH and IGF-1 concentration, along with the downregulation of both GHR and IGF1, in this study. Similarly, lower expressions of GHR and IGF1 in Malabari goats have been reported by [15]. Likewise, ref. [71] also reported similar findings of lower expressions of GHR and IGF1 in Salem black goats. These authors attributed these downregulated GHR and IGF1 during heat stress exposure to the lower nutritional status of these animals. Thus, the herbal supplement during heat stress that upregulated GHR and IGF1 in the present study proved that the necessary micronutrient supplementation was essential to protect the Kenguri ewes from heat stress. These findings signify the importance of supplementing essential micronutrients to improve the climate resilience in small ruminants. The significant interaction between heat treatment and diet clearly demonstrates the positive impact of herbal feed additives on GHR and IGF-1 expression patterns in heat-stressed Kenguri ewes. These molecular-level results clearly demonstrate the significant role of the herbal supplement in enhancing the growth performance of heat-stressed sheep, presenting it as a viable solution to mitigate heat stress-related economic losses for sheep farmers.
4.7.2. The PRLR Expression Pattern
Heat stress downregulated the prolactin receptor (PRLR), while the herbal supplement significantly upregulated its expression pattern. It was found that PRLR was associated with thermo-tolerance in ruminant livestock [72]. Further, ref. [73] established a lower expression pattern of PRLR in heat-stressed domestic ruminants. These authors opined that the downregulation of PRLR was associated with mechanisms to downregulate some metabolic process directed to reduce heat increment. Thus, in the current study, the downregulation of PRLR could be attributed to the adaptive mechanism of Kenguri ewes to reduce metabolic heat production to cope with extreme external severe heat stress conditions. The herbal supplementation significantly upregulated the heat stress-mediated reduced expression of PRLR. This herbal supplement mediated an increase of PRLR in heat-stressed Kenguri ewes that could promote the acclimatisation process involved in the regulation of several ions, extracellular volume, and body fluid, improving animal responses related to heat dissipation during chronic heat stress exposure [74].
4.7.3. The THR Expression Pattern
It has been demonstrated that the thyroid hormone receptor (THR) is considered an important thermo-tolerant gene in livestock species [72]. THR signalling is required for the normal development of parvalbuminergic neurons (PBNs) linking the thyroid hormone to cardiac and temperature regulation [75]. It has been established that heat stress significantly reduces the THR expression in dairy cows [76]. However, in the present study, neither heat stress nor the herbal supplement influenced the expression pattern of thyroid hormone receptor alpha (THRA). The unaltered expression pattern of THRA could reflect the inherent potential of the Kenguri ewes to keep intact their level of mediating effective thermoregulation. The non-significant effect of both treatment and diet could reflect the better nutritional status of the Kenguri ewes.
5. Conclusions
This study establishes, in detail, the various impacts of heat stress on different growth performance-related variables. While the herbal supplement did not result in beneficial effects on the body weight and allometric measurements in Kenguri ewes during heat stress exposure, it did increase the plasma GH and IGF1 concentrations during heat stress exposure, reflecting its positive role on the endocrine factors controlling growth performance in these ewes. Further, the herbal supplement significantly increased the heat stress-induced reduced levels of plasma T3 and T4. In addition, heat stress significantly reduced the GHR, IGF1, and PRLR expression. However, herbal supplementation enhanced their expression, reflecting its positive role in the molecular mechanisms governing growth performance in Kenguri ewes. Thus, the positive impact of the herbal supplements on most of the variables studied clearly reflects their potential for improving climate resilience in Kenguri ewes. Therefore, from the study, it could be concluded that although the herbal supplements did not bring positive changes in body weight and allometric measurements, it still had a beneficial impact on the endocrinology and genes governing growth performance in Kenguri ewes. Thus, the herbal feed additive used in the study shows promise for relieving heat stress in Kenguri ewes. These results suggest further refinements are needed in identifying the active components in the herbal feed additives, and the dose used in this study should be revisited. While this study points towards the heat stress-protective effects of the herbal supplement, future research should investigate its effects on other sheep breeds in a larger population to determine its broader applicability. In addition, future work should target exploring the exact molecular mechanisms underlying its growth-protective effects and investigate its positive effects in a prolonged feeding study.
