Impact of Parenteral Copper and Zinc Administration on Reproduction, Inflammation, and Antioxidant Responses of Bos indicus Beef Heifers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Treatments, and Samples Collection
2.1.1. Experiment 1
2.1.2. Experiment 2
2.2. Laboratory Analysis
2.3. Statistical Analysis
3. Results
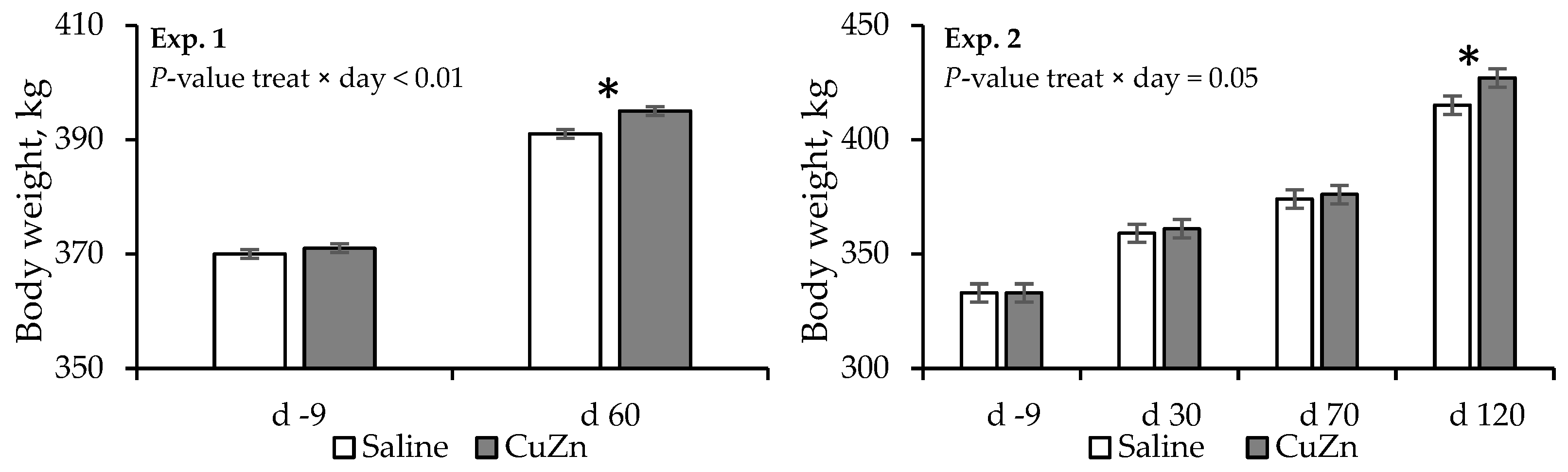
3.1. Experiment 1
3.2. Experiment 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valadares Filho, S.C.; Costa e Silva, L.F.; Gionbelli, M.P.; Rotta, P.P.; Marcondes, M.I.; Chizzotti, M.L.; Prados, L.F. BR—Corte: Tabela Brasileira de Exigências Nutricionais, 3rd ed.; Universidade Federal de Viçosa, DZO: Viçosa, Brazil, 2016. [Google Scholar] [CrossRef]
- Arthington, J.D.; Ranches, J. Trace Mineral Nutrition of Grazing Beef Cattle. Animals 2021, 11, 2767. [Google Scholar] [CrossRef]
- Vedovatto, M.; Moriel, P.; Cooke, R.F.; Costa, D.S.; Faria, F.J.C.; Cortada Neto, I.M.; Pereira, C.D.S.; Bento, A.L.D.L.; De Almeida, R.G.; Santos, S.A.; et al. Effects of a Single Trace Mineral Injection on Body Parameters, Ovarian Structures, Pregnancy Rate and Components of the Innate Immune System of Grazing Nellore Cows Synchronized to a Fixed-Time AI Protocol. Livest. Sci. 2019, 225, 123–128. [Google Scholar] [CrossRef]
- Corah, L.R.; Ives, S. The Effects of Essential Trace Minerals on Reproduction in Beef Cattle. Vet. Clin. N. Am. Food Anim. Pract. 1991, 7, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.W. Trace element deficiencies in cattle. Vet. Clin. N. Am. Food Anim. Pract. 1991, 7, 153–215. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.H.; Hancock, D.D.; Conrad, H.R. Vitamin E and selenium for reproduction of the dairy cow. J. Dairy Sci. 1984, 67, 123–132. [Google Scholar] [CrossRef]
- Van Emon, M.; Sanford, C.; McCoski, S. Impacts of bovine trace mineral supplementation on maternal and offspring production and health. Animals 2020, 10, 2404. [Google Scholar] [CrossRef]
- Hostetler, C.E.; Kincaid, R.L.; Mirando, M.A. The role of essential trace elements in embryonic and fetal development in livestock. Vet. J. 2003, 166, 125–139. [Google Scholar] [CrossRef]
- Nazari, A.; Dirandeh, E.; Ansari-Pirsaraei, Z.; Deldar, H. Antioxidant levels, copper and zinc concentrations were associated with postpartum luteal activity, pregnancy loss and pregnancy status in Holstein dairy cows. Theriogenology 2019, 133, 97–103. [Google Scholar] [CrossRef]
