Effects of Dietary Chromium Supplementation During Late Lactation on Productive Performance, Milk Composition, and Immune and Antioxidant Responses in Dairy Cows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mineral
2.2. Animals and Installation
2.3. Experimental Design and Diet
2.4. Data and Sample Collection
2.5. Feed Composition Analysis
2.6. Hemogram
2.7. Serum Biochemistry
2.8. Oxidative Status
2.9. Chromium Concentration in Feed, Milk, and Blood Serum
2.10. Milk Quality
2.11. Productive Performance
2.12. Statistical Analyses
3. Results
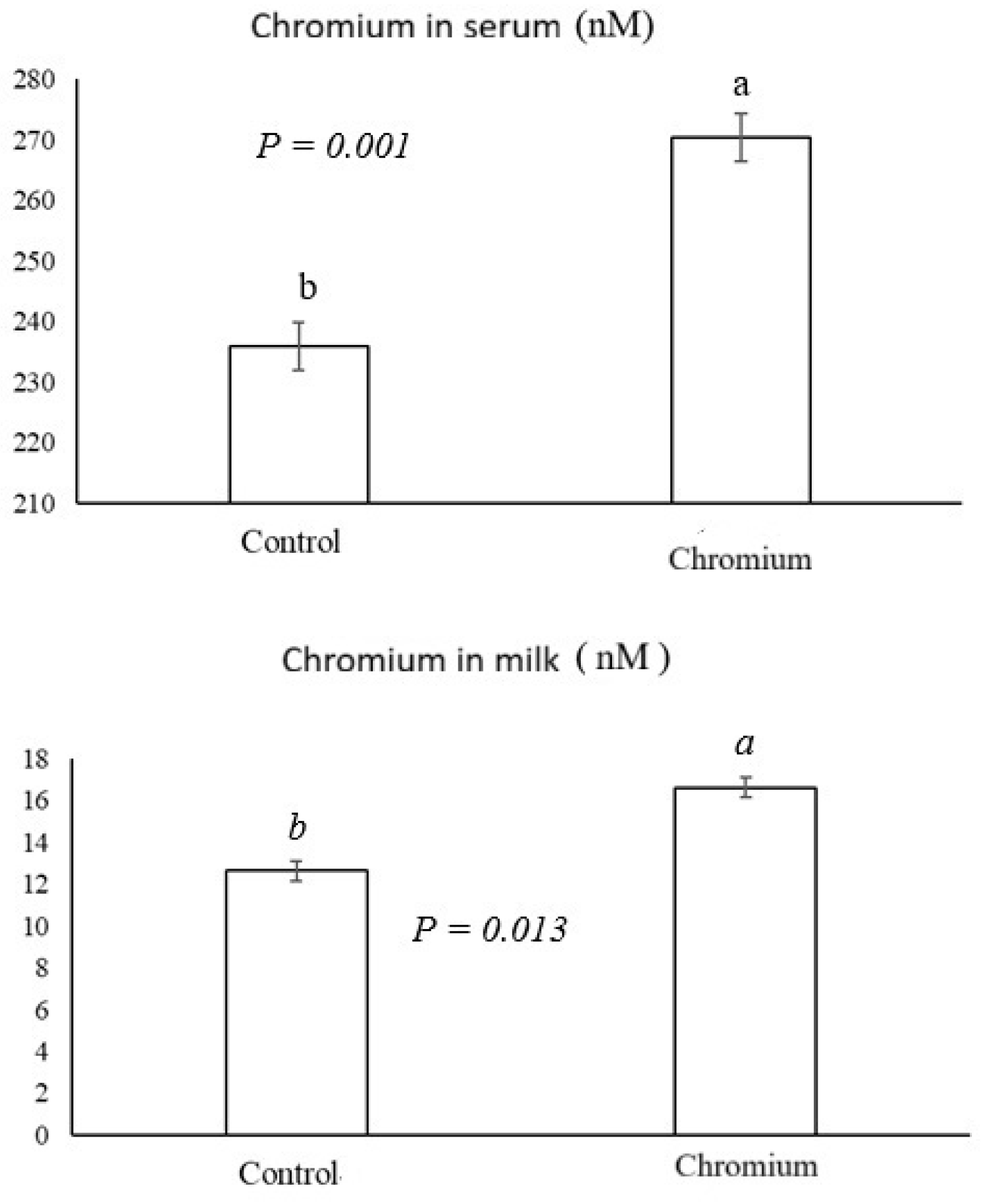
3.1. Chromium Concentration in Milk and Serum
3.2. Productive Performance
3.3. Milk Quality
3.4. Hemogram and Biochemistry
3.5. Oxidative Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knight, C.H. Lactação e gestação em vacas leiteiras: A flexibilidade evita extremos nutricionais. Proc. Nutr. Soc. 2001, 60, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Salamonczyk, E.; Gulinski, P. Somatic Cell Level in Dairy Cows’ Milk During Extended Lactation/Poziom Komorek Somatycznych W Mleku Krow W Laktacji Przedluzonej. Ann. Anim. Sci. 2013, 13, 859. [Google Scholar] [CrossRef][Green Version]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Danieli, B.; Schogor, A.L. Uso de aditivos na nutrição de ruminantes. Rev. Veterinária Zootec. 2020, 27, 1–13. [Google Scholar] [CrossRef]
- Soffa, D.R.; Stewart, J.W.; Arneson, A.G.; Dias, N.W.; Mercadante, V.R.G. Reproductive and lactational responses of multiparous dairy cattle to short-term postpartum chromium supplementation during the summer months. JDS Commun. 2023, 4, 161–165. [Google Scholar] [CrossRef]
- Shan, Q.; Ma, F.; Huang, Q.; Wo, Y.; Sun, P. Chromium yeast promotes milk protein synthesis by regulating ruminal microbiota and amino acid metabolites in heat-stressed dairy cows. Anim. Nutr. 2025, 20, 120–130. [Google Scholar] [CrossRef]
- NASEM—National Academies of Sciences, Engineering, and Medicine. Nutrient Requirements of Dairy Cattle: Eighth Revised Edition; The National Academies Press: Washington, DC, USA, 2021. [Google Scholar] [CrossRef]
- Soltan, M.A. Effect of dietary chromium supplementation on productive and reproductive performance of early lactating dairy cows under heat stress. J. Anim. Physiol. Anim. Nutr. 2010, 94, 264–272. [Google Scholar] [CrossRef]
