Breed-Specific Anaesthetic Mortality in Dogs: Evidence from an Analysis of 55,019 Cases
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- FCI group and section. Breeds were mapped to the Fédération Cynologique Internationale (FCI) groups and sections [12].
- Brachycephalic conformation (yes/no). Defined a priori using a phenotype-based list grounded in the brachycephalic obstructive airway syndrome literature [3,4,6,13,14,15]. The following breeds in our dataset were classified as brachycephalic: Affenpinscher, American Bull Dog, American Cocker Spaniel, American Pit Bull Terrier, American Staffordshire Terrier, Boxer, Boston Terrier, Bull Arab, Bullmastiff, Bull Terrier, Brussels Griffon/Griffon Belge/Petit Brabançon, Bulldog, French Bulldog, Olde English Bulldogge, Italian Cane Corso, Cavalier King Charles Spaniel, Chihuahua, Chow Chow, Continental Bulldog, Dogue de Bordeaux, Dogo Argentino, English Mastiff, Japanese Chin, Lhasa Apso, Löwchen, Mastiff, Miniature Bull Terrier, Neapolitan Mastiff, Newfoundland, Pekingese, Presa Canario, Pug, Pyrenean Mastiff, Rottweiler, Shar Pei, Shih Tzu, Staffordshire Bull Terrier, Tibetan Spaniel, Tosa, and Yorkshire Terrier.
- MDR1-associated breeds (yes/no). A breed-level proxy for ABCB1-1Δ/nt230(del4) carriage based on published genotype surveys and case series [16]. The following breeds were considered potential carriers of this genetic mutation: Australian Shepherd, Black Mouth Cur, Bearded Collie, Border Collie, Chinook, Collie (Smooth and Rough), English Shepherd, Whippet, Old English Sheepdog, Shetland Sheepdog, Silken Windhound, Wäller, McNab, and White Swiss Shepherd Dog.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| ABCB1 | ATP-binding cassette sub-family B member 1 (P-glycoprotein) gene |
| ASA | American Society of Anesthesiologists physical status classification |
| CEEA | Comité de Ética en Experimentación Animal (Ethics Committee) |
| CIs | Confidence intervals |
| FCI | Fédération Cynologique Internationale |
| HC3 | Heteroscedasticity-consistent covariance estimator, type 3 |
| MDR1 | Multidrug resistance gene 1 (historical name for ABCB1) |
| nt230(del4) | Four-base-pair deletion in ABCB1 (also written ABCB1-1Δ) |
| RR | Relative risk |
References
- Redondo, J.I.; Otero, P.E.; Martínez-Taboada, F.; Doménech, L.; Hernández-Magaña, E.Z.; Viscasillas, J. Anaesthetic Mortality in Dogs: A Worldwide Analysis and Risk Assessment. Vet. Rec. 2024, 195, e3604. [Google Scholar] [CrossRef]
- Bainbridge, D.; Martin, J.; Arango, M.; Cheng, D.; for the EPCOR (EPiCOR) Group. Perioperative and Anaesthetic-Related Mortality in Developed and Developing Countries: A Systematic Review and Meta-Analysis. Lancet 2012, 380, 1075–1081. [Google Scholar] [CrossRef]
- Gruenheid, M.; Aarnes, T.K.; McLoughlin, M.A.; Simpson, E.M.; Mathys, D.A.; Mollenkopf, D.F.; Wittum, T.E. Risk of Anesthesia-Related Complications in Brachycephalic Dogs. J. Am. Vet. Med. Assoc. 2018, 253, 301–306. [Google Scholar] [CrossRef]
- Downing, F.; Gibson, S. Anaesthesia of Brachycephalic Dogs. J. Small Anim. Pract. 2018, 59, 725–733. [Google Scholar] [CrossRef]
- Mealey, K.L.; Owens, J.G.; Freeman, E. Canine and Feline P-glycoprotein Deficiency: What We Know and Where We Need to Go. J. Vet. Pharmacol. Ther. 2023, 46, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fédération Cynologique Internationale (FCI). Breed-Specific Instructions (BSI) Regarding Exaggerations in Pedigree Dogs. 2025. Available online: https://www.fci.be/en/Breed-Specific-Instructions-BSI-regarding-exaggerations-in-pedigree-dogs-5859.html (accessed on 25 September 2025).
