Changes in Serum Thiol-Disulphide Homeostasis in Sheep with Gastrointestinal Nematodes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sampling
2.3. Faecal Examination
2.4. Analysis
2.4.1. Blood Samples
2.4.2. Thiol-Disulphide Homeostasis
2.4.3. Oxidative Status Biomarkers
2.4.4. Inflammatory Biomarkers
2.4.5. Statistical Analysis
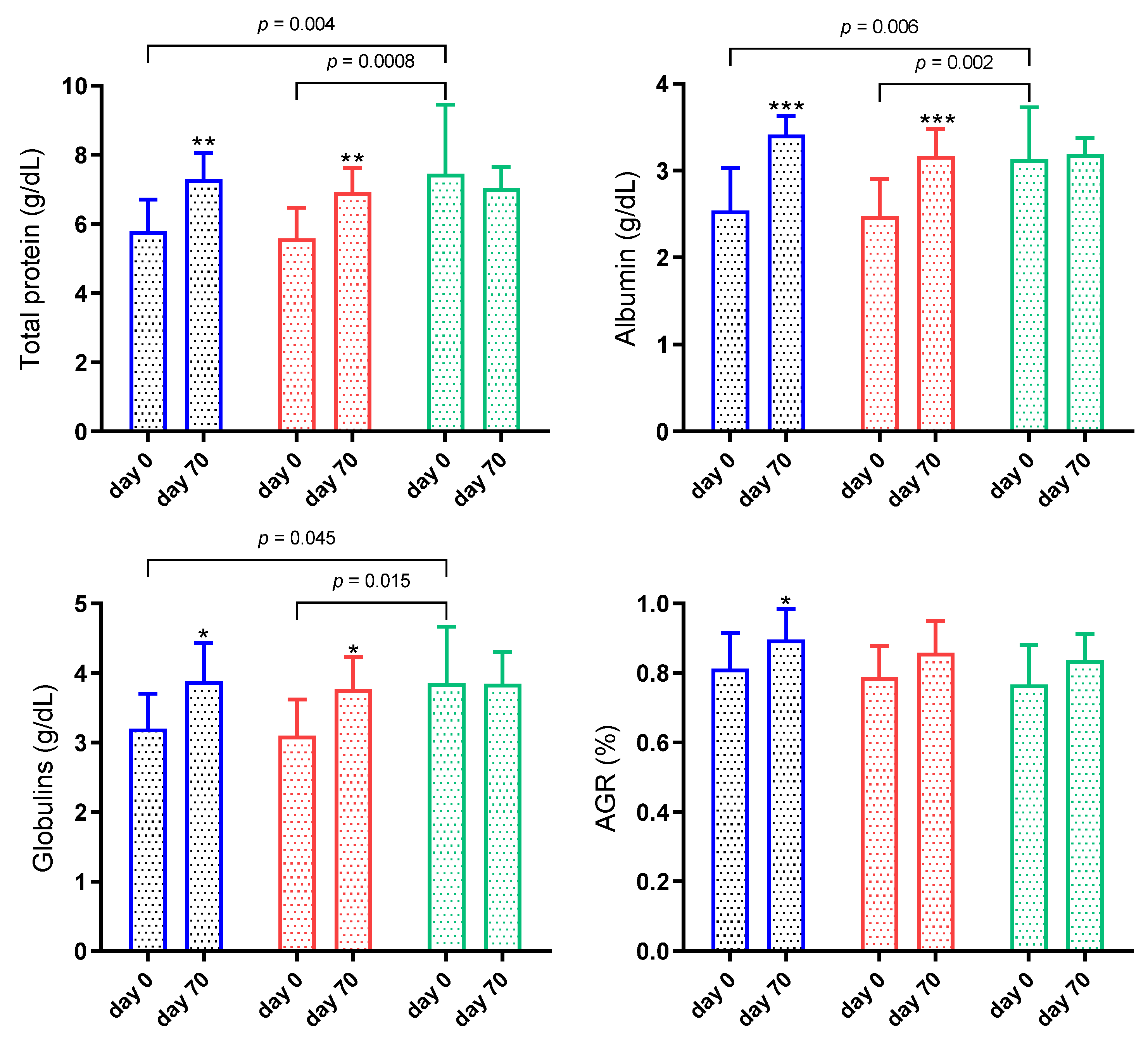
3. Results
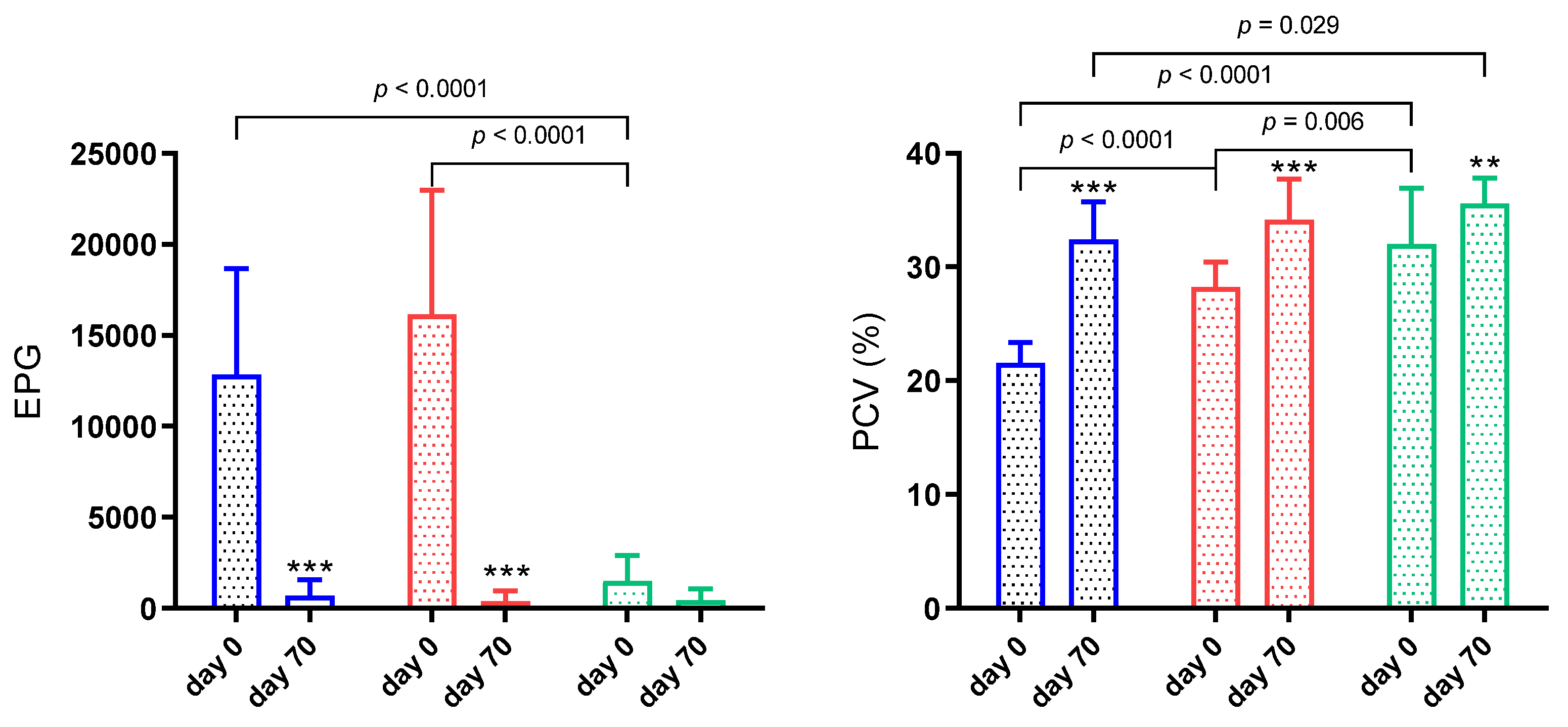
3.1. Animals
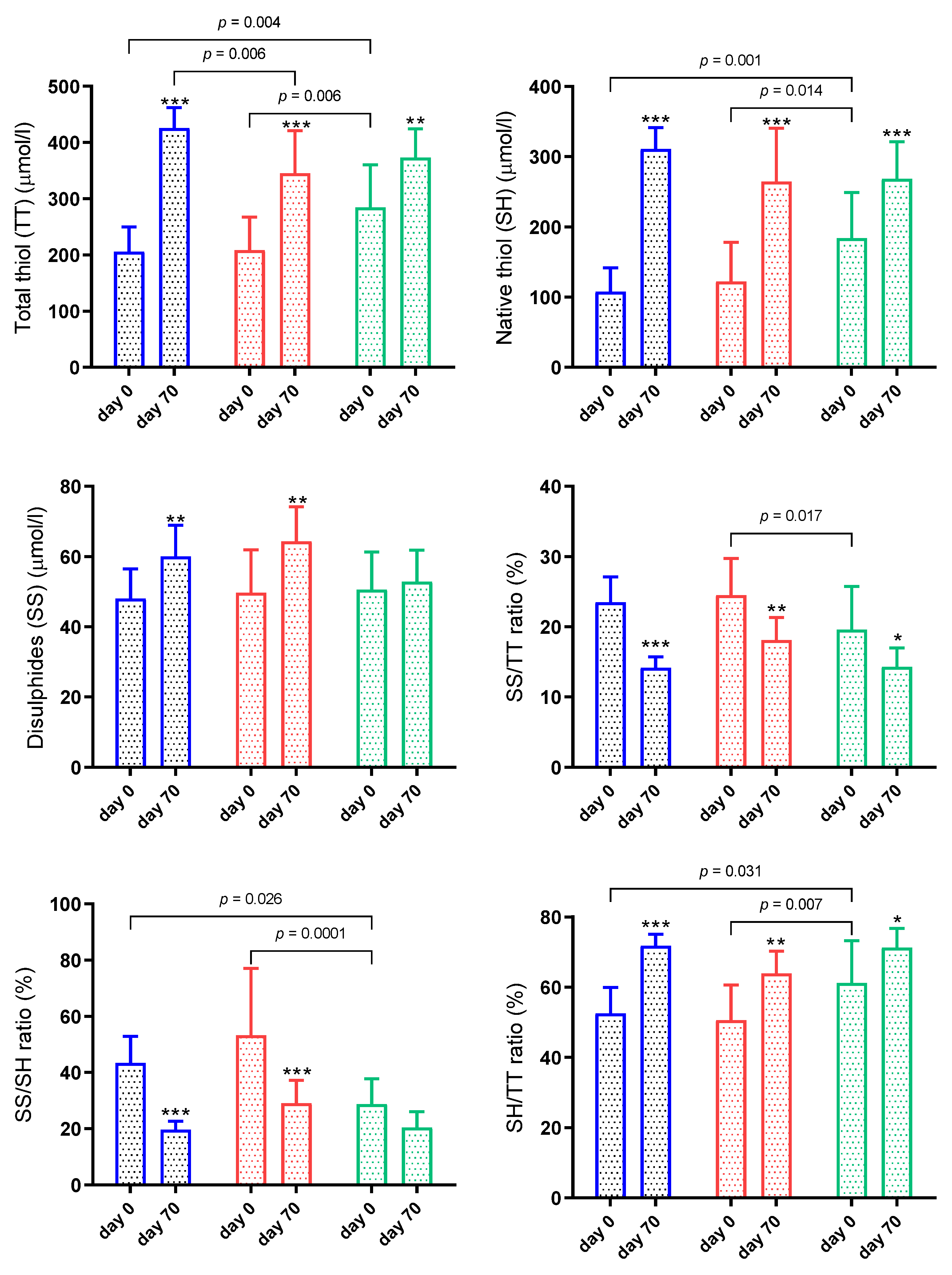
3.2. Thiol-Disulphide Homeostasis
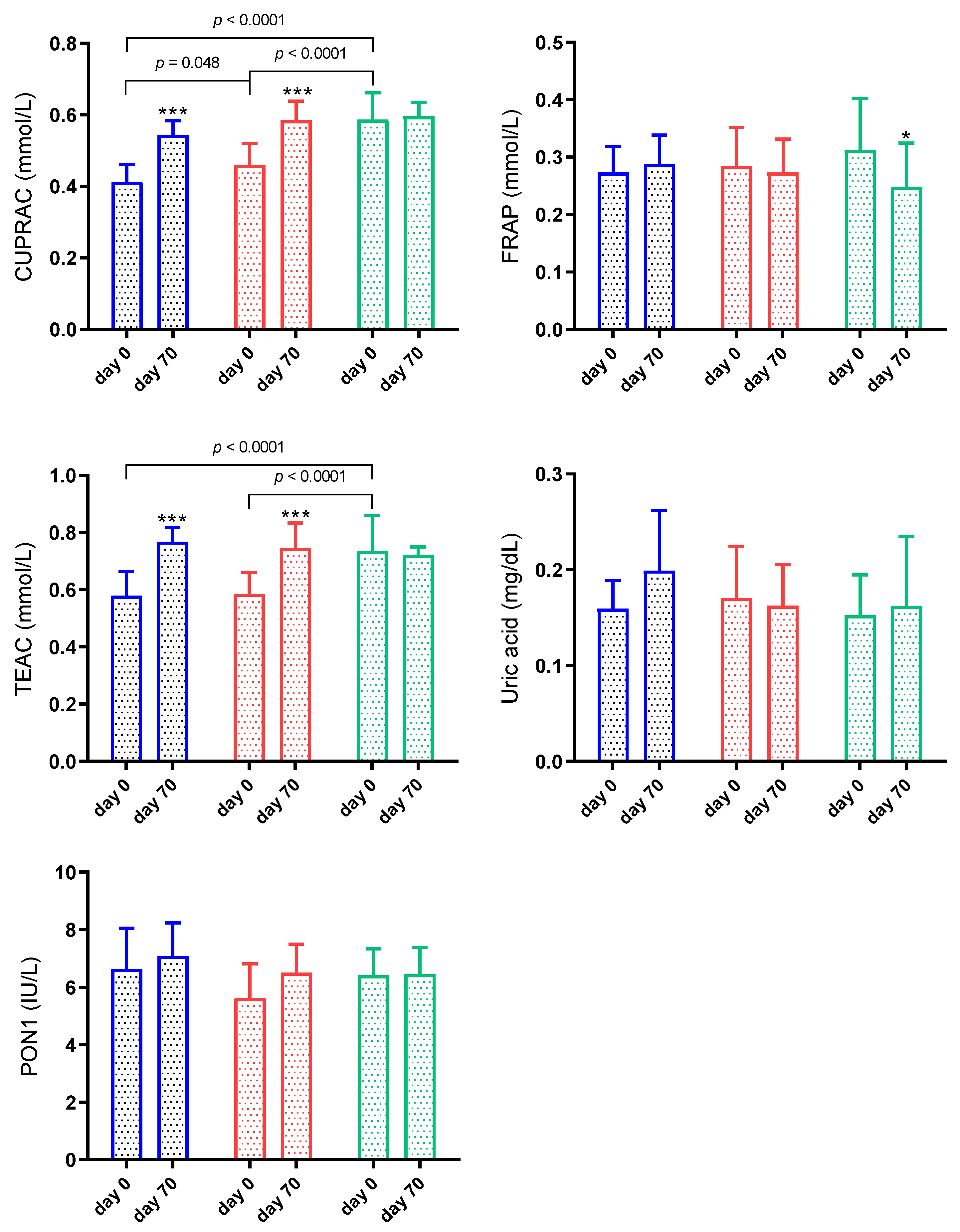
3.3. Antioxidant Biomarkers
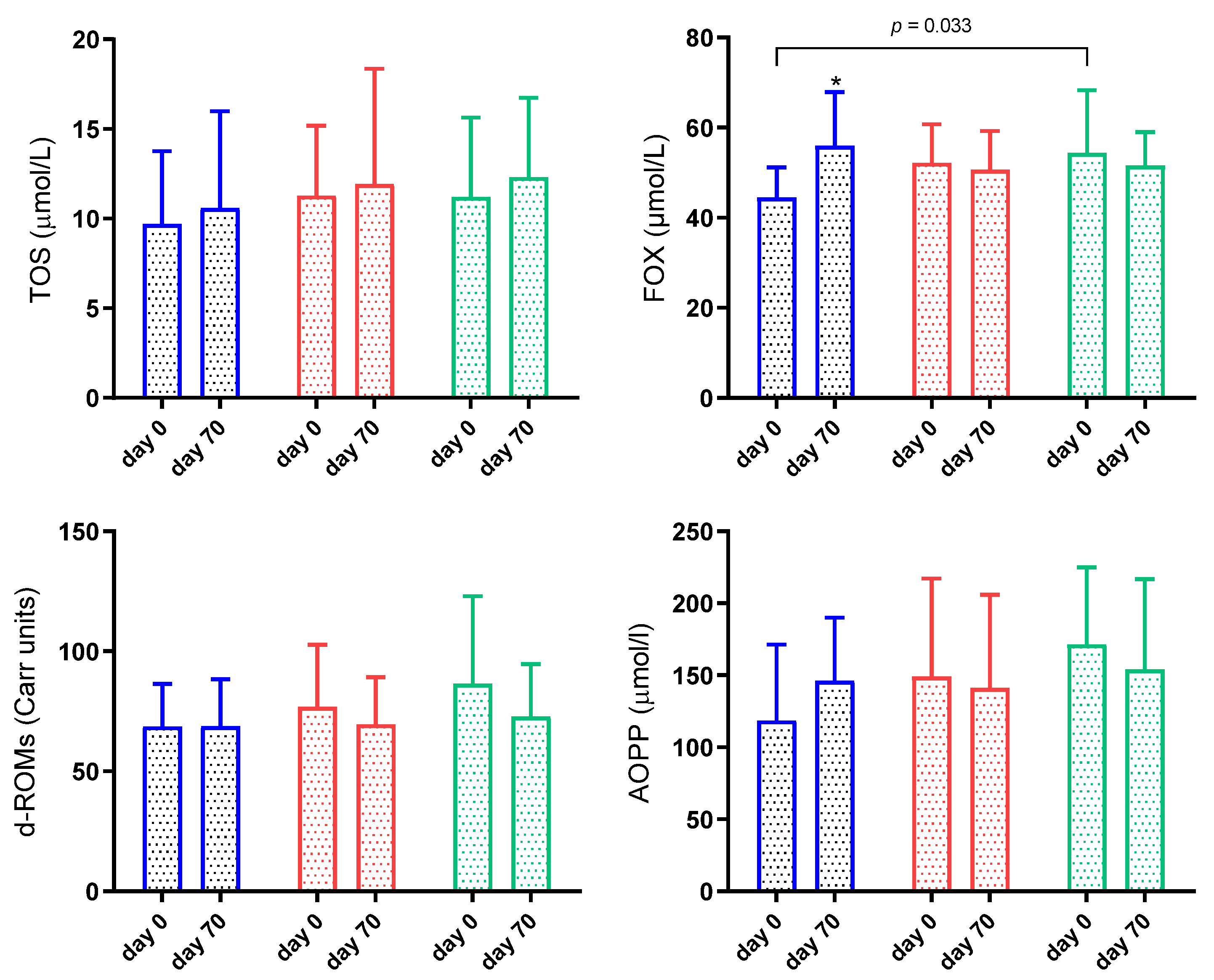
3.4. Oxidant Biomarkers
3.5. Inflammatory Biomarkers
3.6. Correlation Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kloosterman, A.; Parmentier, H.K.; Ploeger, H.W. Breeding cattle and sheep for resistance to gastrointestinal nematodes. Parasitol. Today 1992, 8, 330–335. [Google Scholar] [CrossRef]
- Mcrae, K.M.; Stear, M.J.; Good, B.; Keane, O.M. The host immune response to gastrointestinal nematode infection in sheep. Parasite Immunol. 2015, 37, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Craig, T.M. Gastrointestinal nematodes, diagnosis and control. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Amarante, A.F.T.; Bricarello, P.A.; Rocha, R.A.; Gennari, S.M. Resistance of Santa Ines, Suffolk and Ile de France sheep to naturally acquired gastrointestinal nematode infections. Vet. Parasitol. 2004, 120, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.R.; van Dijk, J. Climate and the epidemiology of gastrointestinal nematode infections of sheep in Europe. Vet. Parasitol. 2012, 189, 8–14. [Google Scholar] [CrossRef]