- Mcnamara, J.P.; Valdez, F. Adipose Tissue Metabolism and Production Responses to Calcium Propionate and Chromium Propionate. J. Dairy Sci. 2005, 88, 2498–2507. [Google Scholar] [CrossRef]
- Kafilzadeh, F.; Shabankareh, H.K.; Targhibi, M.R. Effect of Chromium Supplementation on Productive and Reproductive Performances and Some Metabolic Parameters in Late Gestation and Early Lactation of Dairy Cows. Biol. Trace Elem. Res. 2012, 49, 42–49. [Google Scholar] [CrossRef]
- De Assis, J.R.; Gomes, L.M. Effects of chromium supplementation on the metabolism and performance of dairy cows. Zootec. Pesqui. Prat. Conteporanea 2021, 8, 108–119. [Google Scholar] [CrossRef]
- Chirivi, M.; Abou-Rjeileh, U.; Gandy, J.; Bhattacharya, S.; Lock, A.L. Chromium and palmitic acid supplementation modulate adipose tissue insulin sensitivity in postpartum dairy cows. J. Dairy Sci. 2025, 108, 1078–1091. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, R.J.; Allen, M.S. Chromium propionate supplementation during the peripartum period interacts with starch source fed postpartum: Production responses during the immediate postpartum and carryover periods. J. Dairy Sci. 2016, 99, 4453–4463. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, J.; Shen, Y.; Chen, P.; Li, Y. Effects of chromium propionate supplementation on lactation performance, nutrient digestibility, rumen fermentation patterns, and antioxidant status in Holstein cows under heat stress. Anim. Feed Sci. Technol. 2023, 305, 115765. [Google Scholar] [CrossRef]
- Aragon, V.E.F.; Graça, D.S.; Norte, A.L. Supplemental high chromium yeast and reproductive performance of grazing primiparous zebu cows. Arq. Bras. Med. Veterinária Zootec. 2001, 53, 624–628. [Google Scholar]
- Silva, D.J.; Queiroz, A.C. Análise de Alimentos: Métodos Químicos e Biológicos, 3rd ed.; UFV: Viçosa, Brazil, 2002. [Google Scholar]
- Association of Official Analytical Collaboration. AOAC Official Method 2001.11. Protein (Crude) in Animal Feed, Forage (Plant Tissue), Grain, and Oilseeds: Block Digestion Method Using Copper Catalyst and Steam Distillation into Boric Acid. In Official Methods of Analysis of Aoac International, 22nd ed.; Latimer, G.W., Jr., Ed.; Oxford University Press: Oxford, UK, 2023. [Google Scholar] [CrossRef]