- Wooldridge, L.K.; Nardi, M.E.; Ealy, A.D. Zinc supplementation during in vitro embryo culture increases inner cell mass and total cell numbers in bovine blastocysts. J. Anim. Sci. 2019, 97, 4946–4950. [Google Scholar] [CrossRef]
- Palomares, R.; Ferrer, M.; Jones, L. Role of trace minerals in cow’s reproductive function and performance: A clinical theriogenology perspective. Clin. Theriogenol. 2024, 16, 10529. [Google Scholar] [CrossRef]
- Del Río-Avilés, A.; Correa-Calderón, A.; Avendaño-Reyes, L.; Macías-Cruz, U.; Thomas, M.G.; Mark Enns, R.; Speidel, S.E.; Sánchez-Castro, M.A.; Zamorano-Algandar, R.; López-Castro, P.A.; et al. Mineral supplementation (injectable) improved reproductive performance in Holstein cows managed in a warm summer environment. Reprod. Dom. Anim. 2022, 57, 839–848. [Google Scholar] [CrossRef]
- Avendaño-Reyes, L.; González-López, M.; López-Baca, Á.; López-Rincón, G.; Prado-Rebolledo, O.F.; Mellado, M.; Hernández-Rivera, J.A.; Macías-Cruz, U.; Castañeda-Bustos, V.J.; García-Casillas, A.C. Ovarian activity and reproductive responses of lactating Angus cows due to a mineral supplementation throughout a timed AI protocol. Trop. Anim. Health Prod. 2023, 55, 308. [Google Scholar] [CrossRef]
- Vedovatto, M.; Moriel, P.; Cooke, R.F.; Costa, D.S.; Faria, F.J.C.; Cortada Neto, I.M.; Bento, A.L.L.; Rocha, R.F.A.T.; Ferreira, L.C.L.; Almeida, R.G.; et al. Effects of a Single Trace Mineral Injection at Beginning of Fixed-Time AI Treatment Regimen on Reproductive Function and Antioxidant Response of Grazing Nellore Cows. Anim. Reprod. Sci. 2019, 211, 106234. [Google Scholar] [CrossRef]
- Hernandez, G.P.; Ferreira, M.F.L.; Santos, A.C.R.; Bohnert, D.; Ranches, J. Effects of Trace Mineral Injections on Measures of Growth and Trace Mineral Status of Primiparous Cows and their Calves. Transl. Anim. Sci. 2024, 8, txae068. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine (NASEM). Nutrient Requirements of Beef Cattle Model, 8th ed.; National Academic Press: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- Weiss, W.P.; Conrad, H.R.; St. Pierre, N.R. A Theoretically-Based Model for Predicting Total Digestible Nutrient Values of Forages and Concentrates. Anim. Feed Sci. Technol. 1992, 39, 95–110. [Google Scholar] [CrossRef]
- Herd, D.B.; Sprott, L.R. Body Condition, Nutrition and Reproduction of Beef Cows; Texas A&M University System, Texas Agricultural Extension Service: College Station, TX, USA, 1998. [Google Scholar]