- Karlsson, L.J.E.; Greeff, J.C. Genetic aspects of sheep parasitic diseases. Vet. Parasitol. 2012, 189, 104–112. [Google Scholar] [CrossRef]
- Santos, M.C.; Xavier, J.K.; Amarante, M.R.V.; Bassetto, C.C.; Amarante, A.F.T. Immune response to Haemonchus contortus and Haemonchus placei in sheep and its role on parasite specificity. Vet. Parasitol. 2014, 203, 127–138. [Google Scholar] [CrossRef]
- Shaw, R.J.; Morris, C.A.; Wheeler, M.; Tate, M.; Sutherland, I.A. Salivary IgA: A suitable measure of immunity to gastrointestinal nematodes in sheep. Vet. Parasitol. 2012, 186, 109–117. [Google Scholar] [CrossRef]
- Roeber, F.; Jex, A.R.; Gasser, R.B. Impact of gastrointestinal parasitic nematodes of sheep, and the role of advanced molecular tools for exploring epidemiology and drug resistance-An Australian perspective. Parasites Vectors 2013, 6, 1. [Google Scholar] [CrossRef]
- Grosskopf, H.M.; Grosskopf, R.K.; Biazus, A.H.; Leal, M.L.R.; Bottari, N.B.; Alves, M.S.; Schetinger, M.R.C.; Morsch, V.M.; Machado, G.; Baldissera, M.D.; et al. Supplementation with copper edetate in control of Haemonchus contortus of sheep, and its effect on cholinesterase’s and superoxide dismutase activities. Exp. Parasitol. 2017, 173, 34–41. [Google Scholar] [CrossRef]