- Ali, S.F.; Lebel, C.P.; Bondy, S.C. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology 1992, 13, 637–648. [Google Scholar]
- Jentzsch, A.M.; Bachmann, H.; Fürst, P.; Biesalski, H.K. Improved analysis of malondialdehyde in human body fluids. Free Radic. Biol. Med. 1996, 20, 251–256. [Google Scholar] [CrossRef]
- Suzuki, K. Assay method for myeloperoxidase in human polymorphonuclear 1132 leukocytes. Anal. Biochem. 1983, 132, 345–352. [Google Scholar] [CrossRef]
- Mccord, J.M.; Fridovich, I. Superoxide dismutase: An enzymatic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Klein, G.S.; Leal, K.W.; Rodrigues, C.A.; Draszevski, T.M.R.; Brunetto, A.L.R.; Vitt, M.G.; Klein, M.S.; Cauduro, V.H.; Flores, E.M.M.; da Silva, G.B. Organic Zinc and Selenium Supplementation of Late Lactation Dairy Cows: Effects on Milk and Serum Minerals Bioavailability, Animal Health and Milk Quality. Animals 2025, 15, 499. [Google Scholar] [CrossRef]
- Glombowsky, P.; Soldá, N.M.; Molosse, V.L.; Deolindo, G.L.; Sulzbach, M.M.; Bottari, N.B.; Schetinger, M.R.C.; Zotti, C.A.; Solivo, G.; Vedovatto, M.; et al. Chromium in the Diet of Dairy Calves: Benefits for Growth Performance, Feed Efficiency, Digestibility, and Health. Biol. Trace Elem. Res. 2024, 202, 5036–5050. [Google Scholar] [CrossRef]
- Malik, M.I.; Jonker, A.; Raboisson, D.; Song, B.; Rashid, M.A.; Sun, X. Effects of dietary chromium supplementation on blood biochemical parameters in dairy cows: A multilevel meta-analytical approach. J. Dairy Sci. 2024, 107, 301–316. [Google Scholar] [CrossRef]
- An-Qiang, L.; Zhi-Sheng, W.; An-Guo, Z. Effect of chromium picolinate supplementation on early lactation performance, rectal temperatures, respiration rates and plasma biochemical response of Holstein cows under heat stress. Pak. J. Nutr. 2009, 8, 940–945. [Google Scholar]
- Giometti, J.; Chiacchio, S.B.; Albas, A.; Pardo, P.E.; Bremer Neto, H.; Giometti, A.I.; Reis, L.S.L.S. Suplementação com levedura de crômio eleva a concentração sérica de crômio em bovinos. Arch. Zootec. 2011, 60, 821–824. [Google Scholar] [CrossRef][Green Version]