- Matthews, N.S.; Mohn, T.J.; Yang, M.; Spofford, N.; Marsh, A.; Faunt, K.; Lund, E.M.; Lefebvre, S.L. Factors Associated with Anesthetic-Related Death in Dogs and Cats in Primary Care Veterinary Hospitals. J. Am. Vet. Med. Assoc. 2017, 250, 655–665. [Google Scholar] [CrossRef]
- Itami, T.; Aida, H.; Asakawa, M.; Fujii, Y.; Iizuka, T.; Imai, A.; Iseri, T.; Ishizuka, T.; Kakishima, K.; Kamata, M.; et al. Association between Preoperative Characteristics and Risk of Anaesthesia-Related Death in Dogs in Small-Animal Referral Hospitals in Japan. Vet. Anaesth. Analg. 2017, 44, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Shoop-Worrall, S.J.; O’Neill, D.G.; Viscasillas, J.; Brodbelt, D.C. Mortality Related to General Anaesthesia and Sedation in Dogs under UK Primary Veterinary Care. Vet. Anaesth. Analg. 2022, 49, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Brodbelt, D.C.; Blissitt, K.J.; Hammond, R.A.; Neath, P.J.; Young, L.E.; Pfeiffer, D.U.; Wood, J.L.N. The Risk of Death: The Confidential Enquiry into Perioperative Small Animal Fatalities. Vet. Anaesth. Analg. 2008, 35, 365–373. [Google Scholar] [CrossRef]
- Brodbelt, D.C.; Pfeiffer, D.U.; Young, L.E.; Wood, J.L.N. Results of the Confidential Enquiry into Perioperative Small Animal Fatalities Regarding Risk Factors for Anesthetic-Related Death in Dogs. J. Am. Vet. Med. Assoc. 2008, 233, 1096–1104. [Google Scholar] [CrossRef]
- Fédération Cynologique Internationale (FCI). FCI Breeds Nomenclature. 2025. Available online: https://fci.be/nomenclature/ (accessed on 25 September 2025).
- Packer, R.M.; Tivers, M.S. Strategies for the Management and Prevention of Conformation-Related Respiratory Disorders in Brachycephalic Dogs. Vet. Med. Res. Rep. 2015, 6, 219–232. [Google Scholar] [CrossRef]
- Liu, N.-C.; Troconis, E.L.; Kalmar, L.; Price, D.J.; Wright, H.E.; Adams, V.J.; Sargan, D.R.; Ladlow, J.F. Conformational Risk Factors of Brachycephalic Obstructive Airway Syndrome (BOAS) in Pugs, French Bulldogs, and Bulldogs. PLoS ONE 2017, 12, e0181928. [Google Scholar] [CrossRef]
- Doyle, C.R.; Aarnes, T.K.; Ballash, G.A.; Wendt-Hornickle, E.L.; Baldo, C.F.; Johnson, R.A.; Wittum, T.E.; McLoughlin, M.A. Anesthetic Risk during Subsequent Anesthetic Events in Brachycephalic Dogs That Have Undergone Corrective Airway Surgery: 45 Cases (2007–2019). J. Am. Vet. Med. Assoc. 2020, 257, 744–749. [Google Scholar] [CrossRef]
- Washington State University Veterinary Clinical Pharmacology Laboratory (WSU VCSL). Dog Breeds Commonly Affected by MDR1 Mutation. 2021. Available online: https://prime.vetmed.wsu.edu/2021/10/19/breeds-commonly-affected-by-mdr1-mutation/ (accessed on 25 September 2025).
