Simple Summary
Granivorous murids, namely striped field (Apodemus agrarius), yellow-necked (Apodemus flavicollis), and harvest (Micromys minutus) mice, occur in a variety of habitats and live syntopically in agricultural areas. Agroecosystems may be quite complex isotopically with δ15N values being influenced by many internal and external fluxes. Using isotopic (δ15N and δ13C) compositions from hair samples, we analysed isotopic niches of granivores in apple and plum orchards, raspberry and currant plantations, and nearby meadows in Lithuania. As the main hypothesis, we expected differences in the isotopic niches of these species (being a proxy for their diet), minimising interspecific competition. Striped field and yellow-necked mice were trapped in every habitat. Therefore, syntopic co-occurrence of granivores depended on the presence of harvest mice in the apple orchards, raspberry plantations, and meadows that served as control habitats. All species were fully separated according to δ15N values, presuming different amounts of food of animal origin in their diet. The separation of species according to δ13C was not expressed in all habitats. The core dietary niches of these species were fully separated in the apple orchards and raspberry plantations. Intraspecific differences of the isotopic niche were not present in any of the three species: that is, resources were equally used by males and females, adults, subadults, and juveniles.
Abstract
In agricultural habitats, diets and trophic positions of syntopic granivorous small mammals are not known sufficiently. Agroecosystems may be quite complex isotopically and the most complex situation concerns the nitrogen-15 isotope as δ15N values are influenced by many internal and external fluxes. We analysed the isotopic niches of striped field (Apodemus agrarius), yellow-necked (Apodemus flavicollis), and harvest (Micromys minutus) mice living sympatrically and syntopically in apple and plum orchards, raspberry and currant plantations, and nearby meadows that were used as control habitats. Carbon (δ13C) and nitrogen (δ15N) stable isotope ratios from hair samples were used as a proxy for their diet. As the main hypothesis, we expected differences in the isotopic niches of these three species, minimising interspecific competition. All species were fully separated according to δ15N values, presuming different amounts of food of animal origin in their diet. The separation of species according to δ13C was not expressed in all habitats. The core dietary niches of these species were fully separated in the apple orchards and raspberry plantations. Intraspecific differences of the isotopic niche were not present in any of the three species: that is, resources were equally used by males and females, adults, subadults, and juveniles.
1. Introduction
Three granivorous rodents, namely the striped field mouse Apodemus agrarius (Pallas, 1771), the yellow-necked mouse Apodemus flavicollis (Melchior, 1834), and the harvest mouse Micromys minutus (Pallas, 1771), all occur in a wide variety of habitats but favour areas of tall, dense vegetation [1,2,3,4]. These closely related species are common in Central and Eastern Europe [5,6,7,8]. In Lithuania, populations of the above mentioned granivores may occur syntopically in moist habitats [9,10] but little is known about their diet and interspecific relationships.
Diets link animals with their environment and have an influence on physiological, behavioural, and morphological traits [11]. In the temperate climate zone, most rodent species belong to three groups according to their diet: granivores (Apodemus and Micromys species), herbivores (Microtus and Arvicola species), and omnivores (Clethrionomys and Mus species) [12,13]. Pineda-Munoz and Alroy in 2014 identified granivory as a readily identifiable diet type in mammals [14]. The ecological or evolutionary relevance of granivory is evidenced by the fact that several rodent species have modified incisors for shaving and husking seeds [15]. These structures evolved in species in the families of Cricetidae and Muridae [16].
Small mammals are not only part of food chains in agroecosystems but also are pests of many crops [17]. Their negative effects are not limited to their damage of agricultural crops but rather expand to their role in the distribution of weed seeds and the fact that they are carriers of various pathogens [18]. Granivorous animals play a dual role in plant regeneration [19], both enhancing it by dispersing seeds and reducing it by often consuming large quantities of seeds [20]. Although granivorous rodents are known for hoarding seeds, their ability to consume foods other than seeds allows them to survive variation in resource availability [21]. As a result, the quantity of seeds consumed by granivores may depend critically on the presence of other nearby resources in the environment that serve to attract or distract foraging granivores [22].
In commercial orchards and berry plantations in Lithuania, the dominant species is the common vole (Microtus arvalis), while A. agrarius and A. flavicollis are subdominants, although they dominate in some cases [23]. M. minutus is a rare species in commercial gardens, represented by just a few individuals [10].
With high dietary and microhabitat overlaps, some small mammal species could coexist primarily only due to passive, mutually avoiding and amicable interactions [24]. Another opportunity for coexistence is niche partitioning [25], which may be based on time [26,27], space or habitats [28,29], or resources [30,31].
Resource use or diet is one of the main dimensions of a niche [32]. An assessment of resource use may concern consumed food items (relevance or frequency) using methods of analysis such as assessing stomach contents, scats, cheek pouches, food caches, etc. [33]. In recent years, diet has been analysed by modern tools such as DNA, fatty acid, or stable isotope analyses [34,35,36,37]. We used stable carbon and nitrogen (δ13C and δ15N) isotope analysis as a reliable tool to investigate the trophic ecology of vertebrate animals including various rodent species [9,23,30,36,37,38,39].
