Morinda officinalis Oligosaccharides Protect Against LPS-Induced Uterine Damage and Endometrial Inflammation in Mice and Bovine Endometrial Epithelial Cells
Simple Summary
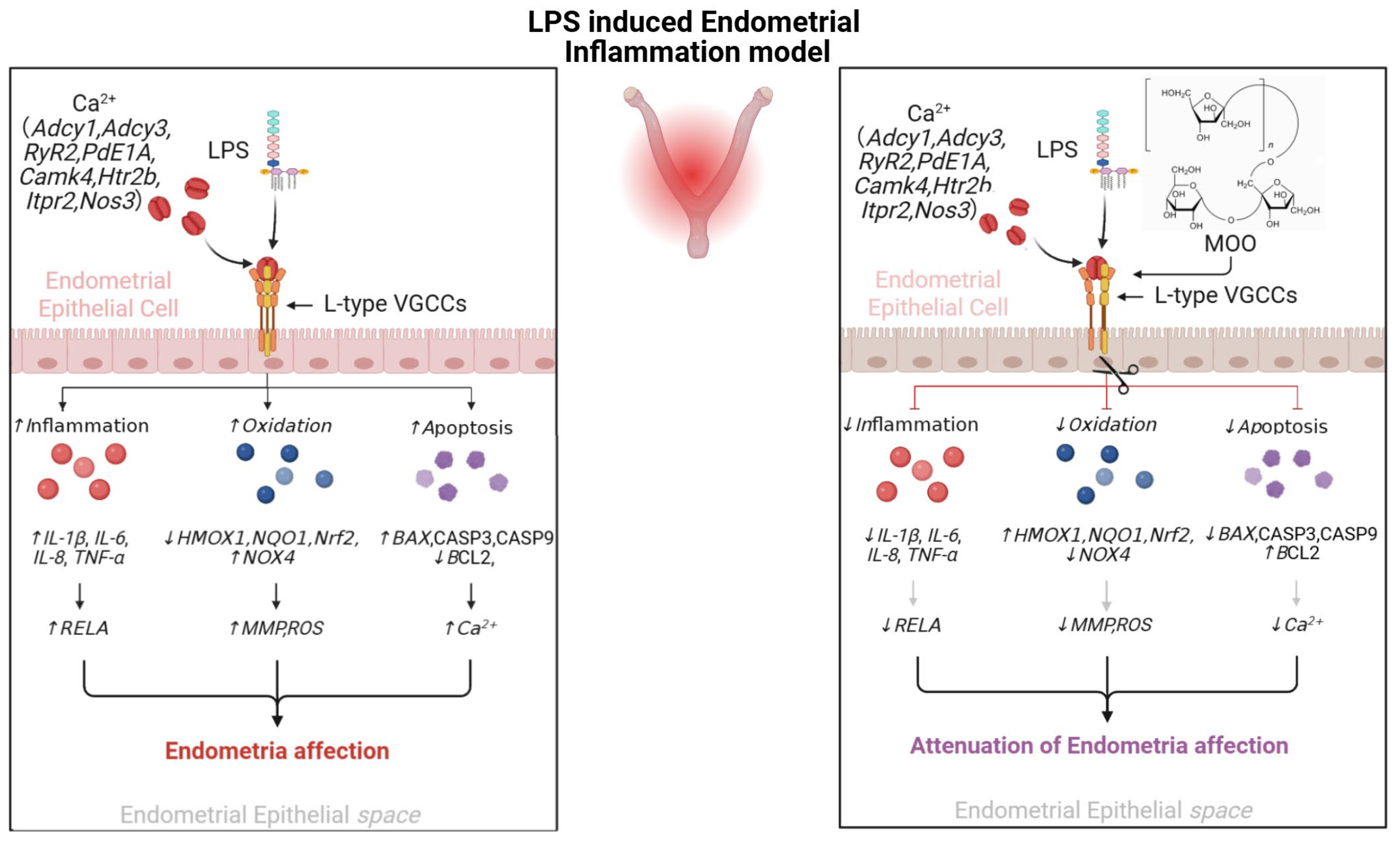
Abstract
1. Introduction
2. Materials and Methods
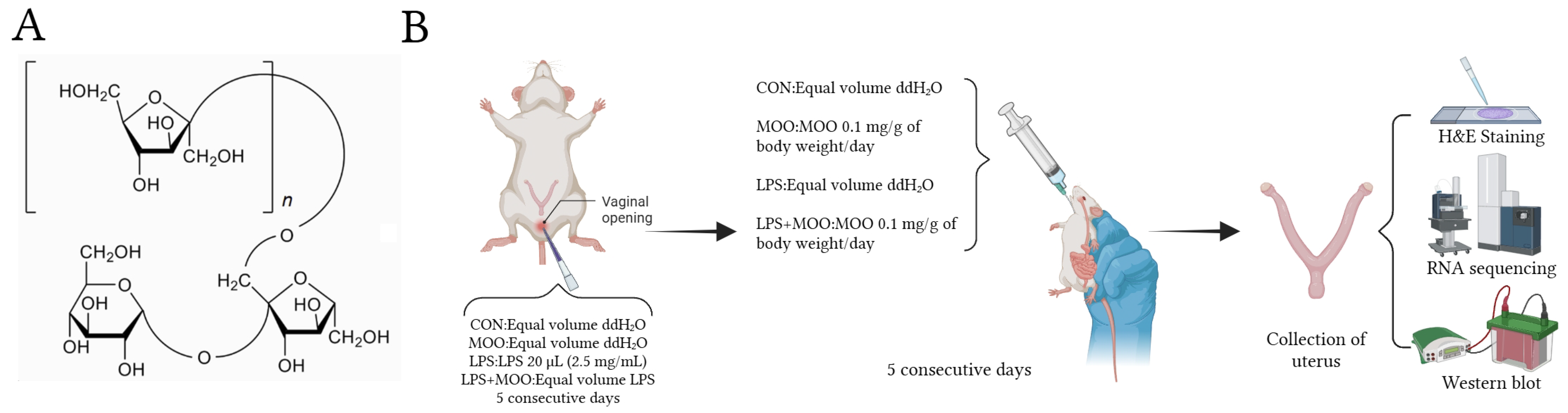
2.1. Experimental Animal Groups and Treatments
2.2. H and E Staining
2.3. RNA Sequencing and Bioinformatic Analysis
2.4. RNA Extraction and Real-Time Quantitative PCR
2.5. Western Blot
2.6. Cell Culture and Drug Treatment
2.7. Measurement of Cell Viability
2.8. Assessment of Reactive Oxygen Species
2.9. Detection of Mitochondrial Membrane Potential
2.10. Measurement of Intracellular Ca2+
2.11. Statistical Analysis
3. Results
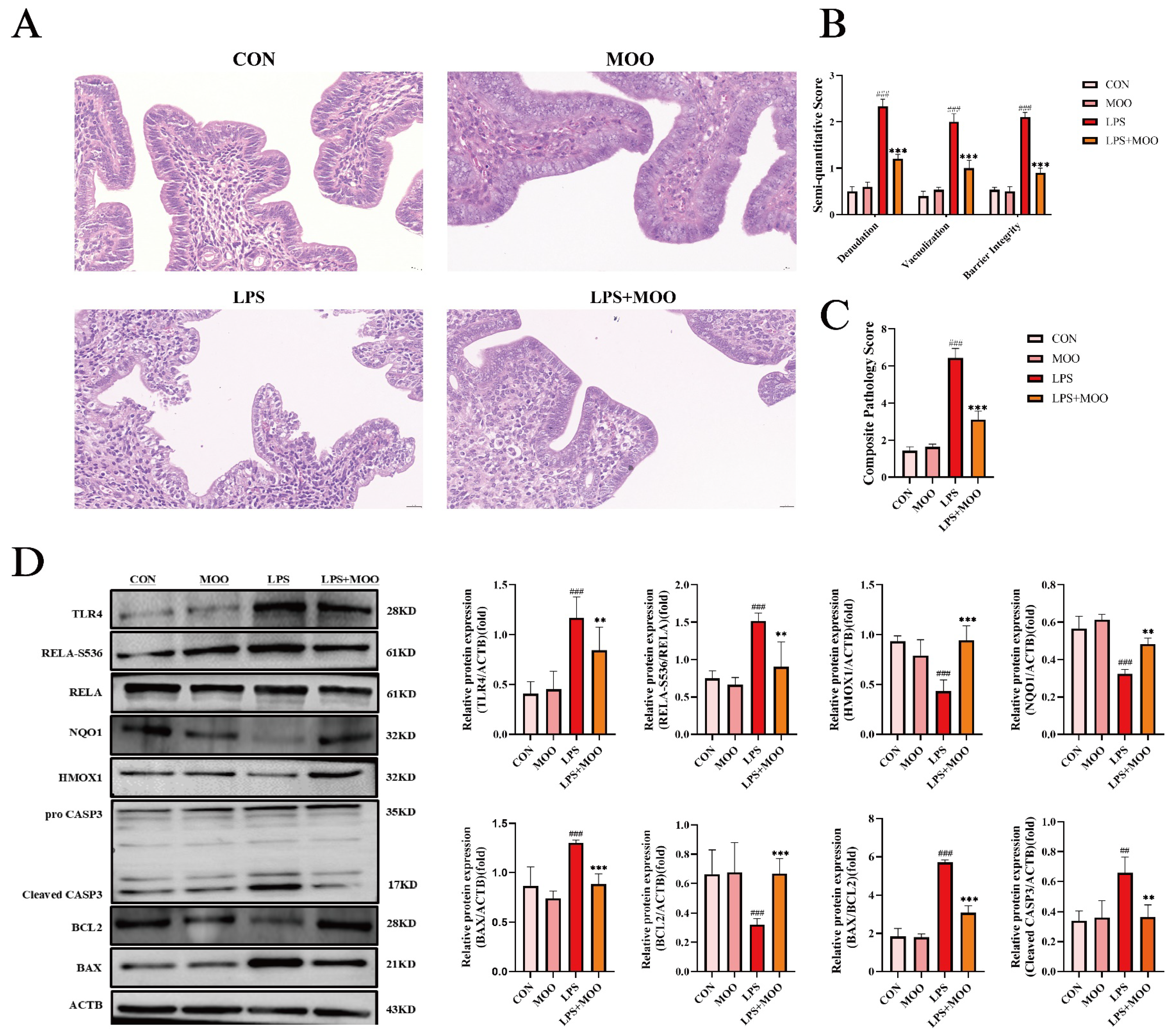
3.1. MOO Alleviates LPS-Induced Uterine Tissue Damage in Mice
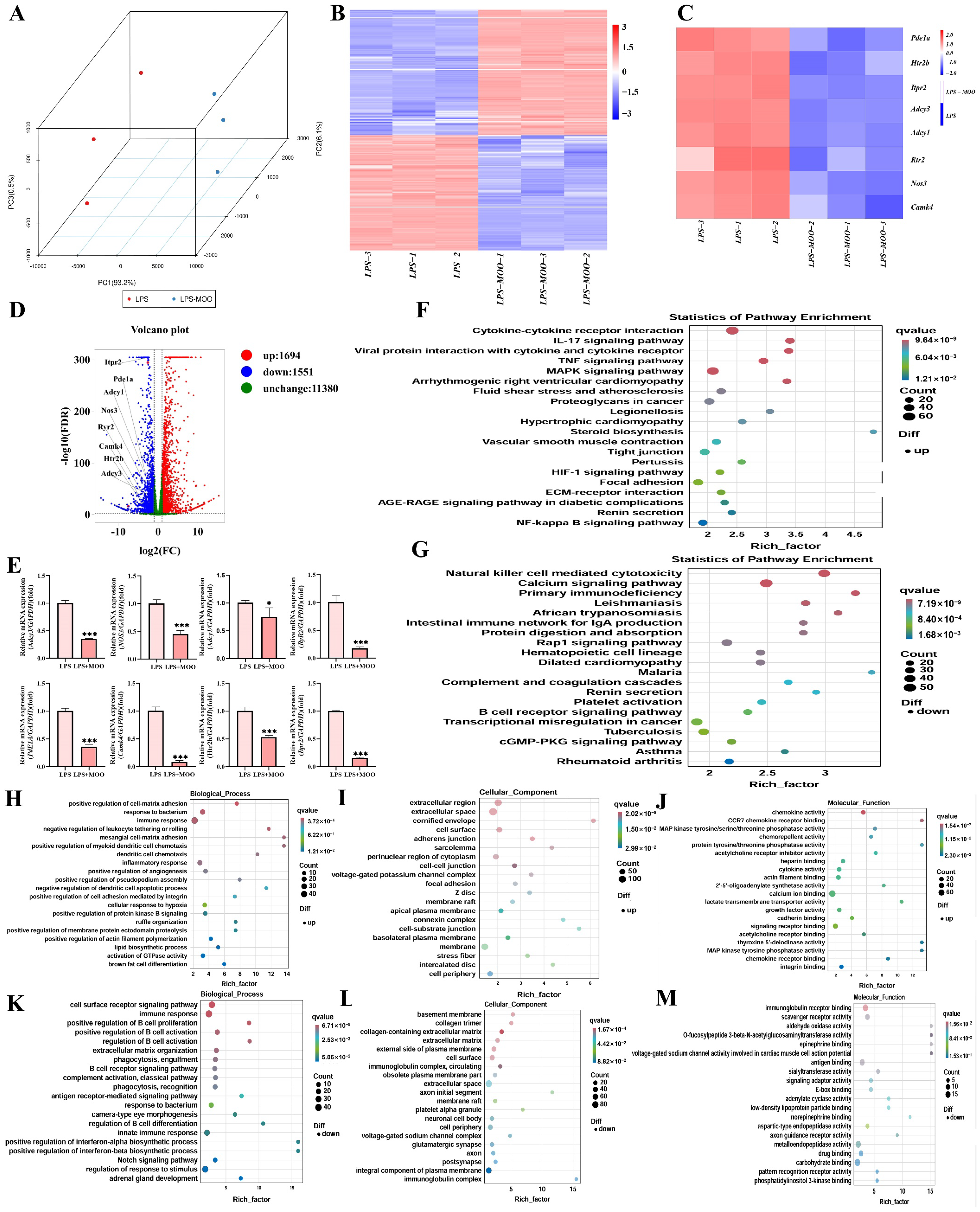
3.2. Transcriptomic Analysis of the Effects of MOO on LPS-Induced Uterine Injury in Mice
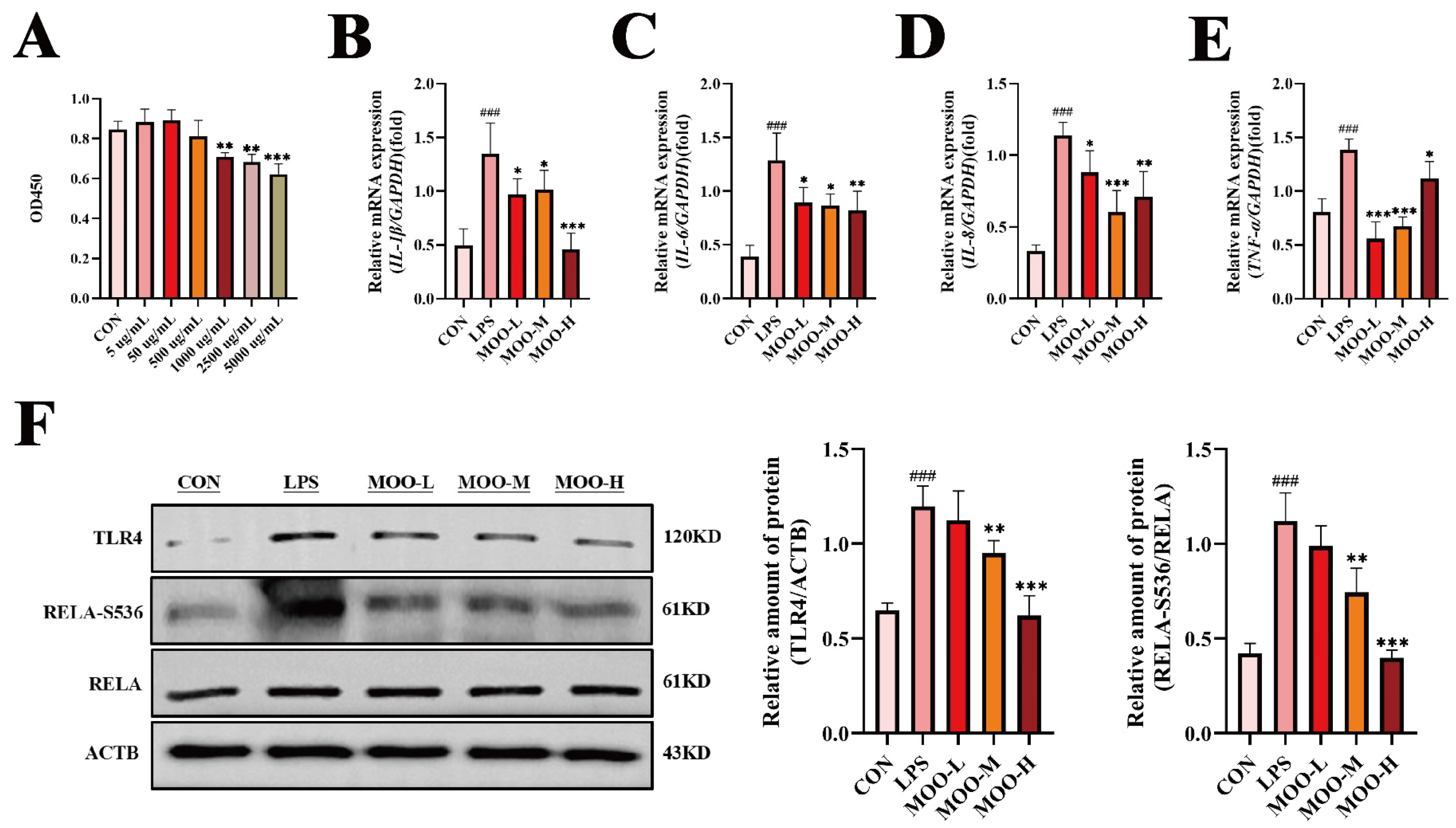
3.3. MOO Alleviates LPS-Induced Inflammation in BENDs
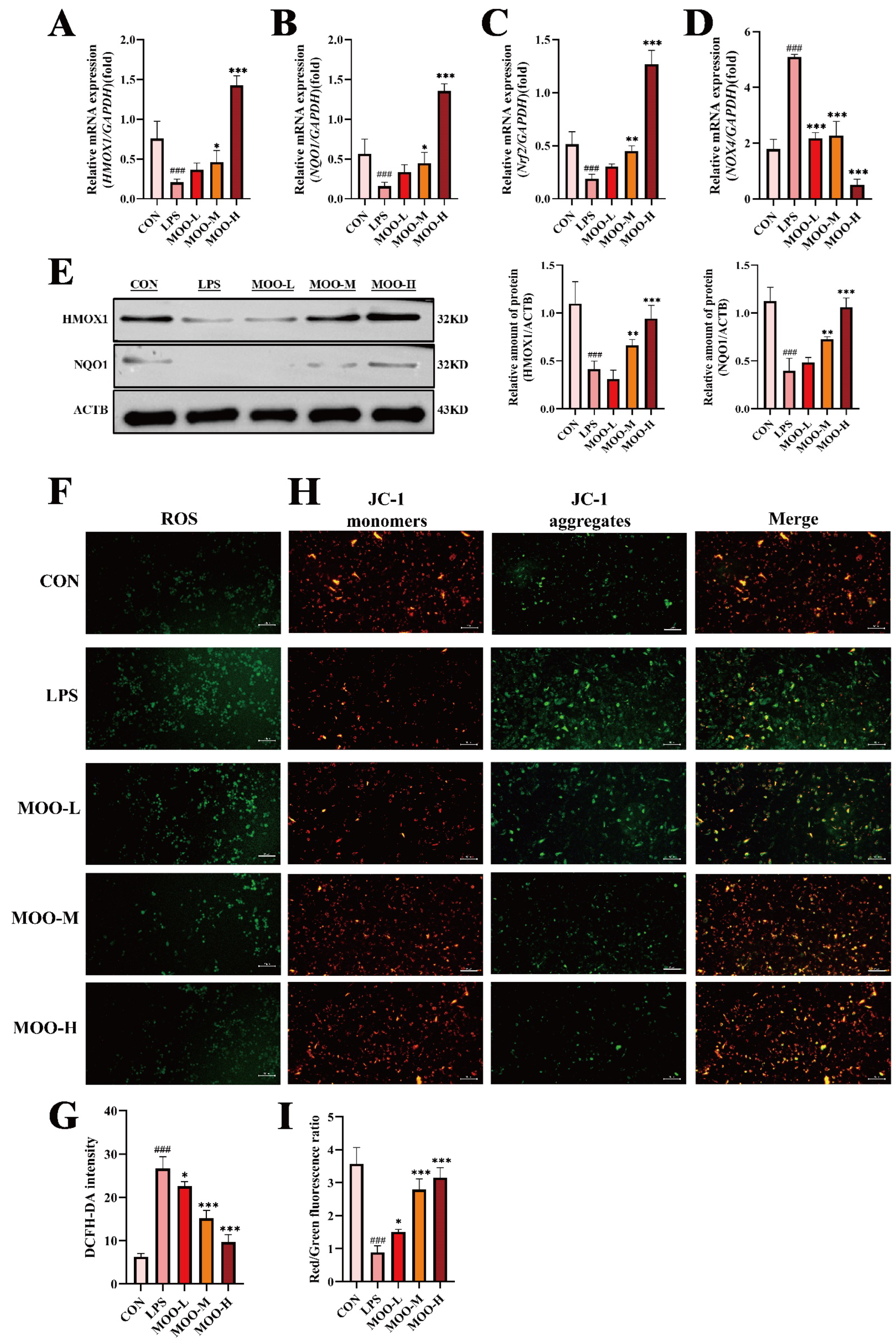
3.4. MOO Alleviates LPS-Induced Oxidative Stress in BENDs
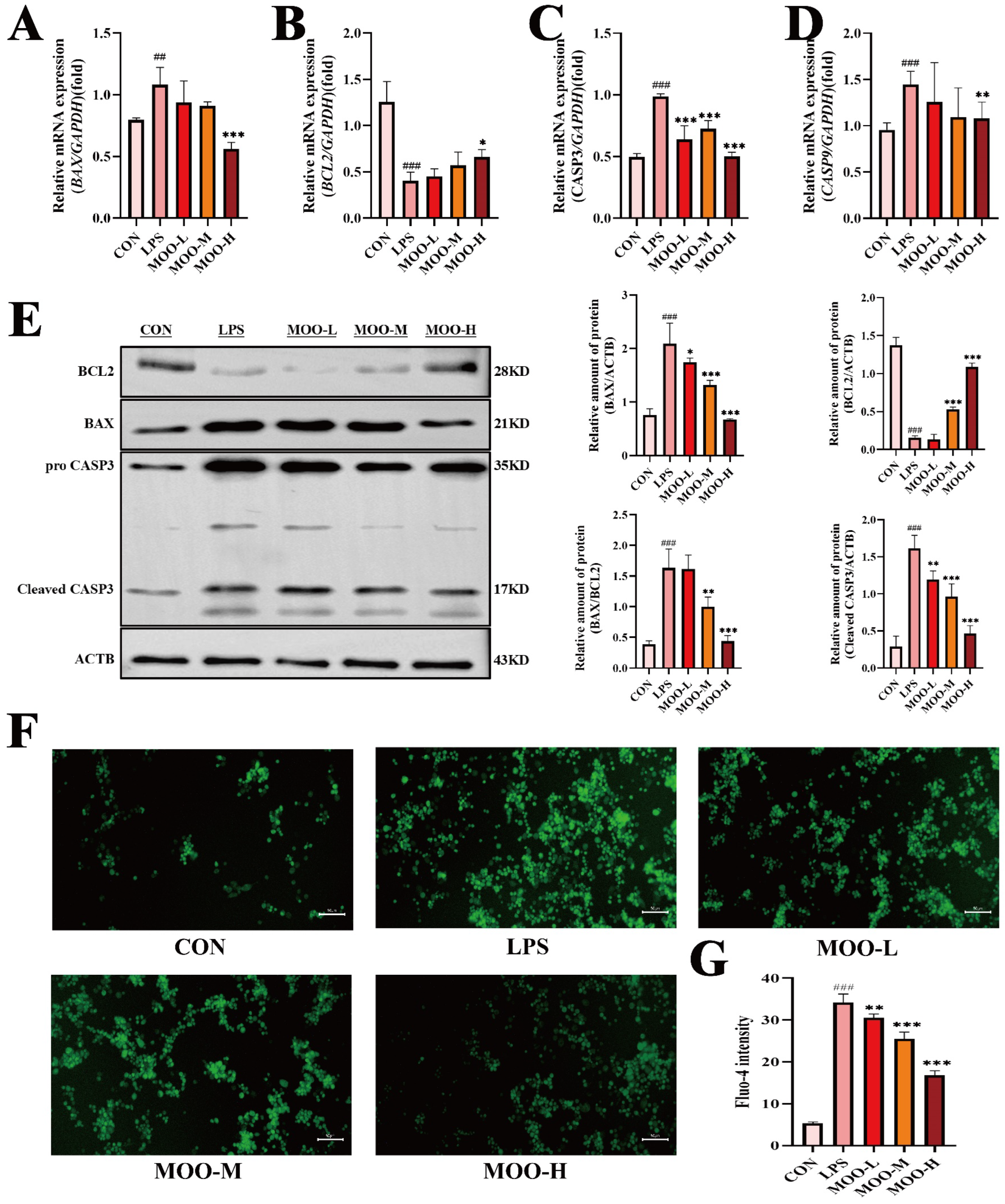
3.5. MOO Mitigates LPS-Induced Apoptosis in BENDs
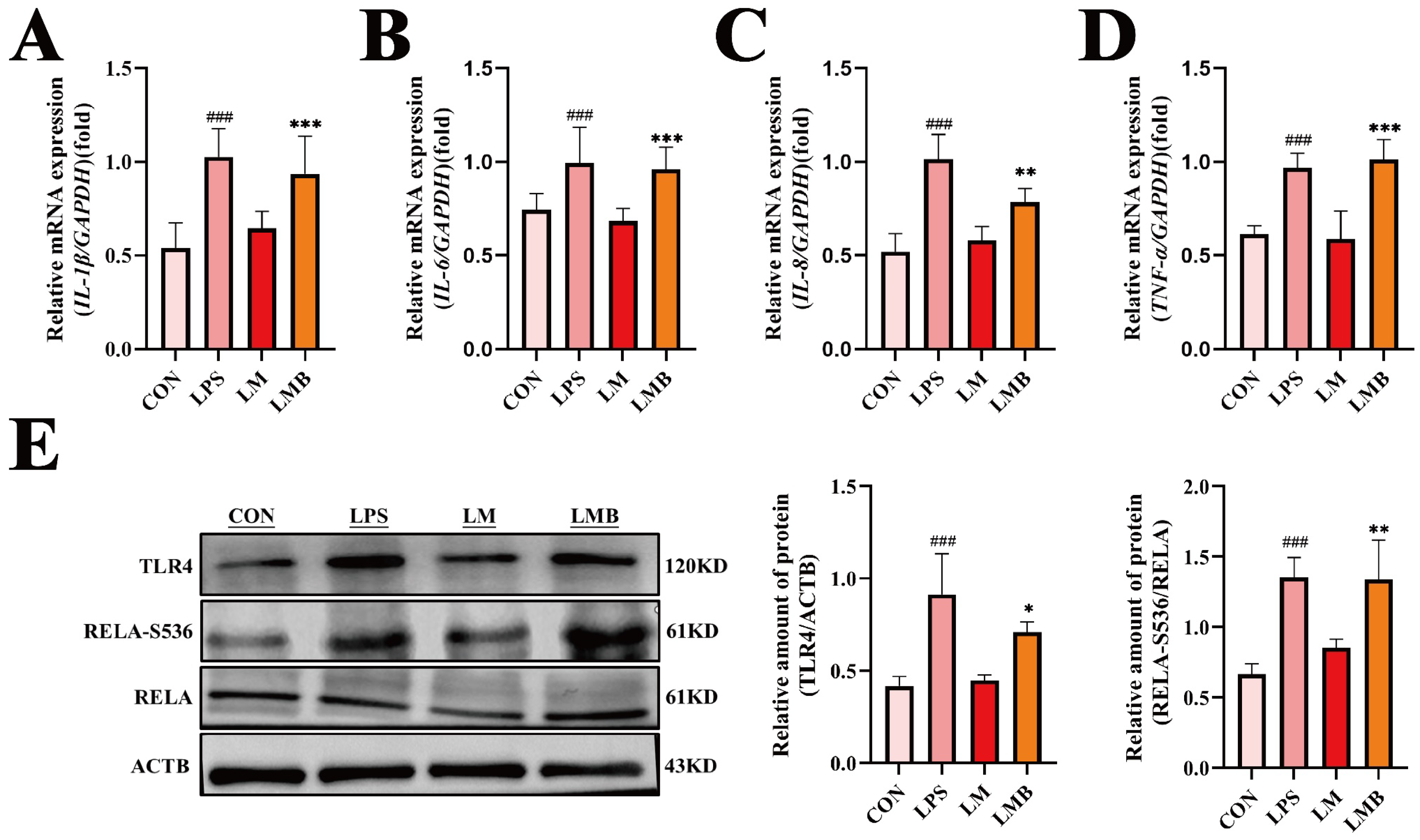
3.6. Activation of Calcium Signaling Impedes the Anti-Inflammatory Effects of MOO in LPS-Treated BENDs
3.7. Activation of Calcium Signaling Impairs the Protective Effects of MOO Against LPS-Induced Oxidative Stress in BENDs
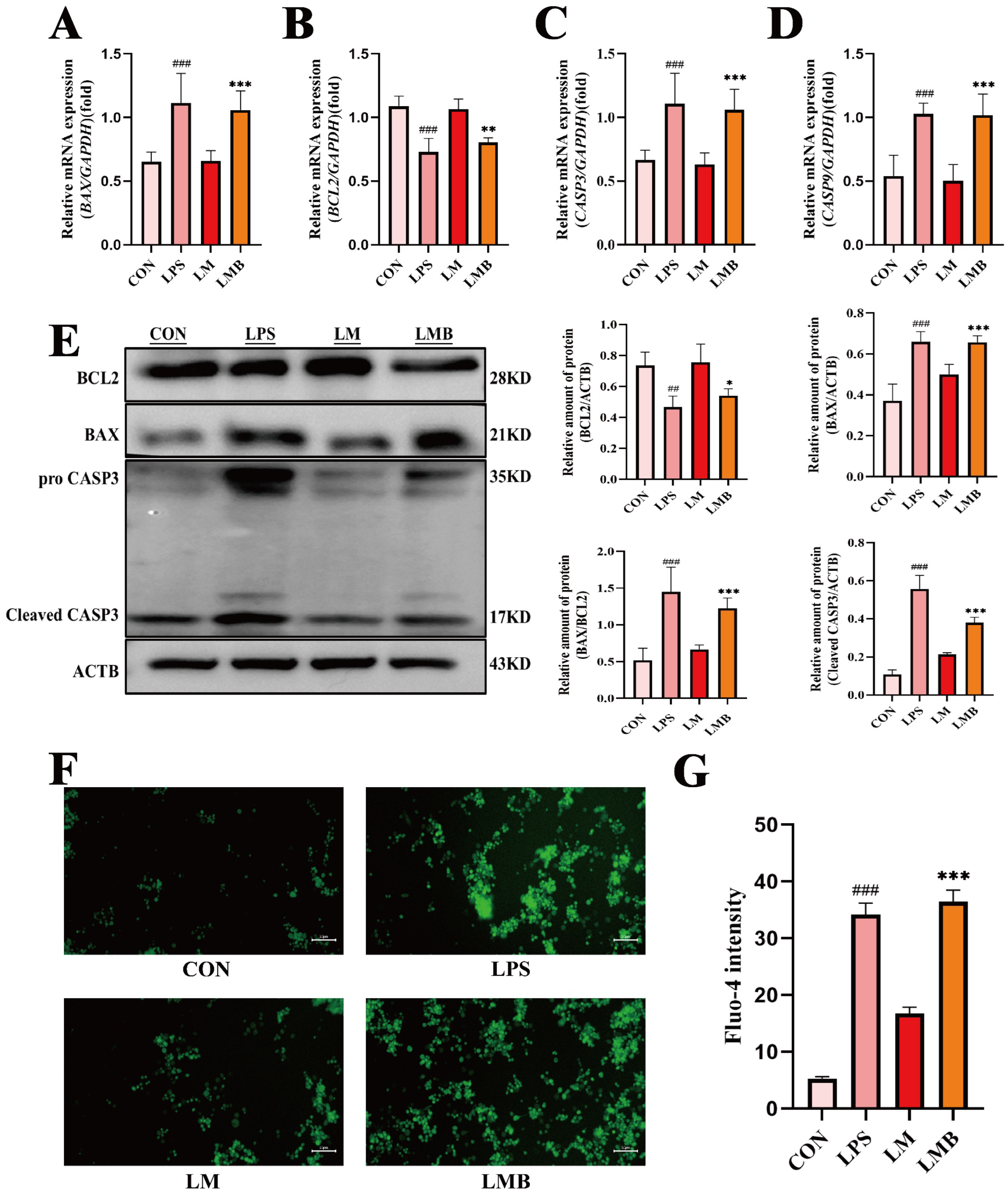
3.8. Activation of Calcium Signaling Impairs the Anti-Apoptotic Effects of MOO in LPS-Induced BENDs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MOO | Morinda officinalis oligosaccharides |