- Gil, L.; Redondo, J.I. Canine Anaesthetic Death in Spain: A Multicentre Prospective Cohort Study of 2012 Cases. Vet. Anaesth. Analg. 2013, 40, e57–e67. [Google Scholar] [CrossRef]
- Ekenstedt, K.J.; Crosse, K.R.; Risselada, M. Canine Brachycephaly: Anatomy, Pathology, Genetics and Welfare. J. Comp. Pathol. 2020, 176, 109–115. [Google Scholar] [CrossRef]
- Oda, A.; Wang, W.H.; Hampton, A.K.; Robertson, J.B.; Posner, L.P. Perianesthetic Mortality in English Bulldogs: A Retrospective Analysis in 2010–2017. BMC Vet. Res. 2022, 18, 198. [Google Scholar] [CrossRef]
- Ovbey, D.H.; Wilson, D.V.; Bednarski, R.M.; Hauptman, J.G.; Stanley, B.J.; Radlinsky, M.G.; Larenza, M.P.; Pypendop, B.H.; Rezende, M.L. Prevalence and Risk Factors for Canine Post-Anesthetic Aspiration Pneumonia (1999–2009): A Multicenter Study. Vet. Anaesth. Analg. 2014, 41, 127–136. [Google Scholar] [CrossRef]
- Johnson, L.R.; Pollard, R.E. Tracheal Collapse and Bronchomalacia in Dogs: 58 Cases (7/2001–1/2008). J. Vet. Intern. Med. 2010, 24, 298–305. [Google Scholar] [CrossRef]
- Kim, M.-R.; Kim, S.-H.; Ryu, M.-O.; Youn, H.-Y.; Choi, J.-H.; Seo, K.-W. A Retrospective Study of Tracheal Collapse in Small-Breed Dogs: 110 Cases (2022–2024). Front. Vet. Sci. 2024, 11, 1448249. [Google Scholar] [CrossRef]
- Redondo, J.I.; Suesta, P.; Serra, I.; Soler, C.; Soler, G.; Gil, L.; Gómez-Villamandos, R.J. Retrospective Study of the Prevalence of Postanaesthetic Hypothermia in Dogs. Vet. Rec. 2012, 171, 374. [Google Scholar] [CrossRef]
- Rose, N.; Kwong, G.P.S.; Pang, D.S.J. A Clinical Audit Cycle of Post-operative Hypothermia in Dogs. J. Small Anim. Pract. 2016, 57, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Glickman, L.T.; Glickman, N.W.; Schellenberg, D.B.; Raghavan, M.; Lee, T. Non-Dietary Risk Factors for Gastric Dilatation-Volvulus in Large and Giant Breed Dogs. J. Am. Vet. Med. Assoc. 2000, 217, 1492–1499. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Case, J.; Boag, A.K.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Gastric Dilation-volvulus in Dogs Attending UK Emergency-care Veterinary Practices: Prevalence, Risk Factors and Survival. J. Small Anim. Pract. 2017, 58, 629–638. [Google Scholar] [CrossRef]
