Effect of an Insulation Device in Preventing Hypothermia during Magnetic Resonance Imaging Examinations for Dogs and Cats under General Anesthesia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
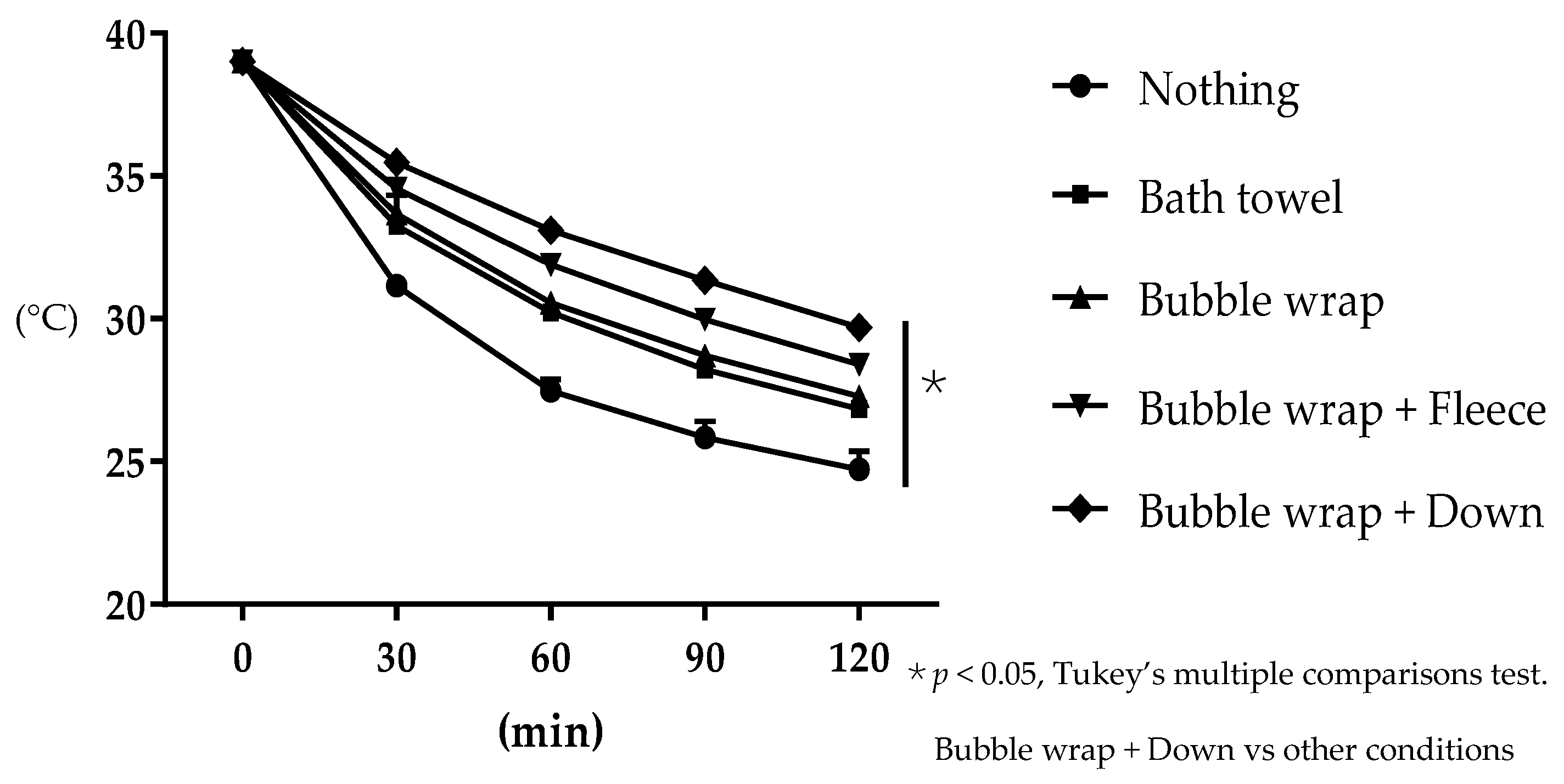
2.1. Preliminary Study—Examination of Heat Insulating Materials Effective in Preventing Hypothermia
2.2. Examination of the Effect of Using a Heat Insulator to Prevent a Decrease in Body Temperature during MRI Exams in Dogs and Cats
2.3. Statistical Analyses
3. Results
3.1. Evaluation of the Effectiveness of the Heat Insulating Materials in Preventing Water Temperature Loss
3.2. Examination of the Heat Insulating Device Effectivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, K.C.; Tamburello, K.R.; Hardie, R.J. Peri-operative Morbidity Associated with Ovariohysterectomy Performed as Part of a Third-Year Veterinary Surgical-Training Program. J. Veter. Med. Educ. 2011, 38, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Redondo, J.I.; Suesta, P.; Serra, I.; Soler, C.; Soler, G.; Gil, L.; Villamandos, R.J.G. Retrospective study of the prevalence of postanaesthetic hypothermia in dogs. Veter. Rec. 2012, 171, 374. [Google Scholar] [CrossRef]
- Redondo, J.I.; Suesta, P.; Gil, L.; Soler, G.; Serra, I.; Soler, C. Retrospective study of the prevalence of postanaesthetic hypothermia in cats. Veter. Rec. 2012, 170, 206. [Google Scholar] [CrossRef]
- Rose, N.; Kwong, G.P.S.; Pang, D.S.J. A clinical audit cycle of post-operative hypothermia in dogs. J. Small Anim. Pr. 2016, 57, 447–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pottie, R.; Dart, C.; Perkins, N. Hodgson Effect of hypothermia on recovery from general anaesthesia in the dog. Aust. Veter. J. 2007, 85, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.R.; Roberts, B.K.; Aronsohn, M. Perioperative hypothermia. J. Veter. Emerg. Crit. Care 2005, 15, 32–37. [Google Scholar] [CrossRef]
- Thomassen, Ø.; Færevik, H.; Østerås, Ø.; Sunde, G.A.; Zakariassen, E.; Sandsund, M.; Heltne, J.K.; Brattebø, G. Comparison of three different prehospital wrapping methods for preventing hypothermia—A crossover study in humans. Scand. J. Trauma Resusc. Emerg. Med. 2011, 19, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madrid, E.; Urrútia, G.; i Figuls, M.R.; Pardo-Hernandez, H.; Campos, J.M.; Paniagua, P.; Maestre, L.; Alonso-Coello, P. Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults. Cochrane Database Syst. Rev. 2016, 4, CD009016. [Google Scholar] [CrossRef]