Our study concentrated on a stable isotope-based evaluation of the trophic niches of three granivores species, namely striped field mouse, yellow-necked mouse, and harvest mouse, these being sympatric and syntopic simultaneously in commercial orchards, berry plantations, and nearby meadows, which are defined as control habitats. As a main hypothesis, we expected differences in their diet (expressed as a proxy by the isotopic space of the species) and that these differences minimise interspecific competition for food resources of the co-occurring species. With more resources available to granivores in this habitat, we expected an overlap of the isotopic niches in the meadows but not in the fruit orchards or berry plantations, as these are resource-scarce. We expected that intraspecific (age or gender based) differences will be present in all investigated habitats as a result of the competition for resources.
2. Materials and Methods
2.1. Study Site
Small mammals were trapped in 2018–2020 at 18 sites in Lithuania. At every site, an agricultural habitat (apple or plum orchard, or currant, raspberry, or highbush blueberry plantation) and a neighbouring control meadow or forest (not subjected to agricultural activity) were investigated (Figure 1a). Commercial habitats were of different age (young, medium-aged, and old) and with different intensities of agricultural measures in their maintenance (intensity defined as low, medium, or high). Additional details on the study sites and habitats are presented in previous publications [10,31].
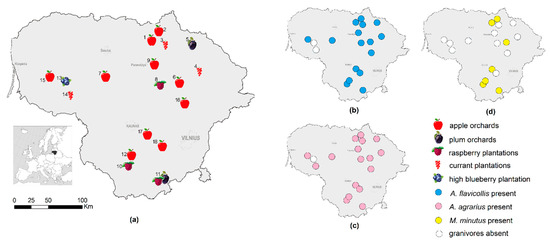
Figure 1.
Commercial fruit orchards and berry plantations. (a) At the trapping sites in Lithuania, 2018–2020. The distribution of the granivorous species: (b) A. flavicollis, (c) A. agrarius, and (d) M. minutus.
2.2. Small Mammal Trapping and Sample Size
Small mammals were snap-trapped two times per year in summer and autumn, covering one or two agricultural habitats and a control habitat at every investigation site (see Figure 1). We used a trap line method, setting 25–100 traps at 5 m intervals per habitat and checking these once per day in the morning [40]. Total trapping effort was 25,503 trap days. The distribution of the trapping effort according to the habitats is shown in Table 1. In total, 168 trapping sessions (three-day long trapping per habitat) were conducted. In three of these sessions, in the high blueberry plantation, no small mammals were trapped. Therefore, this habitat was not analysed.

Table 1.
Sympatry of granivorous rodents in fruit orchards, berry plantations, and control meadows of Lithuania, 2018–2020. Abbreviations: TE, trapping effort, trap days; TS, number of trapping sessions; NSM, number of all small mammals trapped; NH, number of habitats investigated; Af, A. flavicollis; Aa, A. agrarius; Mm, M. minutus; Af + Aa, by two species living syntopically; and All, by all three species living syntopically.
Mice were identified visually when checking the traps and stored in separate plastic bags. Animal age and gender were identified at dissection in the laboratory. Identification of the age of the individual was based on the level of atrophy of the thymus [41], as well as the body mass and status of the sex organs. We used three age categories: adults, subadults, and juveniles [42].
Out of 1450 small mammals trapped in the orchards, plantations, and meadows, 732 were granivores. This group was equally strongly dominated by A. flavicollis (374 individuals, 51.1%) and A. agrarius (346 individuals, 47.3%), while the M. minutus proportion was quite small (12 individuals, 1.6%).
The study was conducted in accordance with Lithuanian (the Republic of Lithuania Law on the Welfare and Protection of Animals No. XI-2271) and European legislation (Directive 2010/63/EU) on the protection of animals and was approved by the Animal Welfare Committee of the Nature Research Centre, protocol number GGT-7. In Lithuania, there is no need or obligation to obtain permission to snap trap small mammals. Trapping was unavoidable as we used the material for the pathogen analysis, collecting of organs (lungs, spleen, brain, etc.), and analysing of breeding parameters (from testes, uterus, and ovaries). In a few cases, trapped animals were killed by cervical dislocation.
2.3. Stable Isotope Analysis
We analysed the carbon and nitrogen stable isotopes in the hair of the trapped rodents. The most numerous species, A. flavicollis and A. agrarius, were sub-sampled in 2020. All suitable individuals of M. minutus were analysed. The distribution of the sampled individuals by species, habitat, age, and gender are presented in Table 2.

Table 2.
Sample sizes by habitat of the three species of granivorous rodents used for stable isotope analysis. Abbreviations: N, number of analysed individuals; m/f, numbers of males and females; and a/s/j, numbers of adults, subadults, and juveniles, respectively.
We used hair clipped from between the shoulders and stored them dry in separate bags for each individual. Dirty (covered by soil or blood) samples were washed in deionized water and methanol, and then dried. Very dirty samples were discarded.
Analyses were conducted at the Centre for Physical Sciences and Technology, Vilnius, Lithuania, using an elemental analyser (EA) (Flash EA1112) coupled to an isotope ratio mass spectrometer (IRMS) (Thermo Delta V Advantage) via a ConFlo III interface (EA-IRMS). The detailed analysis procedure and equipment used are described in [39]. Five percent of the samples were run in duplicates and the obtained results for these samples were averaged.