- Lloyd, K.E.; Fellner, V.; McLeod, S.J.; Fry, R.S.; Krafka, K.; Lamptey, A.; Spears, J.W. Effects of supplementing dairy cows with chromium propionate on milk and tissue chromium concentrations. J. Dairy Sci. 2010, 93, 4774–4780. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.I.; Raboisson, D.; Zhang, X.; Sun, X. Effects of dietary chromium supplementation on dry matter intake and milk production and composition in lactating dairy cows: A meta-analysis. Front. Vet. Sci. 2023, 10, 1076777. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.L.; Waldron, M.R.; Drackley, J.K.; Socha, M.T.; Overton, T.R. Performance of dairy cows as affected by prepartum dietary carbohydrate source and supplementation with chromium throughout the transition period. J. Dairy Sci. 2005, 88, 255–263. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Peng, W.C.; Liu, J.X.; Xu, G.Z.; Wang, D.M. Effect of chromium methionine supplementation on lactation performance, hepatic respiratory rate and anti-oxidative capacity in early-lactating dairy cows. Animal 2021, 15, 100326. [Google Scholar] [CrossRef]
- Andrei, S.; Matei, S.; Rugină, D.; Bogdan, L.; Ştefănuţ, C. Interrelationships between the content of oxidative markers, antioxidative status, and somatic cell count in cow’s milk. Czech J. Anim. Sci. 2016, 61, 407–413. [Google Scholar] [CrossRef]
- Parales-Girón, J.E.; dos Santos Neto, J.M.; Andres Contreras, G.; Lock, A.L. Supplemental palmitic acid and chromium propionate impact production responses during the immediate postpartum in multiparous dairy cows. J. Dairy Sci. 2025, 108, 3613–3626. [Google Scholar] [CrossRef]
- Hayirli, A.; Bremmer, D.R.; Bertics, S.J.; Socha, M.T.; Grummer, R.R. Effect of chromium supplementation on production and metabolic parameters in periparturient dairy cows. J. Dairy Sci. 2001, 84, 1218–1230. [Google Scholar] [CrossRef]
- Yang, W.Z.; Mowat, D.N.; Subiyatno, A.; Liptrap, R.M. Effects of chromium supplementation on early lactation performance of Holstein cows. Can. J. Anim. Sci. 1996, 76, 221–230. [Google Scholar] [CrossRef]
- Lefebvre Hervé, P.; Toutain, P.L.; Serthelon, J.P.; Lassourd, V.; Gardey, L.; Braun, J.P. Pharmacokinetic variables and bioavailabiljty from muscle of creatine kinase in cattle. Am. J. Vet. Res. 1994, 55, 487–493. [Google Scholar] [CrossRef]