Author Contributions
Conceptualization, V.S., C.D. and A.S.; formal analysis, E.B.R., M.V.S., S.V.P. and V.S.; writing—original draft preparation, E.B.R., M.V.S. and J.N.; writing—review and editing, V.S., J.N., C.D., S.V.P., M.V.S., A.S. and F.R.D.; supervision, V.S, C.D. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received external funding from the Indian Council of Agricultural Research under the NICRA project.
Institutional Review Board Statement
The paper was approved for submission by the Institute PME Cell.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data can be obtained on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Indu, S.; Pareek, A. A review: Growth and physiological adaptability of sheep to heat stress under semi–arid environment. Int. J. Emerg. Trends Sci. Technol. 2015, 2, 3188–3198. [Google Scholar] [CrossRef]
- IWTO. 2022 World Sheep Numbers & Wool Production. Available online: https://iwto.org/wp-content/uploads/2022/04/IWTO-Market-Information-Sample-Edition-17.pdf (accessed on 23 September 2024).
- Khani, M.; Fattah, A.; Ebrahimi-Mahmoudabad, S.; Joezy-Shekalgorabi, S. Impact of dietary cation-anion difference on rumen fermentation, digestibility, and blood parameters in Zandi lambs under heat stress. Agrobiol. Rec. 2023, 12, 60–67. [Google Scholar] [CrossRef]
- Yue, S.; Qian, J.; Du, J.; Liu, X.; Xu, H.; Liu, H.; Chen, X. Heat stress negatively influence mammary blood flow, mammary uptake of amino acids and milk amino acids profile of lactating Holstein dairy cows. Pak. Vet. J. 2023, 43, 73–78. [Google Scholar]
- Li, J.B.; Wang, X.; Sun, A.; Li, H.B.; Luo, Y.; He, F.; Yi, K.L. Comparative transcriptomic analysis of spermatozoa from Xiangxi and Simmental bulls under heat stress: Implications for fertility prediction. Pak. Vet. J. 2023, 43, 184–188. [Google Scholar] [CrossRef]
- Hussan, S.J.; Al-hummsod, S.K.; Al-Asadi, M.H. The Impact of Astaxanthin Supplementation on the Production Performance of Laying Hens Exposed to Heat Stress. J. Glob. Innov. Agric. Sci. 2023, 11, 637–642. [Google Scholar]
- Demir, E.; Ceccobelli, S.; Bilginer, U.; Pasquini, M.; Attard, G.; Karsli, T. Conservation and selection of genes related to environmental adaptation in native small ruminant breeds: A review. Ruminants 2022, 2, 255–270. [Google Scholar] [CrossRef]
- Wanjala, G. Genome-Wide Analysis of the Genetic Diversity of Native Sheep Breeds and Determination of Potential Selection Signatures for Climate Change Adaptation. Ph.D. Thesis, Debreceni Egyetem, Debrecen, Hungary, 2024. [Google Scholar]
- Marai, I.F.M.; El-Darawany, A.A.; Fadiel, A.; Abdel-Hafez, M.A.M. Physiological traits as affected by heat stress in sheep—A review. Small Rumin. Res. 2007, 71, 1–12. [Google Scholar] [CrossRef]
- Silanikove, N. Effects of heat stress on the welfare of extensively managed domestic ruminants. Livest. Prod Sci. 2000, 67, 1–18. [Google Scholar] [CrossRef]
- Marai, I.F.M.; Habeeb, A.A.M.; Farghaly, H.M. Productive, physiological and biochemical changes in imported and locally born Friesian and Holstein lactating cows under hot summer conditions of Egypt. Trop. Anim. Health Prod. 1999, 31, 233–243. [Google Scholar] [CrossRef]