As reference materials, we used Caffeine IAEA-600 (δ13C = −27.771 ± 0.043‰, δ15N = 1 ± 0.2‰), Ammonium Sulfate IAEA-N-1 (δ15N = 0.4 ± 0.2‰), and Graphite USGS24 (δ13C = −16.049 ± 0.035‰) provided by the International Atomic Energy Agency (IAEA). These standards were run every 12 samples. Repeated analysis of these reference materials gave a standard deviation of less than 0.08‰ for carbon and 0.2‰ for nitrogen [39].
2.4. Statistical Analyses
Co-occurrence of the granivore species were analysed using the proportions of sites or trapping sessions in the habitats in which two or three granivorous species were co-trapped. A 95% confidence interval was calculated using the Wilson method, while the difference of proportions was evaluated using the chi-square method. As the number of sites in which any of the three species were absent was <10, the G-test for proportions (e.g., https://elem.com/~btilly/effective-ab-testing/g-test-calculator.html, accessed on 10 May 2021) was not used.
The δ13C and δ15N values in the samples were expressed as the arithmetic mean ± 1 SE and the range (min–max). Outliers were not excluded. The normalities of the distributions of the δ15N and δ13C values were tested using Kolmogorov–Smirnov’s D test. Based on the conformity to normal distribution of both isotope values in all species and in all habitats with sample size n ≥ 5, parametric tests were further applied.
The isotopic niches of the species were analysed using the parameters of TA (total area), SEA (standard ellipse area), and SEAc as corrected central ellipses, unbiased for the sample size [43]. The positions of species and intraspecific groups, including those with sample size n < 5, were visualized in isotopic biplots.
We used GLMM to find the influence of the species, habitat type (orchard, plantation, or meadow), habitat age, and intensity of agriculture as categorical factors on the dependent parameters: the δ15N and δ13C values. We also used two continuous predictors, namely year and season (summer or autumn), to control temporal data variability. Hotteling’s two sample T2 test for significance was used to test the significance of the model and eta-squared for the influence of the single factor. Intraspecific differences (between males and females and between age groups) were tested with parametric ANOVA using Wilk’s lambda test for significance. Differences between groups were evaluated with the post-hoc Tukey’s test and differences between pairs of variables with Student t-test.
The isotopic niches of the granivore species were calculated with SIBER [43], which we ran in R version 3.5.0 (https://cran.r-project.org/bin/windows/base/rdevel.html, accessed on 12 September 2020). Isotopic biplots were visualized using SigmaPlot version 12.5 (Systat Software Inc., San Jose, CA, USA). The proportions were evaluated in WinPepi version 11.39 software (Abramson, J., Jerusalem, Izrael). All other calculations were performed using Statistica for Windows version 6 (StatSoft, Inc., Tulsa, OK, USA).
3. Results
3.1. Sympatry and Syntopy of the Granivore Species
At 16 sites, A. flavicollis and A. agrarius were sympatric (trapped at the same site in the same habitat but not necessarily at the same time). All three granivorous species were sympatric in seven sites (all sites where M. minutus was present). Data on the sympatry of A. flavicollis, A. agrarius, and M. minutus are shown in Table 1.
Out of 18 investigation sites, A. agrarius was trapped in 17 (94%, CI = 74–99%) and A. flavicollis in 16 sites (89%, CI = 67–97%), the difference notably not significant. M. minutus was trapped in seven sites (39%, CI = 20–61%), thus the distribution of this species was significantly narrower than that of A. agrarius (χ2 = 9.48, p = 0.002) and A. flavicollis (χ2 = 12.15, p < 0.001). All seven sites with M. minutus were sympatrically inhabited by all three granivore species.
A. flavicollis was most frequently trapped in apple orchards (74% of trapping sessions), significantly exceeding the species’ presence in control meadows (39%, χ2 = 14.25, p < 0.001) and even more significantly than in other habitats, which themselves had no differences between them (Table 3). A. agrarius was present in all habitats including control ones with equal frequency. In M. minutus, the frequency of trapping in apple orchards, raspberry plantations, and control habitats was also equal (differences not significant).

Table 3.
Syntopy of granivorous rodents in commercial orchards, berry plantations, and control habitats. 2018–2020. Abbreviations: TS, number of trapping sessions; NAf, number of habitats inhabited by A. flavicollis; NAa, by A. agrarius; NMm, by M. minutus; NAf + Aa, AAf + Mm, and NAa + Mm, by two species living syntopically; and NAll, by all three species living syntopically.
Data on the syntopy of the granivorous species are presented in Table 3. The syntopy of the species’ pairs and triplets was the same in all habitats. The frequency of occurrence of syntopic A. flavicollis and A. agrarius depended on the presence of the last species, while syntopy with M. minutus was determined by its presence in the apple orchards, raspberry plantations, and control habitats.
3.2. Interspecific and Habitat-Based Differences of Isotopic Niche in Syntopic Granivores
Irrespective of other factors, the widest trophic niche according to δ13C was found in A. agrarius, significantly exceeding that of A. flavicollis (range of −17.41‰ vs. −13.46‰, respectively, t = 4.00, df = 456, p < 0.001). The range of δ13C (10.81‰) in M. minutus did not differ from either of the Apodemus species. The widest trophic niche according to δ15N was characteristic of M. minutus (range 17.62‰), significantly exceeding the range of A. agrarius (15.52‰, t = 3.94, df = 233, p < 0.001) and of A. flavicollis (11.45‰, t = 6.36, df = 243, p < 0.001). The δ15N range in A. agrarius was wider than in A. flavicollis (t = 10.24, df = 456, p < 0.001). Thus, species separation according to δ15N was full, while according to δ13C, it was only partial. The ranges and central positions of the stable isotope ratios of the granivores by habitat are presented in Table S1.
