Effects of High-Frequency Electrical Stunning Current Intensities on Pre-Slaughter Stunning Stress and Meat Lipid Oxidation in Geese
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Geese and Environment
2.2. Electrical Stunning System
2.3. Grouping, Stunning Parameters, and Slaughter
2.4. Sampling
2.5. Serum Variables
2.6. Lipid Oxidation and Antioxidant Capacity in Meat
2.7. Isolation of Total RNA
2.8. Reverse Transcription and cDNA Synthesis
2.9. Real-Time Quantitative PCR
2.10. Statistical Analysis
3. Results
3.1. Serum Variables
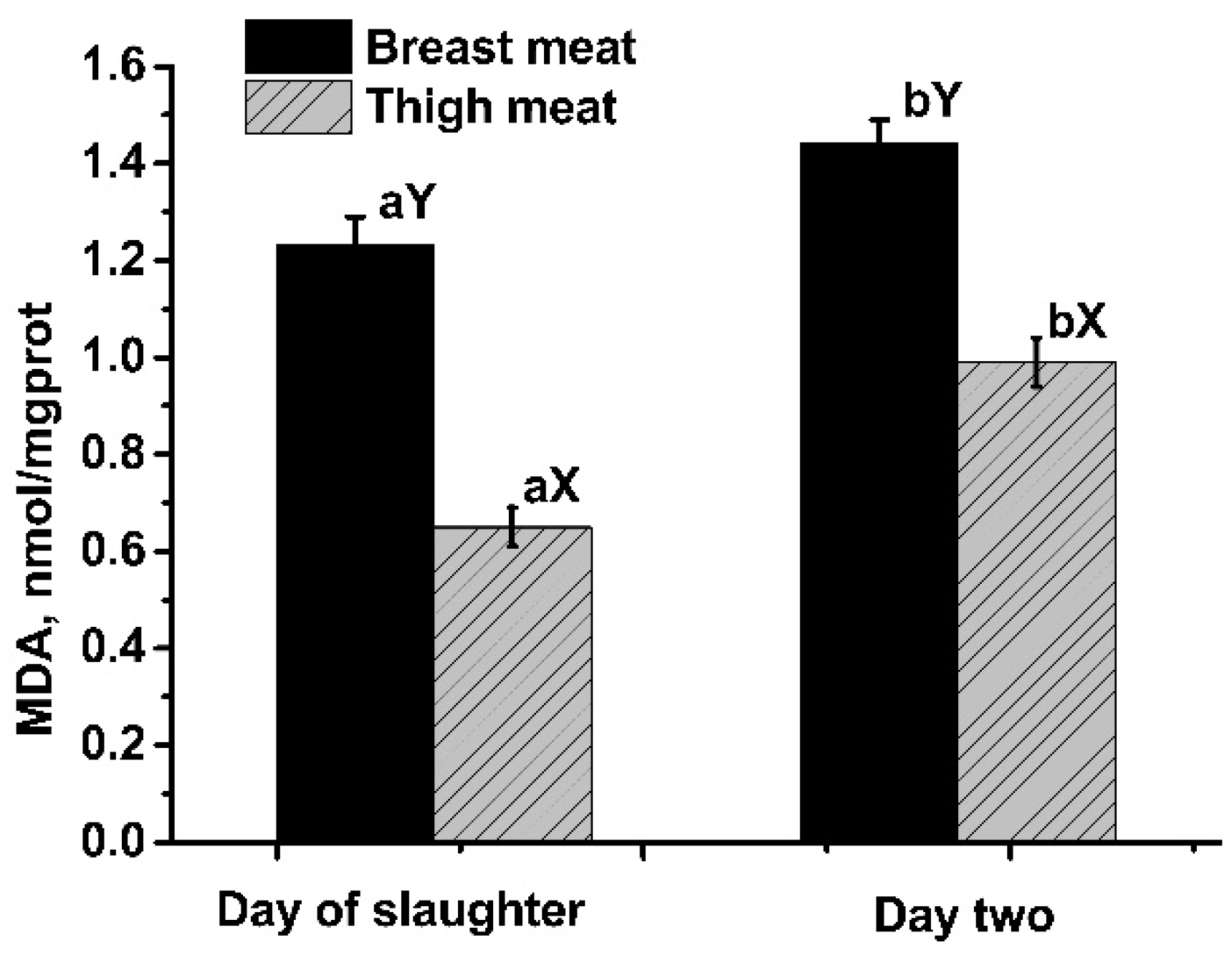
3.2. Lipid Oxidation
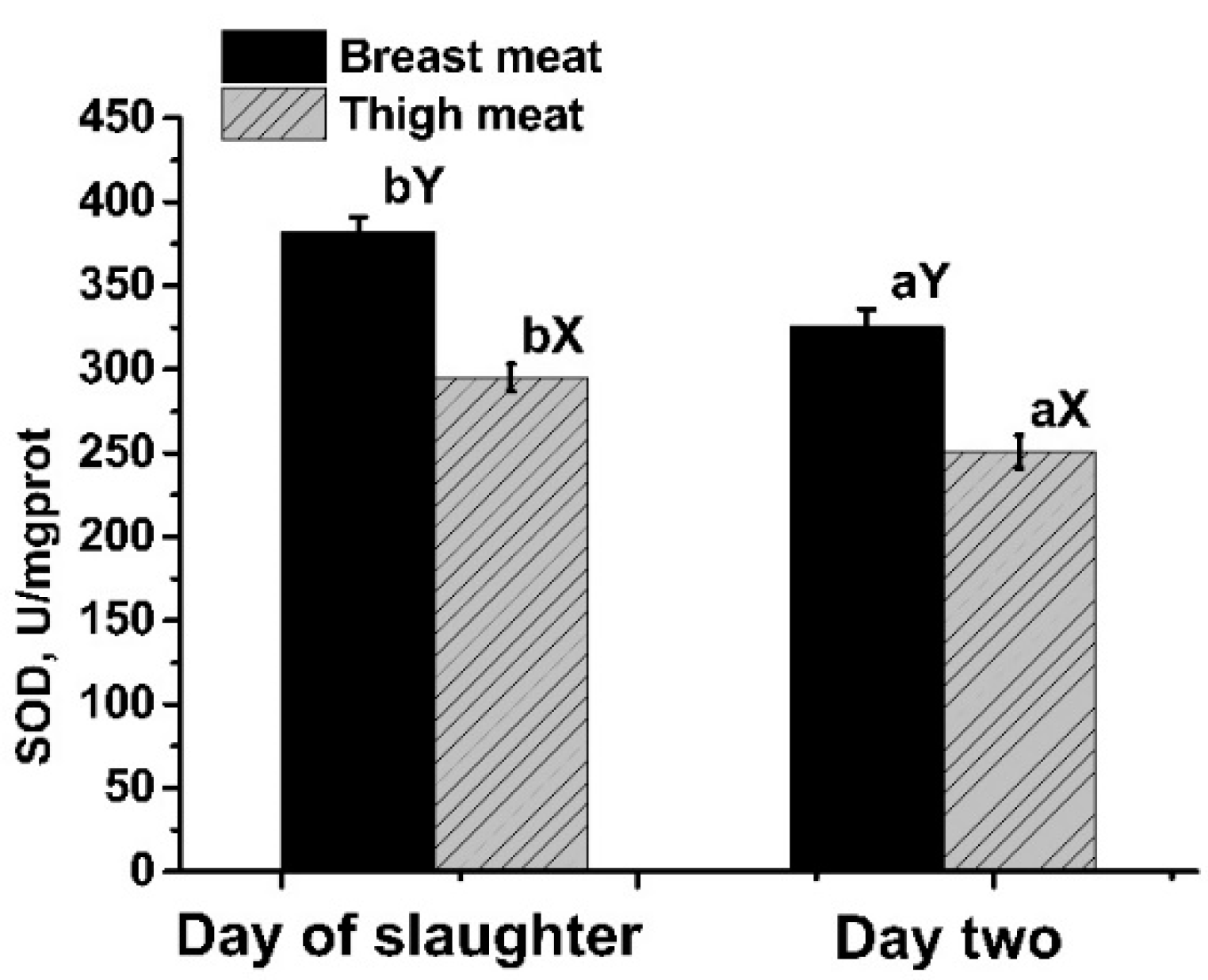
3.3. DPPH·EA and T-SOD Activity
3.4. Gene Expressions in Hypothalamus and Adrenal Gland
4. Discussion
4.1. Stunning Stress
4.2. Lipid Oxidation
4.3. Antioxidant Capacity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, X.K.; Xiao, B.; Liu, L. Economy forecast of goose industry in China from 2018 to 2020. China Poult. 2018, 11, 58–60. (In Chinese) [Google Scholar] [CrossRef]
- Nowicka, K.; Przybylski, W.; Gorska, E.; Jaworska, D.; Wolosiak, R.; Derewiaka, D. Variability in nutritional value of traditional goose meat product. Anim. Sci. Pap. Rep. 2018, 38, 405–420. [Google Scholar]
- Chen, C.; Yu, Q.; Han, L.; Zhang, J.; Guo, Z. Effects of aldehyde products of lipid oxidation on the color stability and metmyoglobin reducing ability of bovine longissimus muscle. J. Anim. Sci. 2018, 89, 810–816. [Google Scholar] [CrossRef]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Hcini, E.; Slima, A.B.; Kallel, I.; Zormati, S.; Traore, A.I.; Gdoura, R. Does supplemental zeolite (clinoptilolite) affect growth performance, meat texture, oxidative stress and production of polyunsaturated fatty acid of turkey poults? Lipids Health Dis. 2018, 17, 177. [Google Scholar] [CrossRef]
- Ying, W.; Jiang, Y.T.; Cao, J.X.; Chen, Y.J.; Gan, N. Study on lipolysis-oxidation and volatile flavour compounds of dry-cured goose with different curing salt content during production. Food Chem. 2016, 190, 33–40. [Google Scholar] [CrossRef]