- Rodrigues, W.B.; Jara, J.P.; Borges, J.C.; Oliveira, L.O.F.; Abreu, U.P.G.; Anache, N.A.; Silva, K.S.; Bezerra, A.O.; Cardoso, C.J.T.; Nogueira, N. Efficiency of mating, artificial insemination, or resynchronisation at different times after first timed artificial insemination in postpartum Nellore cows to produce crossbred calves. Anim. Prod. Sci. 2019, 59, 225–231. [Google Scholar] [CrossRef]
- Speckhart, S.L.; Oliveira Filho, R.V.; Franco, G.A.; Vasconcelos, J.L.M.; Schrick, F.N.; Edwards, J.L.; Pohler, K.G. Short Communication: Influence of estrus activity and reproductive tract size and position scores on fertility in Bos indicus and Bos taurus suckled beef cows. J. Anim. Sci. 2022, 100, skac141. [Google Scholar] [CrossRef]
- Cooke, R.F.; Arthington, J.D.; Austin, B.R.; Yelich, J.V. Effects of Acclimation to Handling on Performance, Reproductive, and Physiological Responses of Brahman-Crossbred Heifers. J. Anim. Sci. 2009, 87, 3403–3412. [Google Scholar] [CrossRef]
- Euclides, V.P.B.; Macedo, M.C.M.; Oliveira, M.P. Avaliação de Diferentes Métodos de Amostragem Para Se Estimar o Valor Nutritivo de Forragem Sob Pastejo. Rev. Soc. Bras. Zootec. 1992, 21, 691–702. [Google Scholar]
- AOAC. Official Methods of Analysis. Association of Official Analytical Chemists, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Martin, J.L.; Vonnahme, K.A.; Adams, D.C.; Lardy, G.P.; Funston, R.N. Effects of Dam Nutrition on Growth and Reproductive Performance of Heifer Calves1. J. Anim. Sci. 2007, 85, 841–847. [Google Scholar] [CrossRef]
- Bordignon, R.; Volpato, A.; Glombowsky, P.; Souza, C.F.; Baldissera, M.D.; Secco, R.; Pereira, W.A.B.; Leal, M.L.R.; Vedovatto, M.; Da Silva, A.S. Nutraceutical Effect of Vitamins and Minerals on Performance and Immune and Antioxidant Systems in Dairy Calves during the Nutritional Transition Period in Summer. J. Therm. Biol. 2019, 84, 451–459. [Google Scholar] [CrossRef]
- Cooke, R.F.; Arthington, J.D. Concentrations of Haptoglobin in Bovine Plasma Determined by ELISA or a Colorimetric Method Based on Peroxidase Activity: Methods to Determine Haptoglobin in Bovine Plasma. J. Anim. Physiol. Anim. Nutr. 2013, 97, 531–536. [Google Scholar] [CrossRef]
- Demetriou, J.A.; Drewes, P.A.; Gin, J.B. Ceruloplasmin. In Clinical Chemistry; Cannon, D.C., Winkelman, J.W., Eds.; Harper and Row: Hagerstown, MD, USA, 1974; pp. 857–864. [Google Scholar]
- Cooke, R.F.; Schubach, K.M.; Marques, R.S.; Peres, R.F.G.; Silva, L.G.T.; Carvalho, R.S.; Cipriano, R.S.; Bohnert, D.W.; Pires, A.V.; Vasconcelos, J.L.M. Effects of Temperament on Physiological, Productive, and Reproductive Responses in Bos Indicus Beef Cows. J. Anim. Sci. 2017, 95, 1–8. [Google Scholar] [CrossRef]