- Ladlow, J.; Liu, N.; Kalmar, L.; Sargan, D. Brachycephalic Obstructive Airway Syndrome. Vet. Rec. 2018, 182, 375–378. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Jackson, C.; Guy, J.H.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Epidemiological Associations between Brachycephaly and Upper Respiratory Tract Disorders in Dogs Attending Veterinary Practices in England. Canine Genet. Epidemiol. 2015, 2, 10. [Google Scholar] [CrossRef]
- Mealey, K.L.; Bentjen, S.A.; Gay, J.M.; Cantor, G.H. Ivermectin Sensitivity in Collies Is Associated with a Deletion Mutation of the Mdr1 Gene. Pharmacogenetics 2001, 11, 727–733. [Google Scholar] [CrossRef]
- Neff, M.W.; Robertson, K.R.; Wong, A.K.; Safra, N.; Broman, K.W.; Slatkin, M.; Mealey, K.L.; Pedersen, N.C. Breed Distribution and History of Canine Mdr1-1Δ, a Pharmacogenetic Mutation That Marks the Emergence of Breeds from the Collie Lineage. Proc. Natl. Acad. Sci. USA 2004, 101, 11725–11730. [Google Scholar] [CrossRef]
- Geyer, J.; Döring, B.; Godoy, J.R.; Leidolf, R.; Moritz, A.; Petzinger, E. Frequency of the Nt230 (Del4) MDR1 Mutation in Collies and Related Dog Breeds in Germany. J. Vet. Pharmacol. Ther. 2005, 28, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Gramer, I.; Leidolf, R.; Döring, B.; Klintzsch, S.; Krämer, E.-M.; Yalcin, E.; Petzinger, E.; Geyer, J. Breed Distribution of the Nt230(Del4) MDR1 Mutation in Dogs. Vet. J. 2011, 189, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Monobe, M.M.; Junior, J.P.A.; Lunsford, K.V.; Silva, R.C.; Bulla, C. Frequency of the MDR1 Mutant Allele Associated with Multidrug Sensitivity in Dogs from Brazil. Vet. Med. Res. Rep. 2015, 6, 111–117. [Google Scholar] [CrossRef]
- Marelli, S.P.; Polli, M.; Frattini, S.; Cortellari, M.; Rizzi, R.; Crepaldi, P. Genotypic and Allelic Frequencies of MDR1 Gene in Dogs in Italy. Vet. Rec. Open 2020, 7, e000375. [Google Scholar] [CrossRef]
- Beckers, E.; Casselman, I.; Soudant, E.; Daminet, S.; Paepe, D.; Peelman, L.; Broeckx, B.J.G. The Prevalence of the ABCB1-1Δ Variant in a Clinical Veterinary Setting: The Risk of Not Genotyping. PLoS ONE 2022, 17, e0273706. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, D.; Hill, K.E.; Mealey, K.L.; Chambers, J.P.; Gieseg, M.A. The Effect of the Canine ABCB1-1Δ Mutation on Sedation after Intravenous Administration of Acepromazine. J. Vet. Intern. Med. 2016, 30, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Magaña, E.Z.; Otero, P.E.; Viscasillas, J.; Martínez-Taboada, F.; Doménech, L.; Redondo, J.I. Inter-Observer Agreement in Classifying Anesthetic Deaths in Cats and Dogs. BMC Vet. Res. 2025, 21, 121. [Google Scholar] [CrossRef] [PubMed]