- Kamanga-Sollo, E.; Pampusch, M.S.; White, M.E.; Hathaway, M.R.; Dayton, W.R. Effects of heat stress on proliferation, protein turnover, and levels of heat shock protein mRNA in cultured porcine muscle satellite cells. J. Anim. Sci. 2011, 89, 3473–3480. [Google Scholar] [CrossRef]
- McManus, C.M.; Lucci, C.M.; Maranhão, A.Q.; Pimentel, D.; Pimentel, F.; Paiva, S.R. Response to heat stress for small ruminants: Physiological and genetic aspects. Livest. Sci. 2022, 263, 105028. [Google Scholar] [CrossRef]
- West, J.W. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Angel, S.P.; Bagath, M.; Sejian, V.; Krishnan, G.; Bhatta, R. Expression patterns of candidate genes reflecting the growth performance of goats subjected to heat stress. Mol. Biol. Rep. 2018, 45, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Song, L.; Dong, Q.; Zhang, A.; Zhang, L.; Wang, Y.; Feng, M.; Li, X.; Li, F.; Sun, X.; et al. Effects of herbal formula on growth performance, apparent digestibility, antioxidant capacity, and rumen microbiome in fattening lambs under heat stress. Environ. Sci. Pollut. Res. 2024, 31, 51364–51380. [Google Scholar] [CrossRef]
- Saleh, A.A.; Soliman, M.M.; Yousef, M.F.; Eweedah, N.M.; El-Sawy, H.B.; Shukry, M.; Wadaan, M.A.; Kim, I.H.; Cho, S.; Eltahan, H.M. Effects of herbal supplements on milk production quality and specific blood parameters in heat-stressed early lactating cows. Front. Vet. Sci. 2023, 10, 1180539. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, L.; Yan, S.; Shi, B. Plant Extracts to Alleviating Heat Stress in Dairy Cows. Animals 2023, 13, 2831. [Google Scholar] [CrossRef]
- Lakhani, P.; Jindal, R.; Nayyar, S. Effect of heat stress on humoral immunity and its amelioration by amla powder (Emblica officinalis) supplementation in buffaloes. J. Anim. Res. 2016, 6, 401–404. [Google Scholar] [CrossRef]
- Verma, P.; Jain, A.K.; Mishra, A.; Jesse, D.D.; Mandal, S.; Gattani, A.; Patel, P.; Singh, P.; Jatav, M. Ameliorative effect of Withania somnifera on haemato-biochemical and hormonal parameters during heat stress in murrah buffalo calves. Int. J. Adv. Biochem. Res. 2024, 8, 80–85. [Google Scholar] [CrossRef]
- Saleh, A.A.K.; Abozed, G.F. Impact of using chamomile flower as a feed additive on reproductive performance and physiological responses of Farafra ewes during heat stress conditions. Egypt. J. Nutr. Feed. 2018, 21, 635–643. [Google Scholar] [CrossRef]
- Kalaitsidis, K.; Sidiropoulou, E.; Tsiftsoglou, O.; Mourtzinos, I.; Moschakis, T.; Basdagianni, Z.; Vasilopoulos, S.; Chatzigavriel, S.; Lazari, D.; Giannenas, I. Effects of cornus and its mixture with oregano and thyme essential oils on dairy sheep performance and milk, yoghurt and cheese quality under heat stress. Animals 2021, 11, 1063. [Google Scholar] [CrossRef]
- Chaturvedi, I.; Dutta, T.K.; Singh, P.K.; Chatterjee, A.; Mandal, D.K.; Bhakat, C.; Mohammad, A.; Das, A.K. Effect of supplementation of phytogenic feed additives on intake, in vitro fermentation, growth performance and carcass traits in weaned Barbari kids reared under intensive feeding. Trop. Anim. Health Prod. 2022, 54, 150. [Google Scholar] [CrossRef] [PubMed]
- Maurya, P.K.; Singh, R.K.; Chandra, G.; Ranjan, K.; Roy, D.; Kumar, P.; Fahim, A.; Sejian, V. The Effect of Silymarin and Selenium-yeast Supplementation on Antioxidant Status, Lipid Peroxidation and Metabolic Hormones in Heat-Stressed Barbari Goats. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Lavanya, M.; Reddy, G.B.; Reddy, M.K.; Anjana, N.S.; Babu, D.S.; Ganguly, B. Anti-Inflammatory Effect of Polyherbal Feed Supplements in Heat Stressed Pigs. Indian Vet. J. 2023, 100, 32–35. [Google Scholar]