The cumulative influence of species, habitat type, habitat age, intensity of agriculture, year, and season was significant for the distribution of both δ13C (F11,256 = 5.08, p < 0.001) and δ15N (F11,256 = 13.23, p < 0.001) values. While analysing univariate results, we did not find the influence of the year (Hotelling’s T2 = 0.001, p = 0.89). Other categorical factors were all significant: species (T2 = 0.44, p < 0.001, eta2 = 0.18), habitat type (T2 = 0.11, p < 0.001, eta2 = 0.05), intensity of agriculture (T2 = 0.09, p < 0.001, eta2 = 0.04), season (T2 = 0.04, p < 0.01, eta2 = 0.04), and habitat age (T2 = 0.04, p < 0.05, eta2 = 0.02).
The univariate analysis disclosed that season and habitat age influences were expressed only for the δ15N variation (F = 7.17, p < 0.01 and F = 3.51, p < 0.05, respectively), while the intensity of agricultural measures were expressed only for the δ13C variation (F = 8.17, p < 0.001). Mice species and habitat type, both being the strongest factors, influenced δ13C and δ15N variation (species: F = 14.39, p < 0.001 and F = 46.12, p < 0.001, respectively; habitat: F = 4.08, p < 0.01 and F = 4.53, p < 0.005, respectively). Therefore, further analysis of the distribution of the δ13C and δ15N values was conducted according to species and habitat (Figure 2).
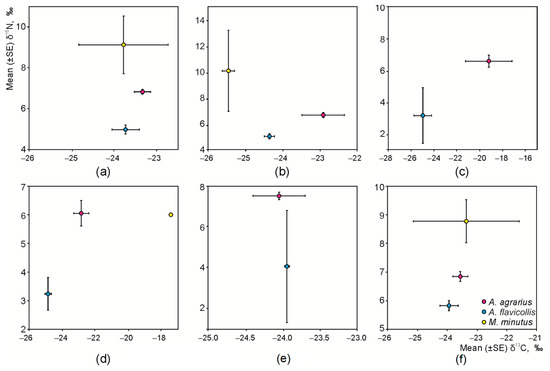
Figure 2.
Distribution of syntopic granivore species according to the stable isotope ratios: (a) irrespective of habitat, (b) apple orchards, (c) plum orchards, (d) raspberry plantations, (e) currant plantations, and (f) meadows, as control habitats.
Separation of species according to δ13C was not expressed in all habitats. Average values of δ13C in the hair of A. agrarius exceeded those of A. flavicollis in the apple orchards (Tukey’s HSD, p < 0.01, Figure 2b), plum orchards (p = 0.06, Figure 2c), and raspberry plantations (p < 0.005, Figure 2d). Despite the species being visually different from the other granivore species (Figure 2b), the average value of δ13C in M. minutus in the apple orchards was significantly smaller than only that in A. flavicollis (p < 0.02). In the control habitats, the variation of δ13C values in M. minutus was very wide (Figure 2f).
According to δ15N, granivorous species were separated irrespective of habitats (Figure 2a). The average values of δ15N in A. agrarius exceeded those of A. flavicollis in apple (Figure 2b, p < 0.001) and plum (Figure 2c, p < 0.10) orchards, in raspberry (Figure 2d, p < 0.002) and currant (Figure 2e, p < 0.01) plantations, and in control habitats (Figure 2f, p < 0.001). The average values of δ15N in M. minutus exceeded those in A. agrarius and A. flavicollis in the apple orchards (Figure 2b, p < 0.01 and p < 0.001, respectively). In the control habitats, the average values of δ15N in M. minutus exceeded those in A. flavicollis (Figure 2f, p = 0.015), while the difference from A. agrarius was not significant (p = 0.17).
The core dietary niches of the three granivorous species, shown as central ellipses in the isotopic space, were fully separated in the apple orchards and raspberry plantations (Figure 3a,b). A small overlap of 3.2% from the total area of the core niche of M. minutus was found with the core dietary niche of A. agrarius in control habitats (Figure 3c). When only two granivorous species were present, namely A. flavicollis and A. agrarius, the core dietary niches were separated in the plum (Figure S1a) and currant orchards (Figure S1b).
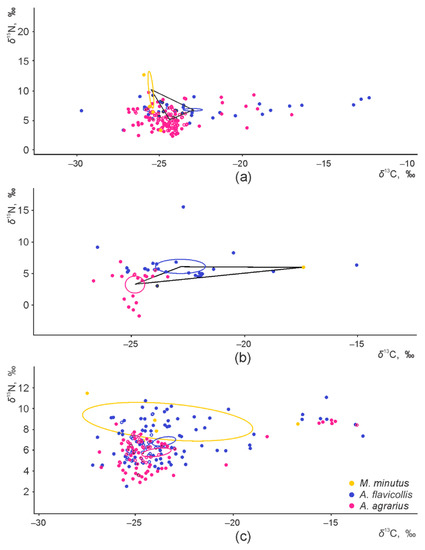
Figure 3.