- Amarante, A.F.T.; Craig, T.M.; Ramsey, W.S.; El-Sayed, N.M.; Desouki, A.Y.; Bazer, F.W. Comparison of naturally acquired parasite burdens among Florida Native, Rambouillet and crossbreed ewes. Vet. Parasitol. 1999, 85, 61–69. [Google Scholar] [CrossRef]
- Almeida, F.A.; Piza, M.L.S.T.; Bassetto, C.C.; Starling, R.Z.C.; Albuquerque, A.C.A.; Protes, V.M.; Pariz, C.M.; Castilhos, A.M.; Costa, C.; Amarante, A.F.T. Infection with gastrointestinal nematodes in lambs in different integrated crop-livestock systems (ICL). Small Rumin. Res. 2018, 166, 66–72. [Google Scholar] [CrossRef]
- Da Silva, F.F.; Bezerra, H.M.F.F.; Feitosa, T.F.; Vilela, V.L.R. Nematode resistance to five anthelmintic classes in naturally infected sheep herds in Northeastern Brazil. Rev. Bras. Parasitol. Veterinária 2018, 27, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Koski, K.G.; Scott, M.E. Gastrointestinal nematodes, trace elements, and immunity. J. Trace Elem. Exp. Med. 2003, 16, 237–251. [Google Scholar] [CrossRef]
- Do Reo Leal, M.L.; De Camargo, E.V.; Henrique Ross, D.; Molento, M.B.; Dos Anjos Lopes, S.T.; Da Rocha, J.B.T. Effect of selenium and vitamin E on oxidative stress in lambs experimentally infected with Haemonchus contortus. Vet. Res. Commun. 2010, 34, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Menzies, M.; Reverter, A.; Andronicos, N.; Hunt, P.; Windon, R.; Ingham, A. Nematode challenge induces differential expression of oxidant, antioxidant and mucous genes down the longitudinal axis of the sheep gut. Parasite Immunol. 2010, 32, 36–46. [Google Scholar] [CrossRef]