- Kaban, G.; Kzlkaya, P.; Breki, B.S.; Hazar, F.Y.; Kaya, M. Microbiological properties and volatile compounds of salted-dried goose. Poult. Sci. 2020, 99, 2293–2299. [Google Scholar] [CrossRef]
- Mézes, M.; Barta, M.; Nagy, G. Comparative investigation on the effect of t-2 mycotoxin on lipid peroxidation and antioxidant status in different poultry species. Res. Vet. Sci. 1999, 66, 19–23. [Google Scholar] [CrossRef]
- Xu, L.; Wu, S.G.; Zhang, H.J.; Zhang, L.; Yue, H.Y.; Ji, F.; Qi, G.H. Comparison of lipid oxidation, messenger ribonucleic acid levels of avian uncoupling protein, avian adenine nucleotide translocator, and avian peroxisome proliferator-activated receptor-γ coactivator-1α in skeletal muscles from electrical- and gas-stunned broilers. Poult. Sci. 2011, 90, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, H.J.; Yue, H.Y.; Wu, S.G.; Yang, H.M.; Qi, G.H.; Wang, Z.Y. Low-current & high-frequency ES increased oxidative stress, lipid peroxidation, and gene transcription of the mitogen-activated protein kinase/nuclear factor-erythroid 2-related factor 2/antioxidant responsive element (MAPK/Nrf2/ARE) signaling pathway in breast muscle of broilers. Food Chem. 2018, 242, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, H.J.; Wan, X.L.; Yang, H.M.; Wang, Z.Y.; Qi, G.H.; Farnell, M. Evaluation of pre-slaughter low-current/high-frequency ES on lipid oxidative stability and antioxidant status in thigh muscle of broilers. Int. J. Food Sci. Technol. 2020, 55, 953–960. [Google Scholar] [CrossRef]
- Zhang, X.; Farnell, M.B.; Lu, Q.; Zhou, X.; Farnell, Y.Z.; Yang, H.; Wan, X.; Xu, L.; Wang, Z. Evaluation of the effects of pre-slaughter high-frequency electrical stunning current intensities on lipid oxidative stability and antioxidant capacity in the liver of yangzhou goose (Anser cygnoides domesticus). Animals 2020, 10, 311. [Google Scholar] [CrossRef]