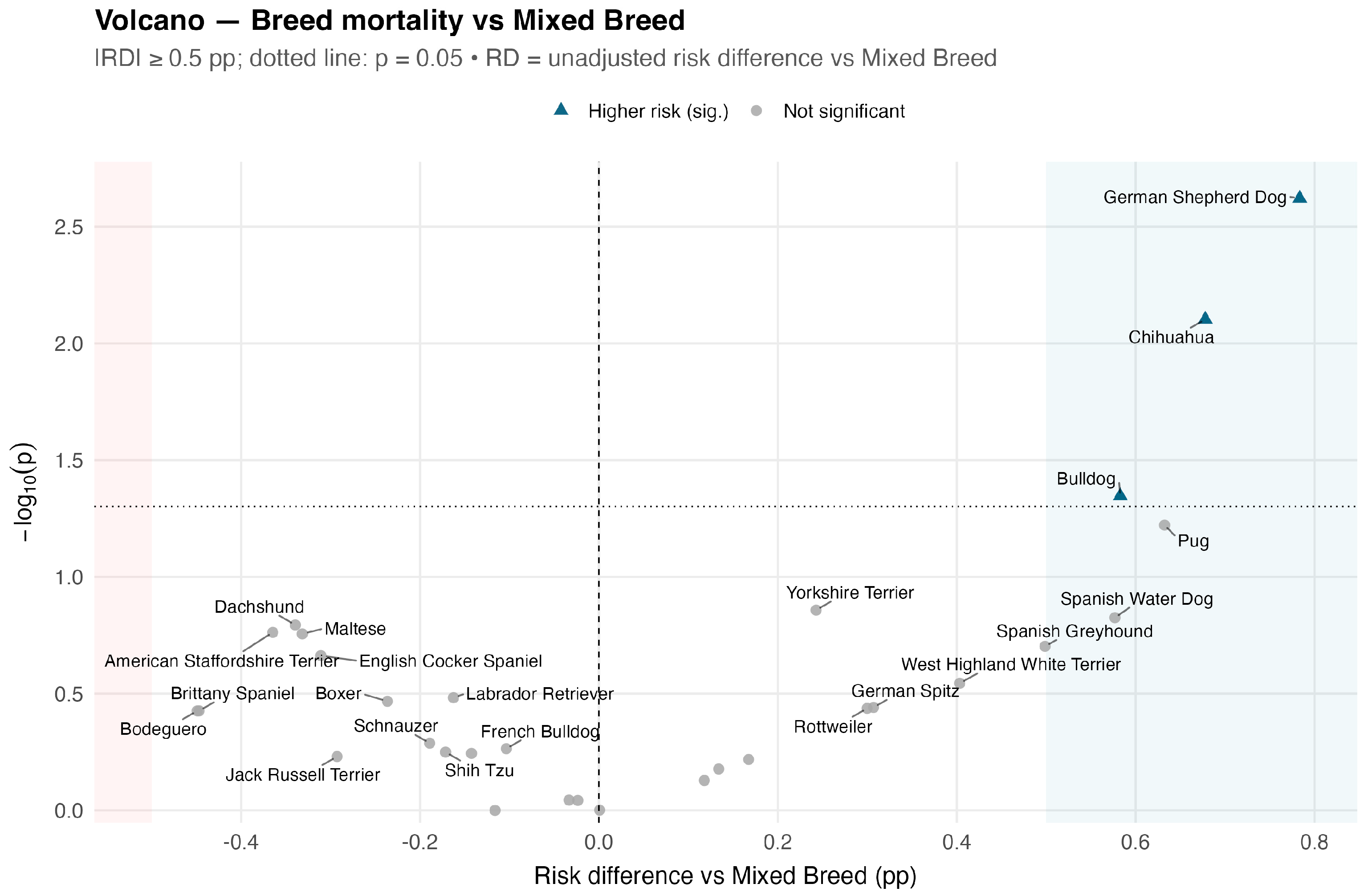


| Breed | Total | Deaths | Unadj. Mortality (%) | 95% CI | p (Unadj.) | Adj. RR (ASA) | Adj. RR 95% CI | p (Adj., ASA) |
|---|---|---|---|---|---|---|---|---|
| Mixed Breed | 16,129 | 109 | 0.68 | 0.56–0.81 | - | 1.00 | - | - |
| Yorkshire Terrier | 3157 | 29 | 0.92 | 0.64–1.32 | 0.139 | 1.24 | 0.84–1.84 | 0.286 |
| Labrador Retriever | 2728 | 14 | 0.51 | 0.31–0.86 | 0.329 | 0.64 | 0.37–1.12 | 0.116 |
| French Bulldog | 2621 | 15 | 0.57 | 0.35–0.94 | 0.544 | 0.85 | 0.50–1.46 | 0.556 |
| Poodle | 1840 | 12 | 0.65 | 0.37–1.14 | 0.907 | 1.01 | 0.56–1.82 | 0.984 |
| Golden Retriever | 1478 | 10 | 0.68 | 0.37–1.24 | 0.997 | 0.85 | 0.45–1.60 | 0.604 |
| Dachshund | 1189 | 4 | 0.34 | 0.13–0.86 | 0.161 | 0.55 | 0.21–1.45 | 0.229 |
| Chihuahua | 1182 | 16 | 1.35 | 0.83–2.19 | 0.008 | 1.80 | 1.08–2.99 | 0.023 |
| German Shepherd Dog | 1165 | 17 | 1.46 | 0.91–2.32 | 0.002 | 1.47 | 0.88–2.44 | 0.139 |
| Maltese | 1162 | 4 | 0.34 | 0.13–0.88 | 0.175 | 0.59 | 0.22–1.59 | 0.300 |
| Boxer | 1138 | 5 | 0.44 | 0.19–1.02 | 0.341 | 0.49 | 0.20–1.20 | 0.119 |
| Beagle | 1125 | 6 | 0.53 | 0.24–1.16 | 0.570 | 0.89 | 0.40–1.96 | 0.770 |
| English Cocker Spaniel | 1096 | 4 | 0.36 | 0.14–0.93 | 0.217 | 0.50 | 0.19–1.35 | 0.172 |
| American Staffordshire Terrier | 964 | 3 | 0.31 | 0.11–0.91 | 0.173 | 0.49 | 0.16–1.47 | 0.201 |
| Border Collie | 934 | 6 | 0.64 | 0.29–1.39 | 0.903 | 1.04 | 0.46–2.37 | 0.928 |
| Bulldog | 874 | 11 | 1.26 | 0.70–2.24 | 0.045 | 1.51 | 0.83–2.76 | 0.177 |
| Schnauzer | 822 | 4 | 0.49 | 0.19–1.24 | 0.516 | 0.59 | 0.22–1.59 | 0.294 |
| Shih Tzu | 793 | 4 | 0.50 | 0.20–1.29 | 0.563 | 0.70 | 0.26–1.92 | 0.492 |
| American Pit Bull Terrier | 741 | 6 | 0.81 | 0.37–1.76 | 0.665 | 1.12 | 0.50–2.54 | 0.782 |
| Pug | 688 | 9 | 1.31 | 0.69–2.47 | 0.060 | 1.85 | 0.94–3.63 | 0.074 |
| Spanish Greyhound | 596 | 7 | 1.17 | 0.57–2.40 | 0.198 | 2.01 | 1.00–4.06 | 0.051 |
| Ibizan Hound | 593 | 5 | 0.84 | 0.36–1.96 | 0.605 | 1.48 | 0.63–3.48 | 0.368 |
| West Highland White Terrier | 556 | 6 | 1.08 | 0.50–2.33 | 0.286 | 1.51 | 0.71–3.23 | 0.289 |
| Miniature Pinscher | 536 | 3 | 0.56 | 0.19–1.63 | 1.000 | 0.94 | 0.31–2.86 | 0.907 |