- Alam, R.T.M.; Hassanen, E.A.A.; El-Mandrawy, S.M.A. Heamonchus contortus infection in sheep and goats: Alterations in haematological, biochemical, immunological, trace element and oxidative stress markers. J. Appl. Anim. Res. 2020, 48, 357–364. [Google Scholar] [CrossRef]
- Baptistiolli, L.; Narciso, L.G.; De Almeida, B.F.M.; Bosco, A.M.; De Souza, J.C.; Torrecilha, R.B.P.; Pereira, P.P.; Figueiredo, R.N.; Garcia, J.F.; Kaneto, C.N.; et al. Systemic oxidative stress in Suffolk and Santa Ines sheep experimentally infected with Haemonchus contortus. Acta Parasitol. 2018, 63, 504–514. [Google Scholar] [CrossRef]
- Machado, V.; Da Silva, A.S.; Schafer, A.S.; Aires, A.R.; Tonin, A.A.; Oliveira, C.B.; Hermes, C.L.; Almeida, T.C.; Moresco, R.N.; Stefani, L.M.; et al. Relationship between oxidative stress and pathological findings in abomasum of infected lambs by Haemonchus contortus. Pathol. Res. Pract. 2014, 210, 812–817. [Google Scholar] [CrossRef]
- Pathak, A.K.; Dutta, N.; Pattanaik, A.K.; Sharma, K.; Banerjee, P.S.; Goswami, T.K. The effect of condensed tannins supplementation through Ficus infectoria and Psidium guajava leaf meal mixture on erythrocytic antioxidant status, immune response and gastrointestinal nematodes in lambs (Ovis aries). Vet. Arh. 2017, 87, 139–156. [Google Scholar]