| LPS | lipopolysaccharide |
| BENDs | Bovine endometrial epithelial cells |
| MO | Morinda officinalis |
| SPF | Specific-pathogen-free |
| FBS | Fetal bovine serum |
| LSD | Least significant difference |
| PCA | Principal component analysis |
| DEGs | Differentially expressed genes |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GO | Gene Ontology |
| MMP | Mitochondrial membrane potential |
References
- Várhidi, Z.; Csikó, G.; Bajcsy, Á.C.; Jurkovich, V. Uterine Disease in Dairy Cows: A Comprehensive Review Highlighting New Research Areas. Vet. Sci. 2024, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.S.; Vieira-Neto, A.; Snodgrass, J.A.; De Vries, A.; Santos, J.E.P. Economic comparison of systemic antimicrobial therapies for metritis in dairy cows. J. Dairy Sci. 2019, 102, 7345–7358. [Google Scholar] [CrossRef]
- Silva, T.V.; de Oliveira, E.B.; Pérez-Báez, J.; Risco, C.A.; Chebel, R.C.; Cunha, F.; Daetz, R.; Santos, J.E.P.; Lima, F.S.; Jeong, K.C.; et al. Economic comparison between ceftiofur-treated and nontreated dairy cows with metritis. J. Dairy Sci. 2021, 104, 8918–8930. [Google Scholar] [CrossRef]
- Pérez-Báez, J.; Silva, T.V.; Risco, C.A.; Chebel, R.C.; Cunha, F.; De Vries, A.; Santos, J.E.P.; Lima, F.S.; Pinedo, P.; Schuenemann, G.M.; et al. The economic cost of metritis in dairy herds. J. Dairy Sci. 2021, 104, 3158–3168. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zhao, L.; Hu, R.; Zhai, Z.; Guo, J.; Zhang, K. Nuciferine protects against lipopolysaccharide-induced endometritis via inhibiting ferroptosis and modulating AMPKα/mTOR/HIF-1α signaling axis. Int. Immunopharmacol. 2023, 124, 110914. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug. Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Fu, K.; Lv, X.; Li, W.; Wang, Y.; Li, H.; Tian, W.; Cao, R. Berberine hydrochloride attenuates lipopolysaccharide-induced endometritis in mice by suppressing activation of NF-κB signal pathway. Int. Immunopharmacol. 2015, 24, 128–132. [Google Scholar] [CrossRef]
- Yang, W.; Jin, M.; Wang, Y.; Zhao, H.; Wang, X.; Guo, Y.; Li, C.; Xiao, B.; Zhang, H.; Kiran, F.; et al. NR1D1 activation alleviates inflammatory response through inhibition of IL-6 expression in bovine endometrial epithelial cells. Int. J. Biol. Macromol. 2024, 283, 137642. [Google Scholar] [CrossRef]
- Yang, D.; Yin, R.; Lei, Q.; Zhu, J.; Nan, S.; Ma, N.; Zhu, H.; Chen, J.; Han, L.; Ding, M.; et al. Baicalin alleviates endometrial inflammatory injury through regulation of tight junction proteins. Food Funct. 2022, 13, 6522–6533. [Google Scholar] [CrossRef]
- Dalal, P.J.; Muller, W.A.; Sullivan, D.P. Endothelial Cell Calcium Signaling during Barrier Function and Inflammation. Am. J. Pathol. 2020, 190, 535–542. [Google Scholar] [CrossRef]
- Jhamat, N.; Niazi, A.; Guo, Y.; Chanrot, M.; Ivanova, E.; Kelsey, G.; Bongcam-Rudloff, E.; Andersson, G.; Humblot, P. LPS-treatment of bovine endometrial epithelial cells causes differential DNA methylation of genes associated with inflammation and endometrial function. BMC Genom. 2020, 21, 385. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiu, R.; Liu, R.; Song, P.; Lin, P.; Chen, H.; Zhou, D.; Wang, A.; Jin, Y. Autophagy Mediates Escherichia Coli-Induced Cellular Inflammatory Injury by Regulating Calcium Mobilization, Mitochondrial Dysfunction, and Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2022, 23, 14174. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Zhang, J.; Feng, X.; Sheng, H.; Hu, H.; Li, F.; Ma, Y.; Hu, Y.; Na, R.; Yang, W.; et al. Analysis of blood biochemistry and non-targeted metabolomics of endometritis in dairy cows. Anim. Reprod. Sci. 2024, 264, 107460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Gao, C.S.; Zhang, H.; Yang, J.; Wang, Y.P.; Pan, L.B.; Yu, H.; He, C.Y.; Luo, H.B.; Zhao, Z.X.; et al. Morinda officinalis oligosaccharides increase serotonin in the brain and ameliorate depression via promoting 5-hydroxytryptophan production in the gut microbiota. Acta Pharm. Sin. B 2022, 12, 3298–3312. [Google Scholar] [CrossRef]
- Zhang, J.H.; Xin, H.L.; Xu, Y.M.; Shen, Y.; He, Y.Q.; Xin, H.-Y.; Lin, B.; Song, H.T.; Juan-Liu; Yang, H.Y.; et al. Morinda officinalis How—A comprehensive review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 213, 230–255. [Google Scholar]
- Wu, Z.Q.; Chen, D.L.; Lin, F.H.; Lin, L.; Shuai, O.; Wang, J.Y.; Qi, L.K.; Zhang, P. Effect of bajijiasu isolated from Morinda officinalis F. C. how on sexual function in male mice and its antioxidant protection of human sperm. J. Ethnopharmacol. 2015, 164, 283–292. [Google Scholar] [CrossRef]
- Yang, L.; Ao, Y.; Li, Y.; Dai, B.; Li, J.; Duan, W.; Gao, W.; Zhao, Z.; Han, Z.; Guo, R. Morinda officinalis oligosaccharides mitigate depression-like behaviors in hypertension rats by regulating Mfn2-mediated mitophagy. J. Neuroinflamm. 2023, 20, 31. [Google Scholar] [CrossRef]
- Faouzi, M.; Wakano, C.; Monteilh-Zoller, M.K.; Dai, B.; Li, J.; Duan, W.; Gao, W.; Zhao, Z.; Han, Z.; Guo, R. Acidic Cannabinoids Suppress Proinflammatory Cytokine Release by Blocking Store-operated Calcium Entry. Function 2022, 3, zqac033. [Google Scholar] [CrossRef]