- A Hirvonen, E.; Niskanen, M. Thermal suits as an alternative way to keep patients warm peri-operatively: A randomised trial. Eur. J. Anaesthesiol. 2011, 28, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Vanni, S.M.D.; Castiglia, Y.M.M.; Ganem, E.M.; Júnior, G.R.R.; Amorim, R.B.; Ferrari, F.; Braz, L.G.; Braz, J.R.C. Preoperative warming combined with intraoperative skin-surface warming does not avoid hypothermia caused by spinal anesthesia in patients with midazolam premedication. Sao Paulo Med. J. 2007, 125, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Machon, R.G.; Raffe, M.R.; Robinson, E.P. Warming with a forced air warming blanket minimizes anesthetic-induced hypothermia in cats. Veter. Surg. 1999, 28, 301–310. [Google Scholar] [CrossRef] [PubMed]
- A Hale, F.; Anthony, J.M. Prevention of hypothermia in cats during routine oral hygiene procedures. Can. Veter. J. La Rev. Veter. Can. 1997, 38, 297–299. [Google Scholar]
- Haskins, S.C. Hypothermia and its prevention during general anesthesia in cats. Am. J. Veter. Res. 1981, 42, 856–861. [Google Scholar]
- Cabell, L.; Perkowski, S.; Gregor, T.; Smith, G. The Effects of Active Peripheral Skin Warming on Perioperative Hypothermia in Dogs. Veter. Surg. 1997, 26, 79–85. [Google Scholar] [CrossRef]
- Kibanda, J.O.; Gurney, M. Comparison of two methods for the management of intraoperative hypothermia in dogs. Veter. Rec. 2012, 170, 392. [Google Scholar] [CrossRef]
- Clark-Price, S.C.; Dossin, O.; Jones, K.R.; Otto, A.N.; Weng, H.-Y. Comparison of three different methods to prevent heat loss in healthy dogs undergoing 90 minutes of general anesthesia. Veter. Anaesth. Analg. 2013, 40, 280–284. [Google Scholar] [CrossRef]
- Tan, C.; Govendir, M.; Zaki, S.; Miyake, Y.; Packiarajah, P.; Malik, R. Evaluation of four warming procedures to minimise heat loss induced by anaesthesia and surgery in dogs. Aust. Veter. J. 2004, 82, 65–68. [Google Scholar] [CrossRef]
- Sakata, H.; Walsh, V.; Chambers, J.P.; Bridges, J.; Sano, H. Effect of insulation with bubble wrap and an absorbent pad on heat loss in anaesthetised cats. New Zealand Veter. J. 2020, 68, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Sessler, D.I. Mild Perioperative Hypothermia. N. Engl. J. Med. 1997, 336, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Khenissi, L.; Covey-Crump, G.; Knowles, T.; Murrell, J. Do heat and moisture exchangers in the anaesthesia breathing circuit preserve body temperature in dogs undergoing anaesthesia for magnetic resonance imaging? Veter. Anaesth. Analg. 2017, 44, 452–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banta, M.R.; Lynott, A.J.; VanSant, M.J.; Bakken, G.S. Partitioning heat loss from mallard ducklings swimming on the air–water interface. J. Exp. Biol. 2004, 207, 4551–4557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurz, A. Physiology of Thermoregulation. Best Pr. Res. Clin. Anaesthesiol. 