- Pivoto, F.L.; Torbitz, V.D.; Aires, A.R.; da Rocha, J.F.X.; Severo, M.M.; Grando, T.H.; Peiter, M.; Moresco, R.N.; da Rocha, J.B.T.; do Rego Leal, M.L. Oxidative stress by Haemonchus contortus in lambs: Influence of treatment with zinc edetate. Res. Vet. Sci. 2015, 102, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Ates, I.; Ozkayar, N.; Inan, B.; Yilmaz, F.M.; Topcuoglu, C.; Neselioglu, S.; Erel, O.; Dede, F.; Yilmaz, N. Dynamic thiol/disulphide homeostasis in patients with newly diagnosed primary hypertension. J. Am. Soc. Hypertens. 2016, 10, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.; Bugday, M.S.; Erel, O. Thiol-disulphide homoeostasis as an oxidative stress marker in men with varicocele. Andrologia 2018, 50, e12982. [Google Scholar] [CrossRef] [PubMed]
- Vural, G.; Gümüşyayla, Ş.; Deniz, O.; Neşelioğlu, S.; Erel, Ö. Relationship between thiol-disulphide homeostasis and visual evoked potentials in patients with multiple sclerosis. Neurol. Sci. 2019, 40, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, K.; Çakırca, G.; Erel, Ö. Impaired thiol/disulphide homoeostasis in children with steroid-sensitive nephrotic syndrome. Int. J. Clin. Pract. 2021, 75, 1–4. [Google Scholar] [CrossRef]
- Çamkerten, İ.; Çamkerten, G.; Erdoğan, H.; Ayan, A.; Erdoğan, S.; Ural, K. Serum thiol disulphide levels among sheep with sarcoptic mange. Kafkas Univ. Vet. Fak. Derg. 2019, 25, 865–868. [Google Scholar] [CrossRef]