- Stokes, R.S.; Volk, M.J.; Ireland, F.A.; Gunn, P.J.; Shike, D.W. Effect of Repeated Trace Mineral Injections on Beef Heifer Development and Reproductive Performance. J. Anim. Sci. 2018, 96, 3943–3954. [Google Scholar] [CrossRef] [PubMed]
- Mundell, L.R.; Jaeger, J.R.J.; Waggoner, W.; Stevenson, J.S.; Grieger, D.M.; Pacheco, L.A.; Bolte, J.W.; Aubel, N.A.; Eckerle, G.J.; Macek, M.J.; et al. Effects of prepartum and postpartum bolus injections of trace minerals on performance of beef cows and calves grazing native range. Prof. Anim. Sci. 2012, 28, 82–88. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Beef Cattle, 7th ed.; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Ahola, J.K.; Baker, D.S.; Burns, P.D.; Mortimer, R.G.; Enns, R.M.; Whittier, J.C.; Geary, T.W.; Engle, T.E. Effect of copper, zinc, and manganese supplementation and source on reproduction, mineral status, and performance in grazing beef cattle over a two-year period. J. Anim. Sci. 2004, 82, 2375–2383. [Google Scholar] [CrossRef] [PubMed]
- Kastelic, J.P.; Bergfelt, D.R.; Ginther, O.J. Relationship between Ultrasonic Assessment of the Corpus Luteum and Plasma Progesterone Concentration in Heifers. Theriogenology 1990, 33, 1269–1278. [Google Scholar] [CrossRef]
- Anchordoquy, J.M.; Anchordoquy, J.P.; Galarza, E.M.; Farnetano, N.A.; Giuliodori, M.J.; Nikoloff, N.; Fazzio, L.E.; Furnus, C.C. Parenteral Zinc Supplementation Increases Pregnancy Rates in Beef Cows. Biol. Trace Elem. Res. 2019, 192, 175–182. [Google Scholar] [CrossRef]
- González-Maldonado, J.; Rangel-Santos, R.; Rodríguez-de Lara, R.; García-Peña, O. Effect of Injectable Trace Mineral Complex Supplementation on Development of Ovarian Structures and Serum Copper and Zinc Concentrations in Over-Conditioned Holstein Cows. Anim. Reprod. Sci. 2017, 181, 57–62. [Google Scholar] [CrossRef]
- Arthington, J.D.; Moriel, P.; Martins, P.G.M.A.; Lamb, G.C.; Havenga, L.J. Effects of Trace Mineral Injections on Measures of Performance and Trace Mineral Status of Pre- and Postweaned Beef Calves1. J. Anim. Sci. 2014, 92, 2630–2640. [Google Scholar] [CrossRef]
- Springman, S.A.; Maddux, J.G.; Drewnoski, M.E.; Funston, R.N. Effects of injectable trace minerals on reproductive performance of beef heifers in adequate trace mineral status. PAS 2018, 34, 649–652. [Google Scholar] [CrossRef]