2008, 22, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, T.; Sessler, D.; Sessler, A.M.; Schroeder, M.; Ozaki, M.; Kurz, A.; Cheng, C. Heat Flow and Distribution during Induction of General Anesthesia. Anesthesiologists 1995, 82, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Raffe, M.R.; Wright, M.; McGrath, C.J.; Crimi, A.J. Body temperature changes during general anesthesia in the dog and cat. Vet. Anesth. 1980, 11, 9–15. [Google Scholar]
- Beal, M.W.; Brown, D.C.; Shofer, F.S. The Effects of Perioperative Hypothermia and the Duration of Anesthesia on Postoperative Wound Infection Rate in Clean Wounds: A Retrospective Study. Veter. Surg. 2000, 29, 123–127. [Google Scholar] [CrossRef]
- Clark-Price, S.C.; Fischer, B.L.; Kirwin, K.L.; Keating, S.C.J.; Auckburally, A.; Flaherty, D. Multicenter study to investigate factors associated with change in rectal temperature during anesthesia in dogs. J. Am. Veter. Med. Assoc. 2021, 258, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Dhupa, N. Hypothermia in dogs and cats. Compendium 2006, 17, 265–271. [Google Scholar]
- Hill, R.C.; Scott, K.C. Energy requirements and body surface area of cats and dogs. J. Am. Veter. Med Assoc. 2004, 225, 689–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jourdan, G.; Didier, C.; Chotard, E.; Jacques, S.; Verwaerde, P. Heated intravenous fluids alone fail to prevent hypothermia in cats under general anaesthesia. J. Feline Med. Surg. 2017, 19, 1249–1253. [Google Scholar] [CrossRef]
- Melling, A.C.; Ali, B.; Scott, E.M.; Leaper, D.J. Effects of preoperative warming on the incidence of wound infection after clean surgery: A randomised controlled trial. Lancet 2001, 358, 876–880. [Google Scholar] [CrossRef]
- Wong, P.F.; Kumar, S.; Bohra, A.; Whetter, D.; Leaper, D.J. Randomized clinical trial of perioperative systemic warming in major elective abdominal surgery. BJS 2007, 94, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, C.F.; Jolliffe, C.T.; Leece, E.A. Effect of prewarming on the body temperature of small dogs undergoing inhalation anesthesia. J. Am. Veter. Med. Assoc. 2015, 247, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Aarnes, T.K.; Bednarski, R.M.; Lerche, P.; Hubbell, J.A. Effect of pre-warming on perioperative hypothermia and anesthetic recovery in small breed dogs undergoing ovariohysterectomy. Can. Veter. J. La Rev. Veter. Can. 2017, 58, 175–179. [Google Scholar]



| No. | Breed | Age (Years) | Sex | Body Weight (kg) | Clinical Signs or Diagnosis | Group |