Central ellipses of the three syntopic granivorous species in the isotopic space, representing fundamental niches in apple orchards (a), raspberry plantations (b), and control habitats (c).
In the apple orchards, control habitats, and raspberry plantations, the total area of the trophic niche was widest in A. agrarius. The central niche in central habitats was widest in M. minutus (see also Figure 3c). In the plum orchards, the widest trophic niche was characteristic to A. flavicollis (Table S2); please note, that area cannot be calculated in the small (n < 5) sample size (Figure S2b, Table S2).
3.3. Intraspecific Differences of the Isotopic Niche in Syntopic Granivores
Irrespective of habitat, intraspecific (age or sex-based) differences of the isotopic niche were not present in any granivorous species (Figure 4); thus, resources were equally used by males and females, adults, subadults, and juveniles of A. flavicollis, A. agrarius, and M. minutus.
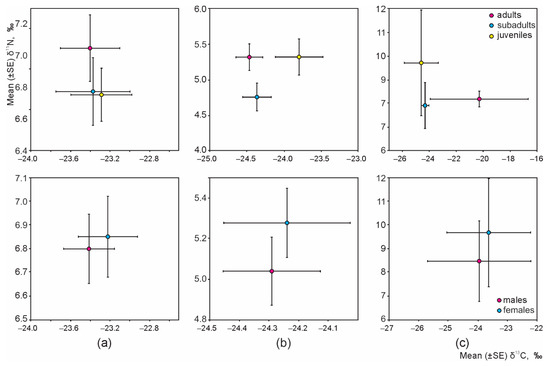
Figure 4.
Intraspecific differences of the isotopic niches of A. flavicollis (a), A. agrarius (b), and M. minutus (c), irrespective of habitat.
4. Discussion
The assembly of the rodent community is inevitably based on the sharing of resources by the species. Resource-sharing is not easy to investigate; therefore, stable carbon and nitrogen isotopes in the tissues or hair of rodents may be used as a proxy, representing the diet of the individual and the trophic niche of the species [44]. Used at the level of animal communities, stable isotope analysis may show not only the dimensions of the trophic niche (e.g., niche width), but also the resource partitioning [9,23,45,46]. Therefore, as mentioned by Post, “stable isotope techniques combine benefits of both the trophic-level and food web paradigms in food web ecology” [47].
Separation of the isotopic niches has been shown in a community of African rodents: in the case of trophic niche overlap, spatial distribution was different [44]. This conforms to the hypothesis that species coexistence should be ensured by the partitioning of resources in space and time if they compete for this resource [25]. The hypothesis was also confirmed for rodents in Africa [44,48], for a flooded meadow in Lithuania [9], and for sympatric/syntopic voles of the genus Microtus in Lithuania [23]. The co-occurrence of species using similar resources has an influence on the trophic niche width and the partitioning of resources [36,37,38,46,48].
In the current study, we found that the isotopic niches (used as a proxy for trophic niches) of the three syntopic granivorous rodent species (A. flavicollis, A. agrarius, and M. minutus) in the commercial orchards, plantations, and control habitats (not subjected to agricultural activities used in orchards and plantations) were separated. The separation according to δ15N was better expressed than the separation according to δ13C. The core dietary niches of these species living syntopically were fully separated in the apple orchards and raspberry plantations, while there was an insignificant overlap of the core niche between M. minutus and A. agrarius in control habitats.
Two granivore species, namely M. minutus and A. agrarius, were not separated in flooded meadows in which resources were abundant for most of the small mammal species from the four guilds-granivores, omnivores, herbivores, and omnivores [9]. From the perspective of co-occurrence, the syntopy of granivores was less expressed than that in herbivore species in the commercial gardens and orchards [23,31]. Therefore, we should presume that these agricultural habitats are richer in resources for herbivores.
The role of the anthropogenic impact on the partitioning of resources in rodents has not been extensively studied, especially in the middle latitudes. For birds, it is known that deforestation in the tropic zone and the resulting loss of habitats influences bird diet and habitat use, with proof obtained by stable isotope analysis: the niches of frugivores, insectivores, nectarivores, and omnivores were narrower in human modified landscapes, while the niches of granivore birds were broader [49].
We agree with the view of Ribeira et al. [50] that more abundant resources result in broader isotopic niches (as a proxy for dietary niche). However, the presence of syntopic species of the same guild (granivores in the current study) seems to have a greater influence than resources. For example, the presence of the brown rat (Rattus norvegicus) affected the diet of the black rat (Rattus rattus) and the house mouse (Mus domesticus), shifting their temporal activities and narrowing trophic niches [51]. In agricultural areas in which resources for granivores are more limited than in flooded meadows, the isotopic niche was wider in A. agrarius (see Reference [9]). In syntopic herbivores from orchards and plantations, the range of δ13C values was not significantly greater than that in herbivores from flooded areas. However, the range of δ15N values was three to four times wider [9,23].
Depending on the habitat and latitude, seasonal changes in the isotopic niche may be expressed [44,50,52], not expressed [23], or absent (this paper).
Resource use may be strongly influenced by competition. In the case of sympatric Keen’s mice (Peromyscus keeni) and dusky shrews (Sorex monticolus), the first being herbivorous (https://animaldiversity.org/accounts/Peromyscus_keeni/, accessed on 5 June 2021) and the second insectivorous (https://animaldiversity.org/accounts/Sorex_monticolus/, accessed on 5 June 2021), their stable carbon and nitrogen values did not differ after a population crash in the mice [53]. Similar interspecific relations were also described among herbivores [54] and omnivorous Peromyscus species [30].