- Caramalac, L.S.; Moriel, P.; Ranches, J.; Silva, G.M.; Arthington, J.D. Comparison of injectable trace minerals vs. adjuvant on measures of innate and humoral immune responses of beef heifers. Livest. Sci. 2021, 251, 104665. [Google Scholar] [CrossRef]
| Items | Exp. 1 (Farm School) | Exp. 2 (Commercial Operation) | Requirement 1 |
|---|---|---|---|
| Forage | |||
| g/kg of DM | |||
| Crude protein | 91.8 | 75.2 | |
| Neutral detergent fiber | 643 | 716 | |
| Acid detergent fiber | 349 | 393 | |
| Lignin | 57.2 | 70.0 | |
| Ether extract | 14.4 | 12.5 | |
| Ash | 102 | 86.3 | |
| Calcium | 1.98 | 1.77 | |
| Phosphorus | 1.93 | 1.78 | |
| Sodium | 0.37 | 0.77 | 1.0 |
| Potassium | 25.9 | 22.5 | 7.0 |
| Magnesium | 2.28 | 2.02 | 2.0 |
| Sulfur | 0.9 | 1.00 | 1.5 |
| TDN 2 | 572 | 546 | |
| Mcal/kg of DM | |||
| NEm 3 | 1.21 | 1.13 | |
| NEg 3 | 0.65 | 0.57 | |
| mg/kg of DM | |||
| Iron | 431 | 109 | 50.0 |
| Manganese | 158 | 91.8 | 40.0 |
| Selenium | 0.11 | 0.12 | 0.10 |
| Zinc | 26.9 | 20.7 | 30.0 |
| Copper | 4.86 | 4.16 | 10.0 |
| Mineral/vitamin supplement 4 | |||
| g/kg of DM | |||
| Calcium | 196 | 140–190 | |
| Phosphorus | 90 | 80 | |
| Sodium | 99 | 110 | |
| Magnesium | 20 | 10 | |
| Sulfur | 20 | 16 | |
| mg/kg of DM | |||
| Fluorine | 900 | 880 | |
| Cobalt | 200 | 61 | |
| Iodine | 180 | 55 | |
| Iron | 2400 | ||
| Manganese | 1670 | 4682 | |
| Selenium | 40 | 11.8 | |
| Zinc | 3000 | 3273 | |
| Copper | 1200 | 1091 | |
| IU/kg of DM | |||
| Vitamin A | 150,000 | ||
| Vitamin D3 | 30,000 | ||
| Vitamin E | 1500 | ||
| Target intake, g/d | 100 | 200 |
| Items | Treatments 1 | SEM | p-Value | ||
|---|---|---|---|---|---|
| Saline | CuZn | Trt | Trt × Day | ||
| Exp. 1, n = | 15 | 14 | |||
| Body traits | |||||
| BW change, kg | 20.5 | 24.4 | 1.51 | 0.09 | |
| Body condition score (BCS), 1–9 | 0.52 | 0.63 | |||
| d −9 | 4.81 | 4.90 | 0.14 | ||
| d 60 | 4.81 | 4.78 | 0.14 | ||
| BCS change, 1–9 | 0.00 | −0.15 | 0.17 | 0.52 | |
| Estrus traits | |||||
| Estrus expression score (d 0), 1–3 | 1.80 | 1.42 | 0.23 | 0.25 | |
| Mating rate (d 0), % | 75.3 | 76.4 | 0.12 | 0.95 | |
| Ovarian traits | |||||
| Dominant follicle diameter (d 0), mm | 10.7 | 10.4 | 0.87 | 0.77 | |
| Corpus luteum diameter, mm | |||||
| d 7 | 18.5 | 19.2 | 0.93 | 0.59 | |
| d 14 | 17.3 | 19.4 | 0.81 | 0.10 | |
| Corpus luteum volume, cm3 | |||||
| d 7 | 3.55 | 3.99 | 0.55 | 0.60 | |
| d 14 | 2.99 | 4.00 | 0.44 | 0.15 | |
| Plasma progesterone (d 14), ng/mL | 5.68 | 6.42 | 0.67 | 0.44 | |