- Valentin, A.P.; Tracey, K.J. The cholinergic anti-inflammatory pathway. Brain Behav. Immun. 2005, 19, 493–499. [Google Scholar] [CrossRef]
- Trevisi, E.; Cattaneo, L.; Piccioli-Cappelli, F.; Mezzetti, M.; Minuti, A. International Symposium on Ruminant Physiology: The immunometabolism of transition dairy cows from dry-off to early lactation: Lights and shadows. J. Dairy Sci. 2025, 1008, 7662–7674. [Google Scholar] [CrossRef] [PubMed]
- Hurley, W.L.; Theil, P.K. Perspectives on Immunoglobulins in Colostrum and Milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef]
- Li, Y.; Dai, C.; Xiao, F.; Spears, J.W. Maternal chromium supplementation improves oxidation resistance, immunity, and intestinal morphology of goat kids injected with lipopolysaccharide. J. Integr. Agric. 2024; in press. [Google Scholar] [CrossRef]
- Lashkari, S.; Habibian, M.; Jensen, S.K. A review on the role of chromium supplementation in ruminant nutrition—Effects on productive performance, blood metabolites, antioxidant status, and immunocompetence. Biol. Trace Elem. Res. 2018, 186, 305–321. [Google Scholar] [CrossRef]
- Shan, Q.; Ma, F.T.; Jin, Y.H.; Gao, D.; Li, H.Y.; Sun, P. Chromium yeast alleviates heat stress by improving antioxidant and immune function in Holstein mid-lactation dairy cows. Anim. Feed Sci. Technol. 2020, 269, 114635. [Google Scholar] [CrossRef]
| Variables | Silage | Hay | Concentrate | Pelleted Concentrate |
|---|---|---|---|---|
| Dry matter—DM (%) | 33.67 | 81.83 | 89.05 | 89.15 |
| Mineral matter % DM | 6.64 | 9.21 | 10.07 | 7.43 |
| Crude protein % DM | 3.89 | 7.29 | 24.26 | 19.93 |
| Ether extract % DM | 3.21 | 1.94 | 3.83 | 3.98 |
| NDF, % DM | 59.7 | 69.8 | 29.9 | - |
| Variables | Control | Chromium | SEM | p-Value |
|---|---|---|---|---|
| Milk production, kg | ||||
| D −30 to 1 (pre-experiment) | 24.41 | 23.69 | 0.82 | 0.73 |
| D 1 to 56 (experimental period) | 18.95 | 20.00 | 0.71 | 0.26 |
| Fat-corrected milk (4%FCM) 1 kg | 19.56 b | 21.71 a | 0.48 | 0.05 |
| Persistence of lactation (%) | 77.66 b | 84.42 a | 2.26 | 0.05 |
| Daily dry matter intake, kg | 16.13 | 16.27 | 0.42 | 0.97 |
| Feed efficiency (kg/kg) | 1.17 b | 1.23 a | 0.01 | 0.02 |
| Variables | Control | Chromium | SEM | p: Treat 1 | p: Day 2 | p: Treat × Day 3 |
|---|---|---|---|---|---|---|
| Fat (%) 4 | 4.21 b | 4.57 a | 0.14 | 0.04 | 0.31 | 0.01 |