- Rajaei-Sharifabadi, H.; Ellestad, L.; Porter, T.; Donoghue, A.; Bottje, W.G.; Dridi, S. Noni (Morinda citrifolia) modulates the hypothalamic expression of stress-and metabolic-related genes in broilers exposed to acute heat stress. Front. Genet. 2017, 8, 192. [Google Scholar] [CrossRef]
- Flees, J.; Rajaei-Sharifabadi, H.; Greene, E.; Beer, L.; Hargis, B.M.; Ellestad, L.; Porter, T.; Donoghue, A.; Bottje, W.G.; Dridi, S. Effect of Morinda citrifolia (noni)-enriched diet on hepatic heat shock protein and lipid metabolism-related genes in heat stressed broiler chickens. Front. Physiol. 2017, 8, 919. [Google Scholar] [CrossRef]
- Selvam, R.; Suresh, S.; Saravanakumar, M.; Chandrasekaran, C.V. Alleviation of heat stress by a polyherbal formulation, Phytocee™: Impact on zootechnical parameters, cloacal temperature, and stress markers. Pharmacogn. Res. 2018, 10, 1. [Google Scholar]
- Suryadi, U.; Kustiawan, E.; Prasetyo, A.F.; Imam, S. Effect of agarwood leaf extract on production performance of broilers experiencing heat stress. Vet. World 2021, 14, 1971. [Google Scholar] [CrossRef]
- Al-Hoshani, N.; Almahallawi, R.; Al-Nabati, E.A.; Althubyani, S.A.; Negm, S.; El-lkott, A.F.; Majed, A.; Bajaber, M.; Soliman, A.E. Anthelmintic effects of herbal mixture of selected plants of Apiaceae on Strongylus vulgaris and Fasciola hepatica. Pak. Vet. J. 2024, 44, 437–441. [Google Scholar] [CrossRef]
- Mohammad, L.M.; Kamil, A.M.; Tawfeeq, R.K.; Ahmed, S.J. Ameliorating effects of herbal mixture for dexamethasone induced histological changes in mice. Int. J. Vet. Sci. 2023, 12, 126–131. [Google Scholar] [CrossRef]
- Gavzan, H.; Araghi, A.; Abbasabadi, B.M.; Talebpour, N.; Golshahi, H. Antidepressant effects of a Persian herbal formula on mice with chronic unpredictable mild stress. Avicenna J. Phytomed. 2023, 13, 562. [Google Scholar] [CrossRef]
- Bhatt, N. Herbs and Herbal Supplements, a Novel Nutritional Approach in Animal Nutrition. Iran. J. Appl. Anim. Sci. 2015, 5, 497–516. [Google Scholar]
- Li, Y.; Fang, L.; Xue, F.; Mao, S.; Xiong, B.; Ma, Z.; Jiang, L. Effects of bamboo leaf extract on the production performance, rumen fermentation parameters, and rumen bacterial communities of heat-stressed dairy cows. Anim. BioSci. 2021, 34, 1784. [Google Scholar]
- Zhao, S.; Shan, C.; Wu, Z.; Feng, M.; Song, L.; Wang, Y.; Gao, Y.; Guo, J.; Sun, X. Fermented Chinese herbal preparation: Impacts on milk production, nutrient digestibility, blood biochemistry, and antioxidant capacity of late-lactation cows under heat stress. Anim. Feed. Sci. Technol. 2022, 292, 115448. [Google Scholar] [CrossRef]
- Hashemzadeh, F.; Rafeie, F.; Hadipour, A.; Rezadoust, M.H. Supplementing a phytogenic-rich herbal mixture to heat-stressed lambs: Growth performance, carcass yield, and muscle and liver antioxidant status. Small Rumin. Res. 2022, 206, 106596. [Google Scholar] [CrossRef]
- McDowell, R.E. Improvement of Livestock Production in Warm Climates; W. H. Freeman: New York, NY, USA, 1972. [Google Scholar]