We found that the sympatric and syntopic co-occurrence of granivorous yellow-necked, striped field, and harvest mice in commercial orchards, berry plantations, and nearby meadows is more frequent than that of herbivorous Microtus voles [23]. Our findings show that the analysed agricultural habitats (fruit orchards and berry plantations) are special for syntopic rodent species, therefore requiring further investigation into the isotopic niche, resource partitioning, and influence of guild diversity (also, possibly, species abundances) on trophic ecology.
5. Conclusions
- Syntopic living determines the separation of granivores in the isotopic space with full separation of species according to δ15N values, this presuming different amounts of food of animal origin in their diet.
- The separation of the core dietary niches in the apple orchards and raspberry plantations proved that these agricultural habitats are resource-scarce for granivores.
- Intraspecific differences of the isotopic niche were not present in any of the three granivorous species.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ani11082375/s1: Figure S1: Central ellipses of two syntopic Apodemus species in the isotopic space, representing fundamental niches in plum orchards (a) and currant plantations (b); Table S1: Central position (mean ± SE) and ranges of stable isotope ratios in the hair of A. flavicollis, A. agrarius, and M. minutus in the habitats of commercial orchards, berry plantations, and control meadows in Lithuania, 2018–2020. Abbreviations: AO, apple orchards; PO, plum orchards; RP, raspberry plantations; CP, currant plantations; CH, control habitats; Af, A. flavicollis; Aa, A. agrarius; and Mm, M. minutus; Table S2. Size of isotopic niches of granivorous mice species in agricultural and control habitats: total area (TA), standard ellipse area (SEA), and central ellipses corrected for sample size (SEAc).
Author Contributions
Conceptualisation and investigation, L.B. (Linas Balčiauskas), R.S., A.G., V.S., and L.B. (Laima Balčiauskienė); methodology and formal analysis, L.B. (Linas Balčiauskas), R.S., and A.G.; data curation, V.S. and L.B. (Laima Balčiauskienė); resources, A.G. and V.R.; supervision, project administration, and funding acquisition, L.B. (Linas Balčiauskas) and V.R. All authors have read and agreed to the published version of the manuscript.
Funding
In 2018 and 2019, this study was funded by the Ministry of Agriculture of the Republic of Lithuania, grant number MT-18-3.
Institutional Review Board Statement
The study was approved by the Animal Welfare Committee of the Nature Research Centre, protocol number GGT-7. It was conducted in accordance with Lithuanian (the Republic of Lithuania Law on the Welfare and Protection of Animals No. XI-2271) and European legislation (Directive 2010/63/EU) on the protection of animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
After publication, research data will be available from the corresponding author upon request. The data are not publicly available due to usage in the ongoing study.
Acknowledgments
We thank Jos Stratford for editing the language and Gintautas Vaitonis for the help with graphics.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dickman, C.R. Habitat utilization and diet of the harvest mouse Micromys minutus, in an urban environment. Acta Theriol. 1986, 31, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Liro, A.; Szacki, J. Movements of field mice Apodemus agrarius (Pallas) in a suburban mosaic of habitats. Oecologia 1987, 74, 438–440. [Google Scholar] [CrossRef]
- Herczeg, R.; Horvath, G.F. Species composition and nestedness of small mammal assemblages in two disturbed marshlands. North.-West. J. Zool. 2015, 11, 183–193. [Google Scholar]
- Vecsernyés, F. Autumn habitat selection of the harvest mouse (Micromys minutus Pallas, 1771) in a rural and fragmented landscape. Rev. Suisse Zool. 2020, 126, 111–125. [Google Scholar] [CrossRef]
- Amori, G.; Hutterer, R.; Kryštufek, B.; Yigit, N.; Mitsainas, G.; Palomo, L. Apodemus flavicollis (amended version of 2016 assessment). IUCN Red List Threat. Species 2021, e.T1892A197269879. [Google Scholar] [CrossRef]
- Kaneko, Y.; Kryštufek, B.; Zagarondnyuk, I.; Vohralík, V.; Batsaikhan, N.; Avirmed, D.; Sukhchuluun, G. Apodemus agrarius (errata version published in 2017). IUCN Red List Threat. Species 2016, e.T1888A115057408. [Google Scholar] [CrossRef]
- Kryštufek, B.; Lunde, D.P.; Meinig, H.; Aplin, K.; Batsaikhan, N.; Henttonen, H. Micromys minutus. IUCN Red List Threat. Species 2019, e.T13373A119151882. [Google Scholar] [CrossRef]