| Protein (%) 4 | 3.77 | 3.84 | 0.10 | 0.49 | 0.89 | 0.37 |
| Lactose (%) 4 | 4.40 | 4.47 | 0.08 | 0.54 | 0.75 | 0.66 |
| Total solids (%) 4 | 13.42 | 13.81 | 0.19 | 0.23 | 0.34 | 0.15 |
| Urea (mg/dL) 4 | 14.05 | 15.07 | 1.01 | 0.78 | 0.25 | 0.81 |
| SCC (×103 mL) 4 | 363 a | 115 b | 38.4 | 0.03 | 0.01 | 0.01 |
| Fat (%) 2,3 | ||||||
| d1 | 4.48 | 4.49 | 0.28 | |||
| d14 | 4.08 | 4.38 | 0.31 | |||
| d28 | 4.23 b | 4.60 a | 0.16 | |||
| d42 | 4.27 b | 4.81 a | 0.23 | |||
| d56 | 4.26 | 4.48 | 0.14 | |||
| SCC (×103/mL) 2,3 | ||||||
| d1 | 255 C | 255 A | 36.4 | |||
| d14 | 320 B | 211 AB | 47.9 | |||
| d28 | 521 aA | 185 bB | 52.4 | |||
| d42 | 336 aB | 90.9 aC | 40.2 | |||
| d56 | 274 aC | 95.1 aC | 41.5 |
| Variables | Control | Chromium | SEM | p: Treat 1 | p: Day 2 | p: Treat × Day 2 |
|---|---|---|---|---|---|---|
| Total leukocytes (× 103 μL) 3 | 6.84 | 6.83 | 0.31 | 0.94 | 0.87 | 0.92 |
| Lymphocyte (×103 μL) 3 | 3.87 | 3.81 | 0.18 | 0.92 | 0.90 | 0.82 |
| Granulocytes (×103 μL) 3 | 2.13 | 2.18 | 0.15 | 0.89 | 0.91 | 0.95 |
| Monocyte (×103 μL) 3 | 0.83 | 0.82 | 0.08 | 0.93 | 0.90 | 0.91 |
| Erythrocytes (×106 μL) 3 | 5.19 | 5.07 | 0.02 | 0.71 | 0.65 | 0.76 |
| Hemoglobin (g/dL) 3 | 9.12 | 9.07 | 0.11 | 0.84 | 0.78 | 0.87 |
| Hematocrit (%) 3 | 25.6 | 25.4 | 0.35 | 0.94 | 0.95 | 0.92 |
| Platelets (×103 μL) 3 | 326 | 325 | 12.1 | 0.92 | 0.89 | 0.97 |
| Seric biochemistry | ||||||
| Albumin (g/dL) 3 | 3.29 | 3.36 | 0.04 | 0.88 | 0.79 | 0.82 |
| Total cholesterol (mg/dL) 3 | 133 | 133 | 1.02 | 0.95 | 0.95 | 0.97 |
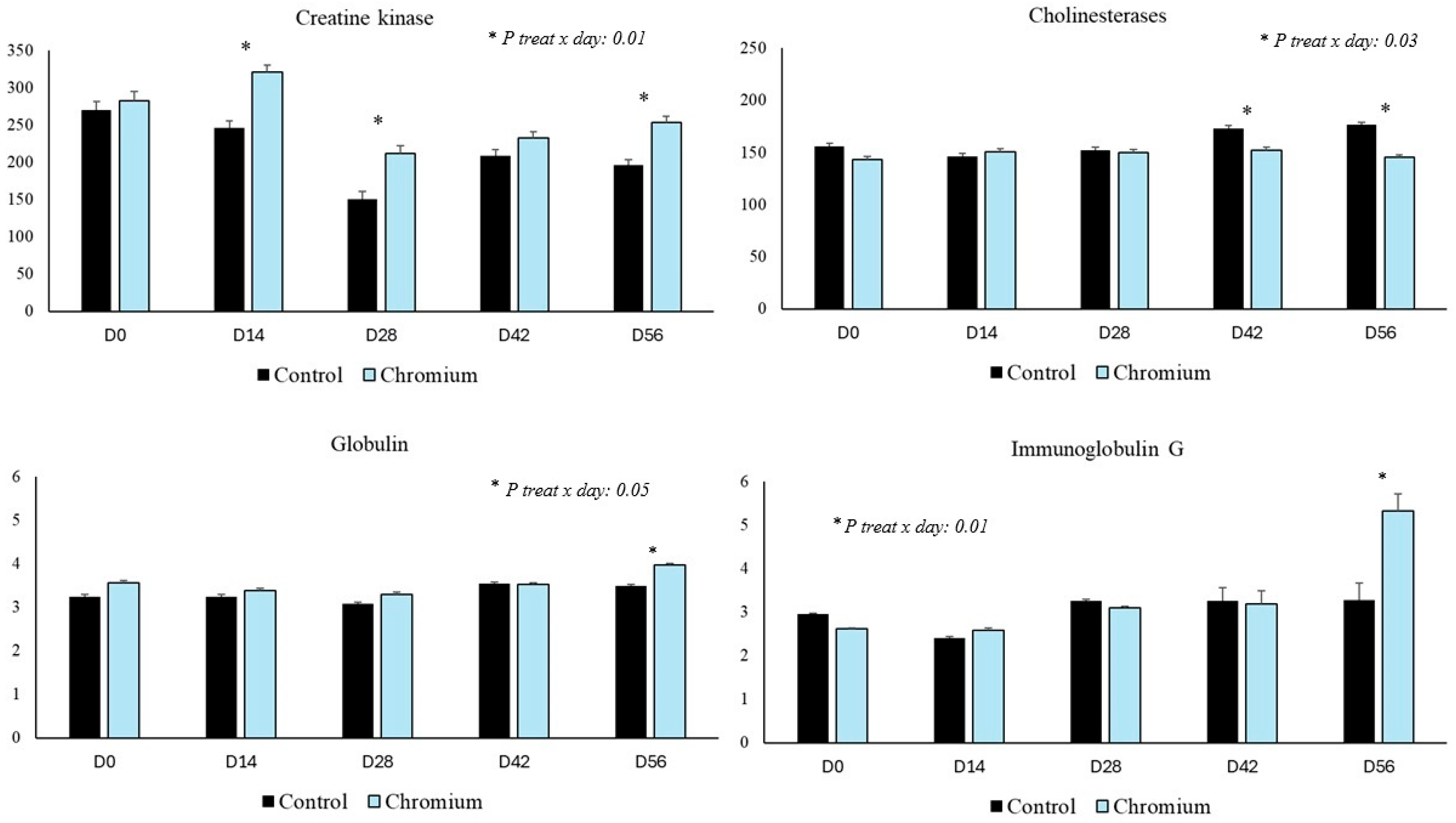
| Creatine kinase (U/L) 3 | 200 a | 254 b | 10.1 | 0.05 | 0.02 | 0.01 |
| Cholinesterase (U/L) 3 | 161 | 149 | 3.05 | 0.07 | 0.05 | 0.03 |
| Glucose (mg/dL) 3 | 62.0 | 60.5 | 1.25 | 0.94 | 0.92 | 0.91 |
| Total protein (g/dL) 3 | 6.62 | 6.90 | 0.08 | 0.45 | 0.32 | 0.20 |
| Ferritin (µg/L) 3 | 466 | 476 | 4.14 | 0.51 | 0.43 | 0.36 |
| C-reactive protein (mg/dL) 3 | 9.99 | 9.96 | 0.07 | 0.98 | 0.97 | 0.98 |
| Triglycerides (mg/dL) 3 | 12.2 | 14.6 | 0.79 | 0.62 | 0.50 | 0.48 |
| Urea (mg/dL) 3 | 30.9 | 33.8 | 1.32 | 0.74 | 0.68 | 0.81 |
| Globulin (g/dL) 3 | 3.33 | 3.54 | 0.06 | 0.09 | 0.06 | 0.05 |
| IgG (mg/dL) 3 | 3.05 a | 3.55 b | 0.03 | 0.03 | 0.01 | 0.01 |