- Murphy, M.T.; Qin, X.; Kaul, S.; Barrientos, G.; Zou, Z.; Mathias, C.B.; Thomas, D.; Bose, D.D. The polyphenol ellagic acid exerts anti-inflammatory actions via disruption of store-operated calcium entry (SOCE) pathway activators and coupling mediators. Eur. J. Pharmacol. 2020, 875, 173036. [Google Scholar] [CrossRef]
- Qing, C.; Wu, Y.; Liu, B.; Wang, C.; Zeng, Z. Ameliorative Effect of Morinda officinalis Oligosaccharides on LPS-Induced Acute Lung Injury. Chem. Biodivers 2024, 21, e202400506. [Google Scholar] [CrossRef]
- Ning, E.-J.; Sun, C.-W.; Wang, X.-F.; Ling, C.; Fei, L.; Li, Z.; Lu, W.; Yan, M.; Jie, Z.; Xiao, L.; et al. Artemisia argyi polysaccharide alleviates intestinal inflammation and intestinal flora dysbiosis in lipopolysaccharide-treated mice. Food Med. Homol. 2024, 1, 9420008. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, Y.; Lu, M.; Xu, M.; Li, Y.; Song, H.; Luo, Y.; Song, J.; Yang, Y.; Wang, X.; et al. Hormonally and chemically defined expansion conditions for organoids of biliary tree Stem Cells. Bioact. Mater. 2024, 41, 672–695. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Chiang, Y.F.; Shih, Y.H.; Chiu, C.H.; Chen, H.Y.; Shieh, T.M.; Wang, K.L.; Huang, T.C.; Hong, Y.H.; Hsia, S.M. Salvia sclarea L. Essential Oil Extract and Its Antioxidative Phytochemical Sclareol Inhibit Oxytocin-Induced Uterine Hypercontraction Dysmenorrhea Model by Inhibiting the Ca(2+)-MLCK-MLC20 Signaling Cascade: An Ex Vivo and In Vivo Study. Antioxidants 2020, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, G.; Fan, W.; Zhang, Y.; Peng, H. TF and TFRC regulate ferroptosis in swine testicular cells through the JNK signaling pathway. Int. J. Biol. Macromol. 