- de Sousa Silva, R.; Furtado, D.A.; Ribeiro, N.L.; Neto, J.P.L.; Rodrigues, R.C.M.; de Oliveira, A.G.; da Costa Silva, J.A.P.; da Silva, M.R.; Mascarenhas, N.M.H.; Marques, J.I.; et al. Physiological variables and estimates of heat exchange in sheep kept at thermoneutral and thermal stress temperatures. Small Rumin. Res. 2024, 237, 107320. [Google Scholar] [CrossRef]
- Mullakkalparambil Velayudhan, S.; Sejian, V.; Devaraj, C.; Manjunathareddy, G.B.; Ruban, W.; Kadam, V.; König, S.; Bhatta, R. Novel insights to assess climate resilience in goats using a holistic approach of skin-based advanced NGS technologies. Int. J. Mol. Sci. 2023, 24, 10319. [Google Scholar] [CrossRef] [PubMed]
- Aleena, J. Comparative assessment of the adaptive capacity of different indigenous breed goats to summer heat stress based on changes in phenotypic traits. Ph.D. Thesis, Academy of Climate Change Education and Research Vellanikkara, Kerala Agricultural University, Kerala, India, 2017. Available online: http://krishikosh.egranth.ac.in/handle/1/5810144116 (accessed on 28 April 2025).
- Das, R.; Sailo, L.; Verma, N.; Bharti, P.; Saikia, J.; Imtiwati; Kumar, R. Impact of heat stress on health and performance of dairy animals: A review. Vet. World 2016, 9, 260–268. [Google Scholar] [CrossRef]
- Avendaño-Reyes, L.; Macías-Cruz, U.; Correa-Calderón, A.; Mellado, M.; Corrales, J.L.; Corrales, G.; Ramirez-Bribiesca, E.; Guerra-Liera, J.E. Biological responses of hair sheep to a permanent shade during a short heat stress exposure in an arid region. Small Rumin. Res. 2020, 189, 106146. [Google Scholar] [CrossRef]
- Mehaba, N.; Coloma-Garcia, W.; Such, X.; Caja, G.; Salama, A.A. Heat stress affects some physiological and productive variables and alters metabolism in dairy ewes. J. Dairy Sci. 2021, 104, 1099–1110. [Google Scholar] [CrossRef]
- Li, F.K.; Yang, Y.; Jenna, K.; Xia, C.H.; Lv, S.J.; Wei, W.H. Effect of heat stress on the behavioral and physiological patterns of Small-tail Han sheep housed indoors. Trop. Anim. Health Prod. 2018, 50, 1893–1901. [Google Scholar] [CrossRef]
- Sejian, V.; Maurya, V.P.; Naqvi, S.M. Adaptability and growth of Malpura ewes subjected to thermal and nutritional stress. Trop. Anim. Health Prod. 2010, 42, 1763–1770. [Google Scholar] [CrossRef]
- Bernabucci, U.; Lacetera, N.; Danieli, P.P.; Bani, P.; Nardone, A.; Ronchi, B. Influence of different periods of exposure to hot environment on rumen function and diet digestibility in sheep. Int. J. Biometeorol. 2009, 53, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.; Beatty, D.; Taylor, E.; Stockman, C.; Maloney, S.; McCarthy, M. Physiology of heat stress in cattle and sheep. Meat Livest. Aust. 2004, 209, 1–36. [Google Scholar]
- Nicolás-López, P.; Macías-Cruz, U.; Mellado, M.; Correa-Calderón, A.; Meza-Herrera, C.A.; Avendaño-Reyes, L. Growth performance and changes in physiological, metabolic and hematological parameters due to outdoor heat stress in hair breed male lambs finished in feedlot. Int. J. Biometeorol. 2021, 65, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Nardone, A.; Ronchi, B.; Valentini, A. Effect of solar radiation on water and food intake and weight gain in “Sarda” and “Comisana” female lambs. In Proceedings of the International Symposium on Animal Husbandry in Warm Climates, Viterbo, Italy, 25–27 October 1990; pp. 149–150. [Google Scholar]