| Jack Russell Terrier | 522 | 2 | 0.38 | 0.11–1.39 | 0.588 | 0.66 | 0.17–2.56 | 0.543 |
| Spanish Water Dog | 479 | 6 | 1.25 | 0.58–2.71 | 0.150 | 2.72 | 1.23–6.01 | 0.013 |
| Bodeguero | 441 | 1 | 0.23 | 0.04–1.27 | 0.375 | 0.39 | 0.05–2.88 | 0.358 |
| Brittany Spaniel | 437 | 1 | 0.23 | 0.04–1.28 | 0.375 | 0.28 | 0.04–2.04 | 0.208 |
| Rottweiler | 410 | 4 | 0.98 | 0.38–2.48 | 0.366 | 1.09 | 0.40–2.94 | 0.871 |
| German Spitz | 407 | 4 | 0.98 | 0.38–2.50 | 0.362 | 1.34 | 0.50–3.59 | 0.562 |
| Group | Total | Deaths | Mortality (%) | 95% CI | p (Unadj.) | Adj. RR (ASA) | Adj. RR 95% CI | p (Adj, ASA) |
|---|---|---|---|---|---|---|---|---|
| Mixed Breed | 16,129 | 109 | 0.68 | 0.56–0.81 | - | 1.00 | - | - |
| Companion and Toy Dogs | 9278 | 65 | 0.70 | 0.55–0.89 | 0.818 | 1.02 | 0.76–1.38 | 0.878 |
| Terriers | 7077 | 52 | 0.73 | 0.56–0.96 | 0.618 | 1.04 | 0.76–1.43 | 0.806 |
| Pinscher and Schnauzer—Molossoid and Swiss Mountain and Cattle Dogs | 6172 | 45 | 0.73 | 0.55–0.97 | 0.667 | 0.85 | 0.60–1.19 | 0.347 |
| Retrievers—Flushing Dogs—Water Dogs | 5883 | 35 | 0.59 | 0.43–0.83 | 0.510 | 0.78 | 0.53–1.13 | 0.188 |
| Sheepdogs and Cattle Dogs (Except Swiss Cattle Dogs) | 3109 | 31 | 1.00 | 0.70–1.41 | 0.054 | 1.24 | 0.84–1.83 | 0.286 |
| Spitz and Primitive Types | 1748 | 14 | 0.80 | 0.48–1.34 | 0.548 | 1.14 | 0.67–1.93 | 0.633 |
| Scent Hounds and Related Breeds | 1636 | 8 | 0.49 | 0.25–0.96 | 0.373 | 0.71 | 0.35–1.43 | 0.333 |
| Pointing Dogs | 1525 | 8 | 0.52 | 0.27–1.03 | 0.487 | 0.59 | 0.29–1.21 | 0.149 |
| Dachshunds | 1189 | 4 | 0.34 | 0.13–0.86 | 0.161 | 0.55 | 0.21–1.45 | 0.224 |
| Sighthounds | 796 | 7 | 0.88 | 0.43–1.80 | 0.497 | 1.48 | 0.72–3.03 | 0.288 |
| Total (all groups) | 54,542 | 378 | 0.69 | 0.62–0.76 | - | - | - | - |
| Section | Total | Deaths | Mortality (%) | 95% CI | p (Unadj.) | Adj. RR (ASA) | Adj. RR 95% CI | p (Adj, ASA) |
|---|---|---|---|---|---|---|---|---|
| Mixed Breed | 16,129 | 109 | 0.68 | 0.56–0.81 | 1.00 | |||
| Molossian Type | 4299 | 31 | 0.72 | 0.51–1.02 | 0.749 | 0.81 | 0.55–1.19 | 0.283 |
| Retrievers | 4236 | 24 | 0.57 | 0.38–0.84 | 0.432 | 0.71 | 0.46–1.10 | 0.122 |
| Small Molossian Type Dogs | 3412 | 25 | 0.73 | 0.50–1.08 | 0.714 | 1.08 | 0.70–1.66 | 0.738 |
| Toy Terriers | 3159 | 29 | 0.92 | 0.64–1.32 | 0.140 | 1.23 | 0.83–1.83 | 0.295 |
| Sheepdogs | 3043 | 30 | 0.99 | 0.69–1.40 | 0.064 | 1.22 | 0.82–1.81 | 0.334 |