| IgA (mg/dL) 3 | 3.47 | 3.45 | 0.02 | 0.96 | 0.92 | 0.89 |
| Insulin (µU/mL) 3 | 7.12 b | 9.35 a | 0.46 | 0.05 | 0.11 | 0.13 |
| Variables | Control | Chromium | SEM | p: Treat 1 |
|---|---|---|---|---|
| ROS (% of fluorescence intensity) 3 | 5.967 | 6.319 | 0.308 | 0.874 |
| TBARS (nmol/mL) 3 | 10.62 | 10.77 | 0.465 | 0.971 |
| MPO (µ moles of quinoneimine/30 min) 3 | 3.757 | 4.359 | 0.425 | 0.625 |
| SOD (units/mg of protein) 3 | 0.169 | 0.289 | 0.012 | 0.262 |
| SOD (units/mg of protein) 2 | ||||
| d1 | 0.182 | 0.166 | 0.009 | |
| d14 | 0.173 | 0.153 | 0.006 | |
| d28 | 0.172 | 0.192 | 0.009 | |
| d42 | 0.177 b | 0.324 a | 0.016 | |
| d56 | 0.161 b | 0.322 a | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turcatto, N.; Deolindo, G.L.; de Vitt, M.G.; Damo, M.; Wandscheer, J.G.W.; Manica, D.; da Silva, G.B.; Bagatini, M.D.; Da Silva, A.S. Effects of Dietary Chromium Supplementation During Late Lactation on Productive Performance, Milk Composition, and Immune and Antioxidant Responses in Dairy Cows. Animals 2025, 15, 3111. https://doi.org/10.3390/ani15213111
Turcatto N, Deolindo GL, de Vitt MG, Damo M, Wandscheer JGW, Manica D, da Silva GB, Bagatini MD, Da Silva AS. Effects of Dietary Chromium Supplementation During Late Lactation on Productive Performance, Milk Composition, and Immune and Antioxidant Responses in Dairy Cows. Animals. 2025; 15(21):3111. https://doi.org/10.3390/ani15213111
Chicago/Turabian StyleTurcatto, Natália, Guilherme Luiz Deolindo, Maksuel Gatto de Vitt, Maisa Damo, João Gustavo Weschenfelder Wandscheer, Daiane Manica, Gilnei Bruno da Silva, Margarete Dulce Bagatini, and Aleksandro Schafer Da Silva. 2025. "Effects of Dietary Chromium Supplementation During Late Lactation on Productive Performance, Milk Composition, and Immune and Antioxidant Responses in Dairy Cows" Animals 15, no. 21: 3111. https://doi.org/10.3390/ani15213111
APA StyleTurcatto, N., Deolindo, G. L., de Vitt, M. G., Damo, M., Wandscheer, J. G. W., Manica, D., da Silva, G. B., Bagatini, M. D., & Da Silva, A. S. (2025). Effects of Dietary Chromium Supplementation During Late Lactation on Productive Performance, Milk Composition, and Immune and Antioxidant Responses in Dairy Cows. Animals, 15(21), 3111. https://doi.org/10.3390/ani15213111