- Berg, C.; Raj, M. A review of different stunning methods for poultry—Animal welfare aspects (stunning methods for poultry). Animals 2015, 5, 1207–1219. [Google Scholar] [CrossRef]
- Huang, J.C. Effects and Mechanism of Electrical Stunning on Meat Quality of Broilers; Nanjing Agricultural University: Nanjing, China, 2015; Available online: http://cdmd.cnki.com.cn/Article/CDMD-10307-1017044285.htm (accessed on 11 August 2021). (In Chinese)
- Xu, L.; Zhang, H.J.; Yue, H.Y.; Wu, S.G.; Yang, H.M.; Wang, Z.Y.; Qi, G.H. Gas stunning with CO2 affected meat colour, lipid peroxidation, oxidative stress, and gene expression of mitogen-activated protein kinases, glutathione s-transferases, and cu/zn-superoxide dismutase in the skeletal muscles of broilers. J. Anim. Sci. Biotechnol. 2018, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Turcsán, Z.; Varga, L.; Szigeti, J.; Turcsán, J.; Csurák, I.; Szalai, M. Effects of electrical stunning frequency and voltage combinations on the presence of engorged blood vessels in goose liver. Poult. Sci. 2003, 82, 1816–1819. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, L.; Yue, H.Y.; Wu, S.G.; Zhang, H.J.; Ji, F.; Qi, G.H. Effect of electrical stunning current and frequency on meat quality, plasma parameters, and glycolytic potential in broilers. Poult. Sci. 2011, 90, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, X.; Leprettre, S.; Dubois, J.P.; Auvergne, A.; Babile, R. The influence of current parameters during the water-bath stunning of overfed geese (Anser anser) on blood loss and on fatty liver and meat downgrading. Anim. Res. 2003, 52, 383–397. [Google Scholar] [CrossRef]
- Xu, L.; Ji, F.; Yue, H.Y.; Wu, S.G.; Zhang, H.J.; Zhang, L.; Qi, G.H. Plasma variables, meat quality, and glycolytic potential in broilers stunned with different carbon dioxide concentrations. Poult. Sci. 2011, 90, 1831–1836. [Google Scholar] [CrossRef]
- Gent, T.C.; Gebhardt-Henrich, S.; Schild, S.; Rahman, A.A.; Toscano, M.J. Evaluation of poultry stunning with low atmospheric pressure, carbon dioxide or nitrogen using a single aversion testing paradigm. Animals 2020, 10, 1308. [Google Scholar] [CrossRef]