- Ramana, D.B.V.; Pankaj, P.K.; Nikhila, M.; Rani, R.; Sudheer, D. Productivity and physiological responses of sheep exposed to heat stress. J. Agrometeorol. (Spec. Issue) 2013, 15, 71–76. [Google Scholar]
- Yancey, J.W.S.; Apple, J.K.; Kegley, E.B.; Godbee, R.G. Effects of Morinda citrifolia (Noni) pulp on growth performance and stress responses of growing cattle. Prof. Anim. Sci. 2013, 29, 420–425. [Google Scholar] [CrossRef]
- Riva, J.; Rizzi, R.; Marelli, S.; Cavalchini, L.G. Body measurements in Bergamasca sheep. Small Rumin. Res. 2004, 55, 221–227. [Google Scholar] [CrossRef]
- Olayode, B.A.; Sowande, O.S.; Bemiji, M.N.; Bawala, T.O.; Ajagbe, O.O.; Oni, A.O. Effect of feed quality restriction on changes in body measurements and carcass characteristics of West African dwarf sheep. Niger. J. Anim. Prod. 2014, 41, 124–138. [Google Scholar] [CrossRef]
- Rana, M.S.; Hashem, M.A.; Sakib, M.N.; Kumar, A. Effect of heat stress on blood parameters in indigenous sheep. J. Bangladesh Agric. Univ. 2014, 12, 91–94. [Google Scholar] [CrossRef]
- Khalil, F.; Yapati, H.; Al Blallam, Z.; Jose, R. Seasonal effects on growth, physiology, hematology and biochemicalprofiles of Naeemi sheep breed. Adv. Anim. Vet. 2022, 10, 2161–2170. [Google Scholar] [CrossRef]
- McManus, C.M.; Faria, D.A.; Lucci, C.M.; Louvandini, H.; Pereira, S.A.; Paiva, S.R. Heat stress effects on sheep: Are hair sheep more heat resistant? Theriogenology 2020, 155, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M. From molecular and cellular to integrative heat defense during exposure to chronic heat. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 131, 475–483. [Google Scholar] [CrossRef]
- Bernabucci, U.; Lacetera, N.; Baumgard, L.H.; Rhoads, R.P.; Ronchi, B.; Nardone, A. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal 2010, 4, 1167–1183. [Google Scholar] [CrossRef]
- Colthorpe, & Curlewis. Hypothalamic dopamine D1 receptors are involved in the stimulation of prolactin secretion by high environmental temperature on the female sheep. J. Neuroendocrinol. 1998, 10, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, M.L.; Kim, J.W.; Collier, R.J.; Crooker, B.A.; Boisclair, Y.R.; Baumgard, L.H.; Rhoads, R.P. Effects of heat stress and nutrition on lactating Holstein cows: II. Aspects of hepatic growth hormone responsiveness. J. Dairy Sci. 2010, 93, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Corona, J.C.; Reyna-Granados, J.R.; Zamorano-Algandar, R.; Sanchez-Castro, M.A.; Thomas, M.G.; Enns, R.M.; Speidel, S.E.; Medrano, J.F.; Rincon, G.; Luna-Nevarez, P. Polymorphisms within the prolactin and growth hormone/insulin-like growth factor-1 functional pathways associated with fertility traits in Holstein cows raised in a hot-humid climate. Trop. Anim. Health Prod. 2018, 50, 1913–1920. [Google Scholar] [CrossRef]
- Bonilla, A.Q.; Hansen, P.J. 110 Developmental changes in actions of insulin-like growth factor-I in the preimplantation bovine embryo-receptor expression and thermotolerance. Reprod. Fertil. Dev. 2008, 21, 155. [Google Scholar] [CrossRef]
- Satrapa, R.A.; Razza, E.M.; Castilho, A.C.S.; Simões, R.A.L.; Silva, C.F.; Nabhan, T.; Pegorer, M.F.; Barros, C.M. Differential Expression of IGF Family Members in Heat-Stressed Embryos Produced In Vitro from OPU-Derived Oocytes of Nelore (Bos indicus) and Holstein (Bos taurus) Cows. Reprod. Domest. Anim. 2013, 48, 1043–1048. [Google Scholar] [CrossRef]