2025, 307, 142369. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, X.; Loor, J.J.; Jiang, Q.; Guo, H.; Zhang, W.; Li, M.; Lv, X.; Yin, Y.; Wen, J.; et al. Role of ORAI calcium release-activated calcium modulator 1 (ORAI1) on neutrophil extracellular trap formation in dairy cows with subclinical hypocalcemia. J. Dairy Sci. 2022, 105, 3394–3404. [Google Scholar] [CrossRef]
- Klopfleisch, R. Multiparametric and semiquantitative scoring systems for the evaluation of mouse model histopathology—A systematic review. BMC Vet. Res. 2013, 9, 123. [Google Scholar] [CrossRef]
- Zheng, L.; Ding, Y.; Fang, S.; Yang, W.; Chen, J.; Ma, J.; Wang, M.; Wang, J.; Zhang, F.; Guo, X.; et al. Potentiated Calcium Carbonate with Enhanced Calcium Overload Induction and Acid Neutralization Capabilities to Boost Chemoimmunotherapy against Liver Cancer. ACS Nano 2024, 18, 27597–27616. [Google Scholar] [CrossRef]
- Lu, Z.; Peng, Q.; Hu, R.; Wang, Y.; Fan, K.; Zhang, T. Naringin attenuates inflammatory injury to the bovine endometrium by regulating the endoplasmic reticulum stress-PI3K/AKT-autophagy axis. Front. Pharmacol. 2024, 15, 1424511. [Google Scholar] [CrossRef]
- Li, H.; Dong, J.; Cui, L.; Liu, K.; Guo, L.; Li, J.; Wang, H. The effect and mechanism of selenium supplementation on the proliferation capacity of bovine endometrial epithelial cells exposed to lipopolysaccharide in vitro under high cortisol background. J. Anim. Sci. 2024, 102, skae021. [Google Scholar] [CrossRef]
- Hao, J.; Wang, H.; Lu, X.; Li, Z.; Zhang, X. TLR4 signalling: The key to controlling EV71 replication and inflammatory response. Front. Cell. Infect. Microbiol. 2024, 14, 1393680. [Google Scholar] [CrossRef]