- De Albuquerque, A.C.A.; Bassetto, C.C.; de Almeida, F.A.; Amarante, A.F.T. Development of Haemonchus contortus resistance in sheep under suppressive or targeted selective treatment with monepantel. Vet. Parasitol. 2017, 246, 112–117. [Google Scholar] [CrossRef]
- Jain, N.C. Hematological techniques. In Schalm’s Vet. Hematol, 4th ed.; Jain, N.C., Ed.; Lea and Febiger: Philadelphia, PA, USA, 1986; pp. 20–86. [Google Scholar]
- Cannas, A. Sheep and Cattle Nutrient Requirement Systems, Ruminal Turnover, and Adaptation of the Cornell Net Carbohydrate and Protein System to Sheep; Cornell University: Ithaca, NY, USA, 2000; ISBN 0599840528. [Google Scholar]
- Van Wyk, J.A.; Bath, G.F. The FAMACHA system for managing haemonchosis in sheep and goats by clinically identifying individual animals for treatment. Vet. Res. 2002, 33, 509–529. [Google Scholar] [CrossRef]
- Papadopoulos, E. Anthelmintic resistance in sheep nematodes. Small Rumin. Res. 2008, 76, 99–103. [Google Scholar] [CrossRef]
- Gordon, H.M.; Whitlock, H. V A new technique for counting nematode eggs in sheep faeces. J. Counc. Sci. Ind. Res. 1939, 12, 50–52. [Google Scholar]
- Roberts, F.H.S.; O’Sullivan, P.J. Methods for egg counts and larval cultures for strongyles infesting the gastro-intestinal tract of cattle. Aust. J. Agric. Res. 1950, 1, 99–102. [Google Scholar] [CrossRef]
- Ueno, H.; Gonçalves, P.C. Manual Para Diagnóstico Das Helmintoses De Ruminantes; Japan International Cooperation Agency: Tokyo, Japan, 1988; Volume 166.