| Bull Type Terriers | 2034 | 13 | 0.64 | 0.37–1.09 | 0.849 | 0.88 | 0.50–1.54 | 0.652 |
| Poodle | 1840 | 12 | 0.65 | 0.37–1.14 | 0.907 | 1.00 | 0.55–1.81 | 0.997 |
| Pinscher and Schnauzer Type | 1606 | 11 | 0.68 | 0.38–1.22 | 0.966 | 0.88 | 0.48–1.61 | 0.680 |
| Bichons and Related Breeds | 1529 | 5 | 0.33 | 0.14–0.76 | 0.104 | 0.53 | 0.22–1.28 | 0.157 |
| Scent Hounds | 1366 | 7 | 0.51 | 0.25–1.05 | 0.475 | 0.79 | 0.38–1.67 | 0.539 |
| Dachshunds | 1189 | 4 | 0.34 | 0.13–0.86 | 0.161 | 0.55 | 0.21–1.45 | 0.224 |
| Chihuahueno | 1182 | 16 | 1.35 | 0.83–2.19 | 0.008 | 1.80 | 1.08–2.99 | 0.024 |
| Flushing Dogs | 1158 | 5 | 0.43 | 0.18–1.01 | 0.322 | 0.58 | 0.24–1.40 | 0.224 |
| Small Sized Terriers | 1156 | 8 | 0.69 | 0.35–1.36 | 0.948 | 1.05 | 0.54–2.06 | 0.882 |
| Continental Pointing Dogs | 985 | 6 | 0.61 | 0.28–1.32 | 0.804 | 0.65 | 0.29–1.49 | 0.311 |
| Tibetan Breeds | 908 | 5 | 0.55 | 0.24–1.28 | 0.653 | 0.79 | 0.32–1.94 | 0.605 |
| Short-Haired Sighthounds | 746 | 7 | 0.94 | 0.46–1.92 | 0.396 | 1.60 | 0.78–3.28 | 0.196 |
| Large and Medium Sized Terriers | 728 | 2 | 0.27 | 0.08–1.00 | 0.244 | 0.49 | 0.12–1.99 | 0.318 |
| Primitive Type—Hunting Dogs | 601 | 6 | 1.00 | 0.46–2.16 | 0.311 | 1.63 | 0.77–3.45 | 0.205 |
| British and Irish Pointers and Setters | 540 | 2 | 0.37 | 0.10–1.34 | 0.589 | 0.46 | 0.11–1.87 | 0.275 |
| Water Dogs | 489 | 6 | 1.23 | 0.56–2.65 | 0.156 | 2.42 | 1.07–5.46 | 0.033 |
| Nordic Sledge Dogs | 415 | 1 | 0.24 | 0.04–1.35 | 0.531 | 0.31 | 0.04–2.27 | 0.249 |
| European Spitz | 408 | 4 | 0.98 | 0.38–2.49 | 0.363 | 1.33 | 0.50–3.56 | 0.570 |
| Asian Spitz and Related Breeds | 299 | 3 | 1.00 | 0.34–2.91 | 0.461 | 1.31 | 0.44–3.90 | 0.627 |
| Related Breeds | 269 | 1 | 0.37 | 0.07–2.08 | 1.000 | 0.40 | 0.05–2.96 | 0.373 |
| Swiss Mountain- and Cattle Dogs | 267 | 3 | 1.12 | 0.38–3.25 | 0.433 | 1.45 | 0.48–4.40 | 0.515 |
| English Toy Spaniels | 235 | 2 | 0.85 | 0.23–3.05 | 0.675 | 0.83 | 0.20–3.41 | 0.792 |
| Japan Chin and Pekingese | 70 | 0 | 0.00 | 0.00–5.20 | 1.000 | 0.00 | 0.00–0.00 | <0.001 |
| Cattle Dogs (Except Swiss Cattle Dogs) | 66 | 1 | 1.52 | 0.27–8.10 | 0.363 | 2.51 | 0.33–19.13 | 0.375 |
| Continental Toy Spaniel and Others | 58 | 0 | 0.00 | 0.00–6.21 | 1.000 | 0.00 | 0.00–0.00 | <0.001 |
| Long-Haired or Fringed Sighthounds | 37 | 0 | 0.00 | 0.00–9.41 | 1.000 | 0.00 | 0.00–0.00 | <0.001 |