- Stefke, K.; Landler, L. Long-term monitoring of rodent and shrew communities in a biodiversity hot-spot in Austria using barn owl (Tyto alba) pellets. Acta Oecol. 2020, 109, 103660. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Skipitytė, R.; Balčiauskienė, L.; Jasiulionis, M. Resource partitioning confirmed by isotopic signatures allows small mammals to share seasonally flooded meadows. Ecol. Evol. 2019, 9, 5479–5489. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L.; Stirkė, V. Mow the Grass at the Mouse’s Peril: Diversity of Small Mammals in Commercial Fruit Farms. Animals 2019, 9, 334. [Google Scholar] [CrossRef] [Green Version]
- Walsh, R.E.; Aprígio Assis, A.P.; Patton, J.L.; Marroig, G.; Dawson, T.E.; Lacey, E.A. Morphological and dietary responses of chipmunks to a century of climate change. Glob. Change Biol. 2016, 22, 3233–3252. [Google Scholar] [CrossRef] [PubMed]
- Abt, K.F.; Bock, W.F. Seasonal variations of diet composition in farmland field mice Apodemus spp. and bank voles Clethrionomys glareolus. Acta Theriol. 1998, 43, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Butet, A.; Delettre, Y.R. Diet differentiation between European arvicoline and murine rodents. Acta Theriol. 2011, 56, 297. [Google Scholar] [CrossRef]
- Pineda-Munoz, S.; Alroy, J. Dietary characterization of terrestrial mammals. P. Roy. Soc. B-Biol. Sci. 2014, 281, 20141173. [Google Scholar] [CrossRef] [PubMed]
- Dayan, T.; Simberloff, D. Morphological relationships among coexisting Heteromyids: An incisive dental character. Am. Nat. 1994, 143, 462–477. [Google Scholar] [CrossRef]
- Brylski, P.; Hall, B. Ontogeny of a macroevolutionary phenotype: The external cheek pouches of geomyoid rodents. Evolution 1988, 42, 391–395. [Google Scholar] [CrossRef]
- Fischer, C.; Gayer, C.; Kurucz, K.; Riesch, F.; Tscharntke, T.; Batáry, P. Ecosystem services and disservices provided by small rodents in arable fields: Effects of local and landscape management. J. Appl. Ecol. 2018, 55, 548–558. [Google Scholar] [CrossRef] [Green Version]
- Luque-Larena, J.J.; Mougeot, F.; Roig, D.V.; Lambin, X.; Rodríguez-Pastor, R.; Rodríguez-Valín, E.; Anda, P.; Escudero, R. Tularemia outbreaks and common vole (Microtus arvalis) irruptive population dynamics in northwestern Spain, 1997–2014. Vector-Borne Zoonot. 2015, 15, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, R.; Crone, E.E. Quantifying the outcome of plant–granivore interactions. Oikos 2012, 121, 20–27. [Google Scholar] [CrossRef]
- Jansen, P.A.; Forget, P.M. Scatterhoarding rodents and tree regeneration. In Nouragues. Dynamics and Plant–Animal Interactions in a Neotropical Rainforest; Bongers, F., Charles-Dominique, P., Forget, P.M., Théry, M., Eds.; Kluver Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 275–288. [Google Scholar]
- Reichman, O.J. Relation of desert rodent diets to available resources. J. Mamm. 1975, 56, 731–751. [Google Scholar] [CrossRef]
- Bartel, S.L.; Orrock, J.L. An omnivorous mesopredator modifies predation of omnivore-dispersed seeds. Ecosphere 2021, 12, e03369. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Skipitytė, R.; Garbaras, A.; Stirkė, V.; Balčiauskienė, L.; Remeikis, V. Stable Isotopes Reveal the Dominant Species to Have the Widest Trophic Niche of Three Syntopic Microtus Voles. Animals 2021, 11, 1814. [Google Scholar] [CrossRef]
- Lancaster, J.; Pillay, N. Behavioral interactions between a coexisting rodent Micaelamys namaquensis and macroscelid Elephantulus myurus. Curr. Zool. 2010, 56, 395–400. [Google Scholar] [CrossRef]
- Pianka, E.R. Niche overlap and diffuse competition. Proc. Natl. Acad. Sci. USA 1974, 71, 2141–2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, E.M.; Paise, G. Temporal niche overlap among insectivorous small mammals. Integr. Zool. 2011, 6, 375–386. [Google Scholar] [CrossRef]
- Castro-Arellano, I.; Lacher, T.E. Temporal niche segregation in two rodent assemblages of subtropical Mexico. J. Trop. Ecol. 2009, 25, 593–603. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, G.; Zhou, Q.; Ma, L.; Wan, X.; Liu, W. Spatial niche partitioning of coexisting small mammals in sand dunes. Ital. J. Zool. 2016, 83, 248–254. [Google Scholar] [CrossRef]
- Magomedov, M.S. Microhabitat partitioning in a rodent community in the arid conditions of the South-western Caspian Lowland. J. Vertebr. Biol. 2021, 70, 20091. [Google Scholar] [CrossRef]
- Stephens, R.B.; Hobbie, E.A.; Lee, T.D.; Rowe, R.J. Pulsed resource availability changes dietary niche breadth and partitioning between generalist rodent consumers. Ecol. Evol. 2019, 9, 10681–10693. [Google Scholar] [CrossRef] [Green Version]
- Stirkė, V.; Balčiauskas, L.; Balčiauskienė, L. Common Vole as a Focal Small Mammal Species in Orchards of the Northern Zone. Diversity 2021, 13, 134. [Google Scholar] [CrossRef]
- Kronfeld, N.; Dayan, T. A new method of determining diets of rodents. J. Mamm. 1998, 79, 1198–1202. [Google Scholar] [CrossRef]