- Wotton, S.; Grist, A.; O’Callaghan, M.; van Klink, E. Head-only stunning of turkeys part 2: Subjective and objective assessment of the application of AC and DC waveforms. Animals 2021, 11, 286. [Google Scholar] [CrossRef]
- Jacobs, L.; Bourassa, D.V.; Boyal, R.S.; Caitlin, E.; Harris, C.E.; Josselson, L.; Campbell, A.; Anderson, G.; Buhr, R.J. Animal welfare assessment of on-farm euthanasia methods for individual, heavy turkeys. Poult. Sci. 2021, 100, 100812. [Google Scholar] [CrossRef]
- Leon, J.A.D.D.; Borges, C.R. Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. J. Vis. Exp. 2020, 159, e61122. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Oyola, M.G.; Handa, R.J. Hypothalamic–pituitary–adrenal and hypothalamic–pituitary–gonadal axes: Sex differences in regulation of stress responsivity. Stress 2017, 20, 476–494. [Google Scholar] [CrossRef]
- Rivier, C.; Rivest, S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: Peripheral and central mechanisms. Biol. Reprod. 1991, 45, 523–532. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- Proudnikov, E.; Hamon, S.; Ott, J.; Kreek, M.J. Association of polymorphisms in the melanocortin receptor type 2 (mc2r, acth receptor) gene with heroin addiction. Neurosci. Lett. 2008, 435, 234–239. [Google Scholar] [CrossRef][Green Version]
- Post, J.; Rebel, J.; Huurne, A.T. Physiological effects of elevated plasma cortisol concentrations in broiler chickens. An alternative means by which to assess the physiological effects of stress. Poult. Sci. 2003, 82, 1313–1318. [Google Scholar] [CrossRef]
- Nanba, K.; Chen, A.X.; Turcu, A.F.; Rainey, W.E. H295R expression of melanocortin 2 receptor accessory protein results in ACTH responsiveness. J. Mol. Endocrinol. 2015, 56, 69–76. [Google Scholar] [CrossRef]