| Pregnancy rate (d 60), % | 40.8 | 45.2 | 0.14 | 0.84 | |
| Conceptus size traits | |||||
| Crown-Rump (d 60), mm | 32.9 | 30.6 | 2.14 | 0.51 | |
| Thoracic (d 60), mm | 15.3 | 13.2 | 1.12 | 0.27 | |
| Items 1 | Treatments 2 | SEM | p-Value | ||
|---|---|---|---|---|---|
| Saline | CuZn | Trt | Trt × Day | ||
| Exp. 1, n = | 10 | 10 | |||
| Serum Cu, μg/dL | 52.1 | 49.2 | 2.60 | 0.47 | 0.61 |
| Serum Zn, μg/dL | 51.9 | 48.1 | 4.60 | 0.58 | 0.91 |
| Serum cortisol, µg/dL | 3.05 | 3.52 | 0.24 | 0.18 | 0.73 |
| Plasma haptoglobin, mg/mL | 0.40 | 0.41 | 0.02 | 0.69 | 0.25 |
| Plasma ceruloplasmin, mg/mL | 16.4 | 16.6 | 0.54 | 0.75 | 0.97 |
| Plasma SOD, U/mL | 86.8 | 69.3 | 13.9 | 0.50 | 0.21 |
| Plasma GSH-px, U/mL | 0.12 | 0.10 | |||
| d −9 | 71.5 | 71.6 | 7.57 | ||
| d 30 | 68.7 b | 94.4 a | 7.57 | ||
| d 60 | 67.6 | 79.7 | 7.57 | ||
| Exp. 2, n = | 10 | 10 | |||
| Serum Cu, μg/dL | 49.0 | 69.9 | 3.79 | <0.01 | 0.28 |
| Serum Zn, μg/dL | 59.8 | 67.8 | 7.72 | 0.56 | 0.17 |
| Plasma SOD, U/mL | 88.0 | 83.3 | 12.0 | 0.78 | 0.96 |
| Plasma GSH-px, U/mL | 70.5 | 74.7 | 4.99 | 0.57 | 0.79 |
| Items 1 | Treatments 2 | SEM | p-Value | ||
|---|---|---|---|---|---|
| Saline | CuZn | Trt | Trt × Day | ||
| Exp. 2, n = | 140 | 143 | |||
| Body traits | |||||
| BW change, kg | |||||
| d −9 to 30 | 24.3 | 26.8 | 8.98 | 0.09 | |
| d 30 to 70 | 13.6 | 14.4 | 5.01 | 0.61 | |
| d 70 to 120 | 42.8 | 47.5 | 26.7 | 0.57 | |
| d −9 to 120 | 76.9 | 87.8 | 27.8 | 0.09 | |
| Body condition score (BCS), 1–9 | 0.34 | 0.44 | |||
| d −9 | 5.56 | 5.58 | 0.05 | ||
| d 30 | 6.07 | 6.10 | 0.05 | ||
| d 70 | 5.94 | 5.92 | 0.07 | ||
| d 120 | 6.53 | 6.70 | 0.07 | ||
| BCS change, 1–9 | |||||
| d −9 to 30 | 0.51 | 0.52 | 0.09 | 0.60 | |
| d 30 to 70 | −0.14 | −0.18 | 0.18 | 0.81 | |
| d 70 to 120 | 0.59 | 0.78 | 0.18 | 0.55 | |
| d −9 to 120 | 0.97 | 1.12 | 0.09 | 0.22 | |
| Estrus traits | |||||
| Estrus expression score, 1–3 | |||||
| d 0 | |||||
| Low BCS | 2.59 | 2.82 | 0.07 | 0.01 | |
| High BCS | 2.67 | 2.62 | 0.09 | 0.68 | |
| Overall | 2.62 | 2.73 | 0.05 | 0.16 | |
| d 39 | |||||
| Low BCS | 2.82 | 2.86 | 0.07 | 0.71 | |
| High BCS | 2.83 | 2.84 | 0.07 | 0.93 | |
| Overall | 2.82 | 2.85 | 0.05 | 0.69 | |
| Mating rate, % | |||||
| d 0 | |||||
| Low BCS | 88.3 | 97.1 | 3.14 | 0.04 | |
| High BCS | 87.8 | 89.9 | 5.07 | 0.72 | |
| Overall | 87.8 | 93.7 | 2.58 | 0.10 | |
| d 39 | |||||
| Low BCS | 100 | 96.0 | 0.02 | 0.23 | |
| High BCS | 100 | 100 | - | - | |