|---|---|---|---|---|---|---|
| 1 | French Bulldog | 12 | Male | 12.6 | Idiopathic epilepsy | Control |
| 2 | Shiba Inu | 12 | Spayed female | 12.7 | Meningioma | Control |
| 3 | Miniature Dachshund | 9 | Castrated male | 5.0 | Cushing syndrome | Control |
| 4 | Chihuahua | 13 | Male | 2.2 | Intranasal adenoma | Control |
| 5 | French Bulldog | 11 | Female | 10.0 | Pituitary adenoma | Control |
| 6 | Boston Terrier | 10 | Male | 7.7 | Pituitary adenoma | Control |
| 7 | Rough Collie | 12 | Male | 24.5 | Nasal discharge/epistaxis | Control |
| 8 | Toy Poodle | 14 | Castrated male | 4.1 | Rathke’s cleft cyst | Control |
| 9 | Mix | 2 | Spayed female | 3.1 | Necrotizing meningoencephalitis | Control |
| 10 | Pomeranian | 13 | Male | 4.0 | Nasal obstruction | Control |
| 11 | Mix | 13 | Castrated male | 4.0 | Cushing syndrome | Control |
| 12 | Boston Terrier | 12 | Spayed female | 7.2 | Chemodectoma | Control |
| 13 | Mix | 5 | Spayed female | 5.2 | Intranasal lymphoma | Control |
| 14 | Miniature Schnauzer | 14 | Spayed female | 5.0 | Meningioma | Control |
| 15 | Shiba Inu | 12 | Spayed female | 14.6 | Peripheral schwannoma | Control |
| 16 | French Bulldog | 11 | Castrated male | 12.6 | Glioma | Control |
| 17 | Shiba Inu | 13 | Male | 11.4 | Change to an aggressive character | Control |
| 18 | Pekingese | 11 | Spayed female | 6.0 | Idiopathic epilepsy | Heat insulating |
| 19 | Pembroke Welsh Corgi | 12 | Male | 11.3 | Pituitary adenoma | Heat insulating |
| 20 | Labrador Retriever | 9 | Female | 35.5 | Intranasal adenocarcinoma | Heat insulating |
| 21 | Papillon | 12 | Castrated male | 3.9 | Meningioma | Heat insulating |
| 22 | Miniature Schnauzer | 12 | Spayed female | 7.0 | Meningioma | Heat insulating |
| 23 | Mix | 2 | Spayed female | 3.6 | Necrotizing meningoencephalitis | Heat insulating |
| 24 | French Bulldog | 9 | Spayed female | 10.0 | Idiopathic epilepsy | Heat insulating |
| No. | Breed | Age (Years) | Sex | Body Weight (kg) | Clinical Signs or Diagnosis | Group |
|---|---|---|---|---|---|---|
| 1 | Mix | 6 | Spayed female | 3.5 | Nystagmus | Control |
| 2 | Mix | 12 | Castrated male | 5.9 | Intranasal adenocarcinoma | Control |
| 3 | Mix | 13 | Spayed female | 5.2 | Torticollis | Control |
| 4 | Mix | 7 | Castrated male | 3.3 | Nasal discharge/epistaxis | Control |
| 5 | Mix | 3 | Castrated male | 3.5 | Intranasal adenocarcinoma | Control |
| 6 | Mix | 19 | Castrated male | 5.1 | Pituitary adenoma | Control |
| 7 | Mix | 6 | Castrated male | 4.0 | Idiopathic epilepsy | Control |
| 8 | Mix | 10 | Spayed female | 3.6 | Intranasal adenocarcinoma | Control |