- Hansson, L. Methods of morphological diet microanalysis in rodents. Oikos 1970, 21, 255–266. [Google Scholar] [CrossRef]
- Franco-Trecu, V.; Drago, M.; Riet-Sapriza, F.G.; Parnell, A.; Frau, R.; Inchausti, P. Bias in diet determination: Incorporating traditional methods in Bayesian mixing models. PLoS ONE 2013, 8, e80019. [Google Scholar] [CrossRef] [Green Version]
- Zarzoso-Lacoste, D.; Bonnaud, E.; Corse, E.; Gilles, A.; Meglecz, E.; Costedoat, C.; Gouni, A.; Vidal, E. Improving morphological diet studies with molecular ecology: An application for invasive mammal predation on island birds. Biol. Conserv. 2016, 193, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Missagia, R.V.; Patterson, B.D.; Perini, F.A. Stable isotope signatures and the trophic diversification of akodontine rodents. Evol. Ecol. 2019, 33, 855–872. [Google Scholar] [CrossRef]
- Verde Arregoitia, L.D.; D’Elía, G. Classifying rodent diets for comparative research. Mammal. Rev. 2021, 51, 51–65. [Google Scholar] [CrossRef]
- Shiels, A.B.; Flores, C.A.; Khamsing, A.; Krushelnycky, P.D.; Mosher, S.M.; Drake, D.R. Dietary niche differentiation among three species of invasive rodents (Rattus rattus, R. exulans, Mus musculus). Biol. Invasions 2013, 15, 1037–1048. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Skipitytė, R.; Jasiulionis, M.; Trakimas, G.; Balčiauskienė, L.; Remeikis, V. The impact of Great Cormorants on biogenic pollution of land ecosystems: Stable isotope signatures in small mammals. Sci. Total Environ. 2016, 565, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Balčiauskas, L. Methods of Investigation of Terrestrial Ecosystems; Part. I. Animal Surveys; VU Leidykla: Vilnius, Lithuania, 2004; p. 183. [Google Scholar]
- Rezzani, R.; Nardo, L.; Favero, G.; Peroni, M.; Rodella, L.F. Thymus and aging: Morphological, radiological, and functional overview. Age 2014, 36, 313–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balciauskas, L.; Balciauskiene, L.; Janonyte, A. Reproduction of the root vole (Microtus oeconomus) at the edge of its distribution range. Turk. J. Zool. 2012, 36, 668–675. [Google Scholar]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef]
- Codron, J.; Duffy, K.J.; Avenant, N.L.; Sponheimer, M.; Leichliter, J.; Paine, O.; Sandberg, P.; Codron, D. Stable isotope evidence for trophic niche partitioning in a South African savanna rodent community. Curr. Zool. 2015, 61, 397–411. [Google Scholar] [CrossRef]
- Polis, G.A. Age structure component of niche width and intraspecific resource partitioning: Can age groups function as ecological species? Am. Nat. 1984, 123, 541–564. [Google Scholar] [CrossRef]
- Sheppard, C.E.; Inger, R.; McDonald, R.A.; Barker, S.; Jackson, A.L.; Thompson, F.J.; Vitikainen, E.I.K.; Cant, M.A.; Marshall, H.H. Intragroup competition predicts individual foraging specialisation in a group-living mammal. Ecol. Lett. 2018, 21, 665–673. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Symes, C.T.; Wilson, J.W.; Woodborne, S.M.; Shaikh, Z.S.; Scantlebury, M. Resource partitioning of sympatric small mammals in an African forest-grassland vegetation mosaic. Austral. Ecol. 2013, 38, 721–729. [Google Scholar] [CrossRef]
- Navarro, A.B.; Magioli, M.; Bogoni, J.A.; Moreira, M.Z.; Silveira, L.F.; Alexandrino, R.; Apolinario da Luz, D.T.; Pizo, M.A.; Silva, W.R.; Cristina de Oliveira, V.; et al. Human-modified landscapes narrow the isotopic niche of neotropical birds. Oecologia 2021, 196, 171–184. [Google Scholar] [CrossRef]
- Ribeiro, J.F.; Guaraldo, A.; Nardoto, G.B.; Santoro, G.; Vieira, E.M. Habitat type and seasonality influence the isotopic trophic niche of small mammals in a neotropical savanna. Hystrix It. J. Mamm. 2019, 30, 30–38. [Google Scholar] [CrossRef]
- Mori, E.; Ferretti, F.; Fattorini, N. Alien war: Ectoparasite load, diet and temporal niche partitioning in a multi-species assembly of small rodents. Biol. Invasions 2019, 21, 3305–3318. [Google Scholar] [CrossRef]
- Hope, A.G.; Gragg, S.F.; Nippert, J.B.; Combe, F.J. Consumer roles of small mammals within fragmented native tallgrass prairie. Ecosphere 2021, 12, e03441. [Google Scholar] [CrossRef]
- Eckrich, C.A.; Flaherty, E.A.; Ben-David, M. Functional and numerical responses of shrews to competition vary with mouse density. PLoS ONE 2018, 13, e0189471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, C.; Guo, Q.; Zhang, Z.; Li, X.; Man, D.; Yuan, S.; Fu, H.; Wu, X.; Jin, G.; Liu, J.; et al. Trophic niche of Brandt’s voles (Lasiopodomys brandtii) and their interspecific relationships with other common rodents in a typical steppe, Inner Mongolia. Acta Theriol. Sinica 2020, 40, 424. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).