| Overall | 100 | 98.0 | 1.24 | 0.25 | |
| Pregnancy rate, % | |||||
| d 30 (1st FTAI) | |||||
| Low BCS | 47.9 | 56.1 | 5.10 | 0.10 | |
| High BCS | 48.0 | 52.8 | 6.88 | 0.61 | |
| Overall | 48.3 | 55.4 | 4.63 | 0.26 | |
| d 70 (2nd FTAI) | |||||
| Low BCS | 38.2 | 28.6 | 9.97 | 0.42 | |
| High BCS | 33.1 | 48.9 | 9.65 | 0.25 | |
| Overall | 36.2 | 38.7 | 7.26 | 0.79 | |
| d 120 (bull) | |||||
| Low BCS | 12.4 | 9.45 | 3.90 | 0.58 | |
| High BCS | 15.0 | 11.4 | 4.71 | 0.59 | |
| Overall | 13.6 | 10.2 | 2.92 | 0.43 | |
| d 120 (FTAIs + bull) | |||||
| Low BCS | 82.3 | 77.3 | 5.44 | 0.40 | |
| High BCS | 80.6 | 87.4 | 6.47 | 0.35 | |
| Overall | 81.7 | 82.1 | 4.12 | 0.94 | |
| Pregnancy loss, % | |||||
| d 30 to 120 | |||||
| Low BCS | 0.00 | 2.50 | 1.81 | 0.33 | |
| High BCS | 3.66 | 3.05 | 3.48 | 0.90 | |
| Overall | 1.50 | 2.77 | 1.79 | 0.61 | |
| d 70 to 120 | |||||
| Low BCS | 7.15 | 1.92 | 8.70 | 0.39 | |
| High BCS | 0.43 | 12.3 | 10.8 | 0.33 | |
| Overall | 3.65 | 16.5 | 6.30 | 0.16 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.G.d.; Vedovatto, M.; Ranches, J.; Martins, E.C.; Ferreira, M.F.; Lima, E.d.A.; Ferreira, L.C.L.; Reis, W.V.A.d.; Franco, G.L. Impact of Parenteral Copper and Zinc Administration on Reproduction, Inflammation, and Antioxidant Responses of Bos indicus Beef Heifers. Animals 2025, 15, 2926. https://doi.org/10.3390/ani15192926
Silva LGd, Vedovatto M, Ranches J, Martins EC, Ferreira MF, Lima EdA, Ferreira LCL, Reis WVAd, Franco GL. Impact of Parenteral Copper and Zinc Administration on Reproduction, Inflammation, and Antioxidant Responses of Bos indicus Beef Heifers. Animals. 2025; 15(19):2926. https://doi.org/10.3390/ani15192926
Chicago/Turabian StyleSilva, Luana Gomes da, Marcelo Vedovatto, Juliana Ranches, Edilane Costa Martins, Matheus Fellipe Ferreira, Eduardo de Assis Lima, Luiz Carlos Louzada Ferreira, Willian Vaniel Alves dos Reis, and Gumercindo Loriano Franco. 2025. "Impact of Parenteral Copper and Zinc Administration on Reproduction, Inflammation, and Antioxidant Responses of Bos indicus Beef Heifers" Animals 15, no. 19: 2926. https://doi.org/10.3390/ani15192926
APA StyleSilva, L. G. d., Vedovatto, M., Ranches, J., Martins, E. C., Ferreira, M. F., Lima, E. d. A., Ferreira, L. C. L., Reis, W. V. A. d., & Franco, G. L. (2025). Impact of Parenteral Copper and Zinc Administration on Reproduction, Inflammation, and Antioxidant Responses of Bos indicus Beef Heifers. Animals, 15(19), 2926. https://doi.org/10.3390/ani15192926