| 9 | Persian | 8 | Castrated male | 3.1 | Nasal discharge/epistaxis | Control |
| 10 | Mix | 13 | Castrated male | 4.9 | Soft tissue sarcoma | Control |
| 11 | Mix | 8 | Castrated male | 5.2 | Idiopathic epilepsy | Control |
| 12 | Mix | 16 | Castrated male | 5.2 | Pituitary adenoma | Control |
| 13 | Mix | 15 | Spayed female | 2.4 | Intranasal lymphoma | Control |
| 14 | Singapura | 10 | Castrated male | 4.9 | Intranasal lymphoma | Control |
| 15 | Mix | 16 | Castrated male | 3.4 | Intranasal adenocarcinoma | Heat insulating |
| 16 | Mix | 6 | Castrated male | 5.6 | Idiopathic epilepsy | Heat insulating |
| 17 | Mix | 6 | Castrated male | 3.3 | Idiopathic epilepsy | Heat insulating |
| 18 | Scottish Fold | 18 | Spayed female | 3.9 | Intranasal lymphoma | Heat insulating |
| 19 | Mix | 15 | Spayed female | 3.6 | Torticollis | Heat insulating |
| 20 | Mix | 17 | Castrated male | 3.3 | Intranasal lymphoma | Heat insulating |
| 21 | Mix | 5 | Castrated male | 6.5 | No clinical signs | Heat insulating |
| 22 | Mix | 5 | Castrated male | 5.2 | No clinical signs | Heat insulating |
| 23 | Sphynx | 8 | Spayed female | 3.2 | Intranasal adenocarcinoma | Heat insulating |
| 24 | Mix | 6 | Castrated male | 4.8 | No clinical signs | Heat insulating |
| 25 | Mix | 5 | Castrated male | 5.0 | No clinical signs | Heat insulating |
| 26 | Mix | 5 | Spayed female | 3.7 | Intranasal lymphoma | Heat insulating |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onozawa, E.; Azakami, D.; Seki, S.; Hamamoto, Y.; Ishioka, K. Effect of an Insulation Device in Preventing Hypothermia during Magnetic Resonance Imaging Examinations for Dogs and Cats under General Anesthesia. Animals 2021, 11, 2378. https://doi.org/10.3390/ani11082378
Onozawa E, Azakami D, Seki S, Hamamoto Y, Ishioka K. Effect of an Insulation Device in Preventing Hypothermia during Magnetic Resonance Imaging Examinations for Dogs and Cats under General Anesthesia. Animals. 2021; 11(8):2378. https://doi.org/10.3390/ani11082378
Chicago/Turabian StyleOnozawa, Eri, Daigo Azakami, Seri Seki, Yuji Hamamoto, and Katsumi Ishioka. 2021. "Effect of an Insulation Device in Preventing Hypothermia during Magnetic Resonance Imaging Examinations for Dogs and Cats under General Anesthesia" Animals 11, no. 8: 2378. https://doi.org/10.3390/ani11082378
APA StyleOnozawa, E., Azakami, D., Seki, S., Hamamoto, Y., & Ishioka, K. (2021). Effect of an Insulation Device in Preventing Hypothermia during Magnetic Resonance Imaging Examinations for Dogs and Cats under General Anesthesia. Animals, 11(8), 2378. https://doi.org/10.3390/ani11082378