| Hairless Dogs | 22 | 0 | 0.00 | 0.00–14.87 | 1.000 | 0.00 | 0.00–0.00 | <0.001 |
| Small Belgian Dogs | 22 | 0 | 0.00 | 0.00–14.87 | 1.000 | 0.00 | 0.00–0.00 | <0.001 |
| Rough-Haired Sighthounds | 13 | 0 | 0.00 | 0.00–22.81 | 1.000 | 0.00 | 0.00–0.00 | <0.001 |
| Nordic Watchdogs and Herders | 10 | 0 | 0.00 | 0.00–27.75 | 1.000 | 0.00 | 0.00–0.00 | <0.001 |
| Primitive Type | 10 | 0 | 0.00 | 0.00–27.75 | 1.000 | 0.00 | 0.00–0.00 | <0.001 |
| Nordic Hunting Dogs | 5 | 0 | 0.00 | 0.00–43.45 | 1.000 | 0.00 | 0.00–0.00 | <0.001 |
| Leash (Scent) Hounds | 1 | 0 | 0.00 | 0.00–79.35 | 1.000 | 0.00 | 0.00–Inf | 1.000 |
| Total (all groups) | 54,542 | 378 | 0.69 | 0.62–0.76 | - | - | - | - |
| Breed | Total | Deaths | Unadjusted Mortality (%) | 95% CI | p (Unadjusted) | Adj. RR (ASA) | Adj. RR 95% CI | p (Adj, ASA) |
|---|---|---|---|---|---|---|---|---|
| Non-brachycephalic | 39,971 | 259 | 0.65 | 0.57–0.73 | - | 1.00 | - | - |
| Brachycephalic | 14,571 | 119 | 0.82 | 0.68–0.98 | 0.036 | 1.19 | 0.96–1.47 | 0.112 |
| Non-MDR1 | 53,130 | 368 | 0.69 | 0.63–0.77 | 1.00 | |||
| MDR1 | 1412 | 10 | 0.71 | 0.39–1.30 | 0.944 | 1.14 | 0.61–2.14 | 0.682 |
| Total (all groups) | 54,542 | 378 | 0.69 | 0.62–0.76 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redondo, J.I.; Martínez-Taboada, F.; Hernández-Magaña, E.Z.; Domenech, L.; Viscasillas, J.; Otero, P.E. Breed-Specific Anaesthetic Mortality in Dogs: Evidence from an Analysis of 55,019 Cases. Animals 2025, 15, 3112. https://doi.org/10.3390/ani15213112
Redondo JI, Martínez-Taboada F, Hernández-Magaña EZ, Domenech L, Viscasillas J, Otero PE. Breed-Specific Anaesthetic Mortality in Dogs: Evidence from an Analysis of 55,019 Cases. Animals. 2025; 15(21):3112. https://doi.org/10.3390/ani15213112
Chicago/Turabian StyleRedondo, José I., Fernando Martínez-Taboada, Eva Zoe Hernández-Magaña, Luis Domenech, Jaime Viscasillas, and Pablo E. Otero. 2025. "Breed-Specific Anaesthetic Mortality in Dogs: Evidence from an Analysis of 55,019 Cases" Animals 15, no. 21: 3112. https://doi.org/10.3390/ani15213112
APA StyleRedondo, J. I., Martínez-Taboada, F., Hernández-Magaña, E. Z., Domenech, L., Viscasillas, J., & Otero, P. E. (2025). Breed-Specific Anaesthetic Mortality in Dogs: Evidence from an Analysis of 55,019 Cases. Animals, 15(21), 3112. https://doi.org/10.3390/ani15213112