- Jin, W.; Ma, R.; Zhai, L.; Xu, X.; Lou, T.; Huang, Q.; Wang, J.; Zhao, D.; Li, X.; Sun, L. Ginsenoside rd attenuates ACTH-induced cortisol secretion by blocking the mc2r-cAMP/PKA/CREB pathway in y1 mouse adrenocortical cells. Life Sci. 2020, 245, 117337. [Google Scholar] [CrossRef]
- Goutas, A.; Papathanasiou, I.; Mourmoura, E.; Tsesmelis, K.; Trachana, V. Oxidative stress response is mediated by overexpression and spatiotemporal regulation of caveolin-1. Antioxidants 2020, 9, 766. [Google Scholar] [CrossRef]
- Huang, J.C.; Yang, J.; Huang, M.; Chen, K.J.; Xu, X.L.; Zhou, G.H. The effects of electrical stunning voltage on meat quality, plasma parameters, and protein solubility of broiler breast meat. Poult. Sci. 2017, 96, 764–769. [Google Scholar] [CrossRef]
- Tang, S.; Yin, B.; Xu, J.; Bao, E. Rosemary reduces heat stress by inducing CRYAB and HSP70 expression in broiler chickens. Oxidative Med. Cell Longev. 2018, 2018, 7014126. [Google Scholar] [CrossRef]
- Montoya Urrea, V.; Bridi, A.M.; Ceballos, M.C.; Paranhos da Costa, M.J.R.; Faucitano, L. Behavior, blood stress indicators, skin lesions and meat quality in pigs transported to slaughter at different loading densities. J. Anim. Sci. 2021, 99, skab119. [Google Scholar] [CrossRef]
- Yasuda, J.; Whitmarsh, A.J.; Cavanagh, J.; Sharma, M.; Davis, R.J. The JIP group of mitogen-activated protein kinase scaffold proteins. Mol. Cell. Biol. 1999, 19, 7245–7254. [Google Scholar] [CrossRef]
- Nogueiras, R.; Sabio, G. Brain JNK and metabolic disease. Diabetologia 2021, 64, 265–274. [Google Scholar] [CrossRef]
- Xu, B.Y.; Tang, X.D.; Chen, J.; Wu, H.B.; Chen, W.S.; Chen, L. Rifampicin induces clathrin-dependent endocytosis and ubiquitin-proteasome degradation of MRP2 via oxidative stress-activated PKC-ERK/JNK/p38 and PI3K signaling pathways in HepG2 cells. Acta Pharmacol. Sin. 2020, 41, 56–64. [Google Scholar] [CrossRef]
- Council Regulation. Council Regulation (EC) No. 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of Killing. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:sa0002 (accessed on 15 May 2021).