- Erel, O.; Neselioglu, S. A novel and automated assay for thiol/disulphide homeostasis. Clin. Biochem. 2014, 47, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.; Guzmán, R.; López-Fernández, E.; Casado, Á. Evaluation of the copper (II) reduction assay using bathocuproinedisulfonic acid disodium salt for the total antioxidant capacity assessment: The CUPRAC–BCS assay. Anal. Biochem. 2009, 392, 37–44. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernández-Ruiz, J.; García-Cánovas, F.; Acosta, M. Inhibition by l-ascorbic acid and other antioxidants of the 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) oxidation catalyzed by peroxidase: A new approach for determining total antioxidant status of foods. Anal. Biochem. 1996, 236, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Tecles, F.; Caldin, M.; Tasca, S.; Cerón, J. Validation of spectrophotometric assays for serum paraoxonase type-1 measurement in dogs. Am. J. Vet. Res. 2012, 73, 34–41. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Arab, K.; Steghens, J.-P. Plasma lipid hydroperoxides measurement by an automated xylenol orange method. Anal. Biochem. 2004, 325, 158–163. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Cesarone, M.R.; Belcaro, G.; Carratelli, M.; Cornelli, U.; De Sanctis, M.T.; Incandela, L.; Barsotti, A.; Terranova, R.; Nicolaides, A. A simple test to monitor oxidative stress. Int. Angiol. 1999, 18, 127–130. [Google Scholar] [PubMed]
- Tecles, F.; Cerón, J.J. Determination of whole blood cholinesterase in different animal species using specific substrates. Res. Vet. Sci. 2001, 70, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Erel, Ö.; Erdoğan, S. Thiol-disulfide homeostasis: An integrated approach with biochemical and clinical aspects. Turkish J. Med. Sci. 2020, 50, 1728–1738. [Google Scholar] [CrossRef]
- Kalem, A.K.; Kayaaslan, B.; Neselioglu, S.; Eser, F.; Hasanoglu, İ.; Aypak, A.; Akinci, E.; Akca, H.N.; Erel, O.; Guner, R. A useful and sensitive marker in the prediction of COVID-19 and disease severity: Thiol. Free Radic. Biol. Med. 2021, 166, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Kara, S.S.; Erel, O.; Demirdag, T.B.; Cura Yayla, B.C.; Gulhan, B.; Neselioglu, S.; Polat, M.; Kalkan, G.; Tapisiz, A.; Tezer, H. Alteration of thiol-disulphide homeostasis in acute tonsillopharyngitis. Redox Rep. 2017, 22, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Comini, M.A. Measurement and meaning of cellular thiol:disufhide redox status. Free Radic. Res. 2016, 50, 246–271. [Google Scholar] [CrossRef] [PubMed]
- Eggers, C.H.; Caimano, M.J.; Malizia, R.A.; Kariu, T.; Cusack, B.; Desrosiers, D.C.; Hazlett, K.R.O.; Claiborne, A.L.; Pal, U.; Radolf, J.D. The coenzyme A disulphide reductase of Borrelia burgdorferi is important for rapid growth throughout the enzootic cycle and essential for infection of the mammalian host. Mol. Microbiol. 2011, 82, 679–697. [Google Scholar] [CrossRef]
- Sebel, Y.; Aksoy, M.; An, I.; Celik, H. Evaluation of thiol/disulphide balance in patients with cutaneous leishmaniasis. Int. J. Clin. Pract. 2021, 75, e14087. [Google Scholar] [CrossRef]
- De Oliveira, P.A.; Riet-Correa, B.; Estima-Silva, P.; Coelho, A.C.B.; dos Santos, B.L.; Costa, M.A.P.; Ruas, J.L.; Schild, A.L. Multiple anthelmintic resistance in Southern Brazil sheep flocks. Rev. Bras. Parasitol. Vet. 2017, 26, 427–432. [Google Scholar] [CrossRef]