- Qiu, M.; Ye, C.; Zhao, X.; Zou, C.; Tang, R.; Xie, J.; Liu, Y.; Hu, Y.; Hu, X.; Zhang, N.; et al. Succinate exacerbates mastitis in mice via extracellular vesicles derived from the gut microbiota: A potential new mechanism for mastitis. J. Nanobiotechnol. 2024, 22, 712. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.F.; Yang, J.; Zou, C.; Tang, R.; Xie, J.; Liu, Y.; Hu, Y.; Hu, X.; Zhang, N.; et al. Selenium alleviates lipopolysaccharide-induced endometritis via regulating the recruitment of TLR4 into lipid rafts in mice. Food Funct. 2020, 11, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Luo, Z.H.; Chen, Z.E.; Qin, Y.R.; Liu, Q.Y.; Ding, P. Quality markers for processed products of Morinda officinalis how based on the “oligosaccharides-spectrum-effect”. J. Pharm. Biomed. Anal. 2022, 208, 114403. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Diling, C.; Jian, Y.; Ting, L.; Guoyan, H.; Hualun, L.; Xiaocui, T.; Guoxiao, L.; Ou, S.; Chaoqun, Z.; et al. Effects of Oligosaccharides from Morinda officinalis on Gut Microbiota and Metabolome of APP/PS1 Transgenic Mice. Front. Neurol. 2018, 9, 412. [Google Scholar] [CrossRef]
- Alamri, H.S.; Alsughayyir, J.; Akiel, M.; Ting, L.; Guo, H.; Hua, L.; Xiao, T.; Guo, L.; Ou, S.; Chao, Z.; et al. Stimulation of calcium influx and CK1α by NF-κB antagonist [6]-Gingerol reprograms red blood cell longevity. J. Food Biochem. 2021, 45, e13545. [Google Scholar] [CrossRef]
- Novakovic, M.M.; Korshunov, K.S.; Grant, R.A.; Martin, M.E.; Valencia, H.A.; Budinger, G.R.S.; Radulovic, J.; Prakriya, M. Astrocyte reactivity and inflammation-induced depression-like behaviors are regulated by Orai1 calcium channels. Nat. Commun. 2023, 14, 5500. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, F.; Yang, L.; Liang, H.; Zhu, Q.; Guo, F.; Yin, X.; Li, J. Morinda officinalis saponins promote osteogenic differentiation of human umbilical cord-derived mesenchymal stem cells via the BMP-SMAD signaling pathway. Am. J. Transl. Res. 2024, 16, 5441–5453. [Google Scholar] [CrossRef]
- Lai, M.; Chen, X.; Feng, J.; Ruan, Z.; Lin, J. Morinda officinalis polysaccharide boosts osteogenic differentiation of bone marrow mesenchymal stem cells by Wnt/β-catenin signaling. Am. J. Transl. Res. 2024, 16, 4492–4503. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Yu, B.; Yu, T.; Jiang, T.; Yang, D.; Peng, H. Morinda officinalis Oligosaccharides Protect Against LPS-Induced Uterine Damage and Endometrial Inflammation in Mice and Bovine Endometrial Epithelial Cells. Animals 2025, 15, 1286. https://doi.org/10.3390/ani15091286
He S, Yu B, Yu T, Jiang T, Yang D, Peng H. Morinda officinalis Oligosaccharides Protect Against LPS-Induced Uterine Damage and Endometrial Inflammation in Mice and Bovine Endometrial Epithelial Cells. Animals. 2025; 15(9):1286. https://doi.org/10.3390/ani15091286
Chicago/Turabian StyleHe, Shiwen, Beibei Yu, Tingting Yu, Tingting Jiang, Diqi Yang, and Hui Peng. 2025. "Morinda officinalis Oligosaccharides Protect Against LPS-Induced Uterine Damage and Endometrial Inflammation in Mice and Bovine Endometrial Epithelial Cells" Animals 15, no. 9: 1286. https://doi.org/10.3390/ani15091286
APA StyleHe, S., Yu, B., Yu, T., Jiang, T., Yang, D., & Peng, H. (2025). Morinda officinalis Oligosaccharides Protect Against LPS-Induced Uterine Damage and Endometrial Inflammation in Mice and Bovine Endometrial Epithelial Cells. Animals, 15(9), 1286. https://doi.org/10.3390/ani15091286