- Busquets, O.; Ettcheto, M.; Cano, A.; Manzine, P.R.; Camins, A. Role of c-jun n-terminal kinases (JNKS) in epilepsy and metabolic cognitive impairment. Int. J. Mol. Sci. 2019, 21, 255. [Google Scholar] [CrossRef] [PubMed]
- Uhlířová, L.; Tůmová, E.; Chodová, D.; Volek, Z.; Machander, V. Fatty acid composition of goose meat depending on genotype and sex. Asian-Australas. J. Anim. Sci. 2019, 32, 137–143. [Google Scholar] [CrossRef]
- Donsbough, A.; Powell, L.; Waguespack, S.; Bidner, T.D.; Southern, L.L. Uric acid, urea, and ammonia concentrations in serum and uric acid concentration in excreta as indicators of amino acid utilization in diets for broilers. Poult. Sci. 2010, 89, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, T.; Liang, Y.; Wang, J.; Gao, Q.; Liu, Y.; Wang, B.; Wang, Y.; Zhao, X.; Zhao, Y.; et al. Comparison of growth, slaughter performance and meat quality of Yangzhou meat geese and their crossbred combinations. J. Yangzhou Univ. (Agric. Life Sci. Ed.) 2016, 37, 58–64. (In Chinese) [Google Scholar] [CrossRef]
- Scope, A.; Schwendenwein, I. Laboratory evaluation of renal function in birds. Vet. Clin. N. Am. Exot. Anim. Pract. 2020, 23, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Kand’ár, R.; Štramová, X.; Drábková, P.; Křenková, J. A monitoring of allantoin, uric acid, and malondialdehyde levels in plasma and erythrocytes after ten minutes of running activity. Physiol. Res. 2014, 63, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yue, H.Y.; Wu, S.G.; Zhang, H.J.; Qi, G.H. Comparison of blood variables, fiber intensity, and muscle metabolites in hot-boned muscles from electrical- and gas-stunned broilers. Poult. Sci. 2011, 90, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the cuzn-sod (SOD1), mn-sod (SOD2), and ec-sod (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
| RNA 1 | Sense | Antisense | Annealing (°C) | Product Size (bp) | GenBank Number |
|---|---|---|---|---|---|
| JNK1 (MAPK8) | ACAGGGGATAGTATGTGCAGCTT | ATGGTCGGCTTAGCTTCTTGAT | 60 °C | 74 | XM_013198744.1 |
| CAV1 | CAACGACGACGTGGTCAAGA | AGAGCCATAGGAATGCCAAAGA | 60 °C | 162 | XM_013190822.1 |
| MC2R | GACCTTCCAATCTAATCAAACACCT | GATGACAGCAATAAGGACAAGCA | 60 °C | 176 | XM_013179521.1 |
| β-actin | CAACGAGCGGTTCAGGTGT | ATGATGGAGTTGAAGGTGGTCT | 60 °C | 96 | XM_013174886.1 |
| GFP | TTGAAGATGGAAGCGTTCAACTAGC | AGTTCATCCATGCCATGTGTAATCC | 60 °C | 196 | * |
| Items 1 | Stunning Methods 2 | SEM | p Values | ||||
|---|---|---|---|---|---|---|---|
| Control | E20mA | E40mA | E70mA | E100mA | |||
| ACTH, pb/mL | 27.10 ab | 28.02 ab | 24.20 a | 32.67 bc | 34.57 c | 1.01 | <0.01 |
| Cortisol, ng/mL | 20.49 | 19.60 | 19.31 | 18.48 | 20.35 | 0.49 | 0.72 |
| CK, U/L | 1347 a | 1741 a | 2081 ab | 3640 c | 2805 bc | 193.6 | <0.01 |