- Kaplan, R.M. Biology, epidemiology, diagnosis, and management of anthelmintic resistance in gastrointestinal nematodes of livestock. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 17–30. [Google Scholar] [CrossRef]
- Do Rego Leal, M.L.; Pivoto, F.L.; Fausto, G.C.; Aires, A.R.; Grando, T.H.; Roos, D.H.; Sudati, J.H.; Wagner, C.; Costa, M.M.; Molento, M.B.; et al. Copper and selenium: Auxiliary measure to control infection by Haemonchus contortus in lambs. Exp. Parasitol. 2014, 144, 39–43. [Google Scholar] [CrossRef]
- Lightbody, J.H.; Stevenson, L.M.; Jackson, F.; Donaldson, K.; Jones, D.G. Comparative aspects of plasma antioxidant status in sheep and goats, and the influence of experimental abomasal nematode infection. J. Comp. Pathol. 2001, 124, 192–199. [Google Scholar] [CrossRef]
- Dede, S.; Deger, Y.; Kahraman, T.; Deger, S.; Alkan, M.; Cemek, M. Oxidation products of nitric oxide and the concentrations of antioxidant vitamins in parasitized goats. Acta Vet. Brno. 2002, 71, 341–345. [Google Scholar] [CrossRef][Green Version]
- Abd Ellah, M.R. Involvement of free radicals in parasitic infestations. J. Appl. Anim. Res. 2013, 41, 69–76. [Google Scholar] [CrossRef]
- Cecchini, S.; Fazio, F. Assessment of total (anti) oxidant status in goat kids. Arch. Anim. Breed. 2021, 64, 139–146. [Google Scholar] [CrossRef]
| Biomarker | Spearman Coefficient |
|---|---|
| PCV | −0.719 *** |
| TT | −0.756 *** |
| SH | −0.747 *** |
| SS | −0.293 * |
| SS/TT ratio | 0.710 *** |
| SS/SH ratio | 0.716 *** |
| SH/TT ratio | −0.721 *** |
| CUPRAC | −0.661 *** |
| FRAP | 0.029 |
| TEAC | −0.686 *** |
| Uric acid | 0.070 |
| PON1 | −0.197 |
| TOS | −0.071 |
| FOX | −0.146 |
| d-ROMs | 0.100 |
| AOPP | −0.187 |
| Total proteins | −0.692 *** |
| Albumin | −0.693 *** |
| Globulin | −0.586 *** |
| AGR | −0.194 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, E.M.d.S.; Fachiolli, D.F.; de Oliveira, R.M.; Almeida, F.A.; Pariz, C.M.; de Lima Meirelles, P.R.; Costa, C.; Tvarijonaviciute, A.; Erel, O.; Neselioglu, S.; et al. Changes in Serum Thiol-Disulphide Homeostasis in Sheep with Gastrointestinal Nematodes. Animals 2021, 11, 2856. https://doi.org/10.3390/ani11102856
Schmidt EMdS, Fachiolli DF, de Oliveira RM, Almeida FA, Pariz CM, de Lima Meirelles PR, Costa C, Tvarijonaviciute A, Erel O, Neselioglu S, et al. Changes in Serum Thiol-Disulphide Homeostasis in Sheep with Gastrointestinal Nematodes. Animals. 2021; 11(10):2856. https://doi.org/10.3390/ani11102856
Chicago/Turabian StyleSchmidt, Elizabeth Moreira dos Santos, Daniele Floriano Fachiolli, Raphaela Moreira de Oliveira, Fabiana Alves Almeida, Cristiano Magalhães Pariz, Paulo Roberto de Lima Meirelles, Ciniro Costa, Asta Tvarijonaviciute, Ozcan Erel, Salim Neselioglu, and et al. 2021. "Changes in Serum Thiol-Disulphide Homeostasis in Sheep with Gastrointestinal Nematodes" Animals 11, no. 10: 2856. https://doi.org/10.3390/ani11102856
APA StyleSchmidt, E. M. d. S., Fachiolli, D. F., de Oliveira, R. M., Almeida, F. A., Pariz, C. M., de Lima Meirelles, P. R., Costa, C., Tvarijonaviciute, A., Erel, O., Neselioglu, S., Ceron, J. J., & Rubio, C. P. (2021). Changes in Serum Thiol-Disulphide Homeostasis in Sheep with Gastrointestinal Nematodes. Animals, 11(10), 2856. https://doi.org/10.3390/ani11102856