- De, K.; Kumar, D.; Singh, A.K.; Kumar, K.; Sahoo, A.; Naqvi, S.M.K. Effect of protection against hot climate on growth performance, physiological response and endocrine profile of growing lambs under semi-arid tropical environment. Trop. Anim. Health Prod. 2017, 49, 1317–1323. [Google Scholar] [CrossRef]
- Altin, T.; Yilmaz, M.; Kiral, F.; Yorulmaz, E.; Asici, G.S.E. Effects of the genotype and the seasons on physiological parameters related with adaptability in sheep in Mediterranean climate conditions. Vet. Zootech. 2018, 76, 1–8. [Google Scholar]
- Nazifi, S.; Saeb, M.; Rowghani, E.; Kaveh, K. The influences of thermal stress on serum biochemical parameters of Iranian fat-tailed sheep and their correlation with triiodothyronine (T3), thyroxine (T4) and cortisol concentrations. Comp. Clin. Path 2003, 12, 135–139. [Google Scholar] [CrossRef]
- Rathwa, S.D.; Vasava, A.A.; Pathan, M.M.; Madhira, S.P.; Patel, Y.G.; Pande, A.M. Effect of season on physiological, biochemical, hormonal, and oxidative stress parameters of indigenous sheep. Vet. World 2017, 10, 650–654. [Google Scholar] [CrossRef]
- Pragna, P.; Sejian, V.; Bagath, M.; Krishnan, G.; Archana, P.R.; Soren, N.M.; Beena, V.; Bhatta, R. Comparative assessment of growth performance of three different indigenous goat breeds exposed to summer heat stress. J. Anim. Physiol. Anim. Nutr. 2018, 102, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Le Roith, D.; Bondy, C.; Yakar, S.; Liu, J.L.; Butler, A. The somatomedin hypothesis: 2001. Endoc. Rev. 2001, 22, 53–74. [Google Scholar] [CrossRef]
- Del Vesco, A.P.; Gasparino, E.; Grieser, D.O.; Zancanela, V.; Voltolini, D.M.; Khatlab, A.S.; Guimarães, S.E.; Soares, M.A.; Neto, A.R.O. Effects of methionine supplementation on the expression of protein deposition-related genes in acute heat stress-exposed broilers. PLoS ONE 2015, 10, e0115821. [Google Scholar] [CrossRef]
- Madhusoodan, A.P.; Sejian, V.; Afsal, A.; Bagath, M.; Krishnan, G.; Savitha, S.T.; Rashamol, V.P.; Devaraj, C.; Bhatta, R. Differential expression patterns of candidate genes pertaining to productive and immune functions in hepatic tissue of heat-stressed Salem Black goats. Biol. Rhythm Res. 2021, 52, 809–820. [Google Scholar] [CrossRef]
- Collier, R.J.; Gebremedhin, K.; Macko, A.R.; Roy, K.S. Genes involved in the thermal tolerance of livestock. In Environmental Stress and Amelioration in Livestock Production; Springer Nature: Heidelberg, Germany, 2012; pp. 379–410. [Google Scholar] [CrossRef]
- Alamer, M. The role of prolactin in thermoregulation and water balance during heat stress in domestic ruminants. Asian J. Anim. Vet. Adv. 2011, 6, 1153–1169. [Google Scholar] [CrossRef]
- Hooper, H.B. Physiological Implications, Cellular Responses and Lactational Performance of Saanen Goats Under Heat Stress. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2019. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014. [Google Scholar] [CrossRef]
- Weitzel, J.M.; Viergutz, T.; Albrecht, D.; Bruckmaier, R.; Schmicke, M.; Tuchscherer, A.; Koch, F.; Kuhla, B. Hepatic thyroid signaling of heat-stressed late pregnant and early lactating cows. J. Endocrinol. 2017, 234, 129–141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).