| Urea, mmol/L | 1.08 b | 0.82 ab | 0.90 b | 0.70 a | 0.88 ab | 0.04 | 0.05 |
| UA, umol/L | 388.0 | 269.0 | 379.5 | 324.2 | 378.6 | 15.12 | 0.06 |
| Items | Stunning Method 1 | SEM | p Values | ||||
|---|---|---|---|---|---|---|---|
| Control | E20mA | E40mA | E70mA | E100mA | |||
| Breast meat | |||||||
| MDAd0, nmol/mgprot | 1.52 | 1.16 | 1.16 | 1.16 | 1.12 | 0.06 | 0.22 |
| MDAd2, nmol/mgprot | 1.62 b | 1.50 b | 1.11 a | 1.51 b | 1.50 b | 0.05 | < 0.01 |
| Thigh meat | |||||||
| MDAd0, nmol/mgprot | 0.83 | 0.52 | 0.60 | 0.67 | 0.64 | 0.04 | 0.17 |
| MDAd2, nmol/mgprot | 1.22 | 0.99 | 0.87 | 1.01 | 0.86 | 0.05 | 0.08 |
| Items 1 | Stunning Method 2 | SEM | p Values | |||||
|---|---|---|---|---|---|---|---|---|
| Control | E20mA | E40mA | E70mA | E100mA | Groups | Linear | ||
| Breast meat | ||||||||
| DPPH·EA, μmolTE/g prot | 0.19 | 0.18 | 0.19 | 0.19 | 0.19 | <0.01 | 0.20 | 0.49 |
| T-SODd0, U/mgprot | 389 | 371.3 | 403.3 | 375.8 | 371.3 | 8.49 | 0.74 | 0.62 |
| T-SODd2, U/mgprot | 360.2 b | 352.9 b | 338.4 ab | 298.2 ab | 277.4 a | 10.62 | <0.01 | <0.01 |
| Thigh meat | ||||||||
| DPPH·EA, μmolTE/g prot | 0.21 b | 0.21 b | 0.19 a | 0.19 a | 0.19 a | <0.01 | 0.02 | <0.01 |
| T-SODd0, U/mgprot | 360.22 b | 352.94 b | 338.35 ab | 298.16 ab | 277.43 a | 10.62 | 0.05 | <0.01 |
| T-SODd2, U/mgprot | 280.1 | 266.1 | 221.2 | 251.2 | 236.4 | 10.03 | 0.38 | 0.18 |
| Items 1 | Stunning Methods 2 | SEM | p Values | ||
|---|---|---|---|---|---|
| Control | E40mA | E100mA | |||
| JNK1 | 1.00 | 0.70 | 0.90 | 0.06 | 0.08 |
| CAV1 | 1.07 | 0.87 | 0.81 | 0.09 | 0.45 |
| MC2R | 0.93 | 0.76 | 0.70 | 0.05 | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Yang, H.; Wan, X.; Zhang, X.; Yang, Z.; Wang, Z. Effects of High-Frequency Electrical Stunning Current Intensities on Pre-Slaughter Stunning Stress and Meat Lipid Oxidation in Geese. Animals 2021, 11, 2376. https://doi.org/10.3390/ani11082376
Xu L, Yang H, Wan X, Zhang X, Yang Z, Wang Z. Effects of High-Frequency Electrical Stunning Current Intensities on Pre-Slaughter Stunning Stress and Meat Lipid Oxidation in Geese. Animals. 2021; 11(8):2376. https://doi.org/10.3390/ani11082376
Chicago/Turabian StyleXu, Lei, Haiming Yang, Xiaoli Wan, Xin Zhang, Zhi Yang, and Zhiyue Wang. 2021. "Effects of High-Frequency Electrical Stunning Current Intensities on Pre-Slaughter Stunning Stress and Meat Lipid Oxidation in Geese" Animals 11, no. 8: 2376. https://doi.org/10.3390/ani11082376
APA StyleXu, L., Yang, H., Wan, X., Zhang, X., Yang, Z., & Wang, Z. (2021). Effects of High-Frequency Electrical Stunning Current Intensities on Pre-Slaughter Stunning Stress and Meat Lipid Oxidation in Geese. Animals, 11(8), 2376. https://doi.org/10.3390/ani11082376







