Quercetin Dietary Supplementation Advances Growth Performance, Gut Microbiota, and Intestinal mRNA Expression Genes in Broiler Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals, Management and the Experimental Design
2.3. Growth Performance
2.4. Sample Collection
2.5. Total Fecal Bacterial Count
2.6. Hemagglutination Inhibition (HI) Assay
2.7. RNA Extraction and RT-PCR
2.8. Histology
2.9. Statistical Analysis
3. Results
3.1. Growth Performance and Survival Percentages
3.2. Total Fecal Bacterial Count
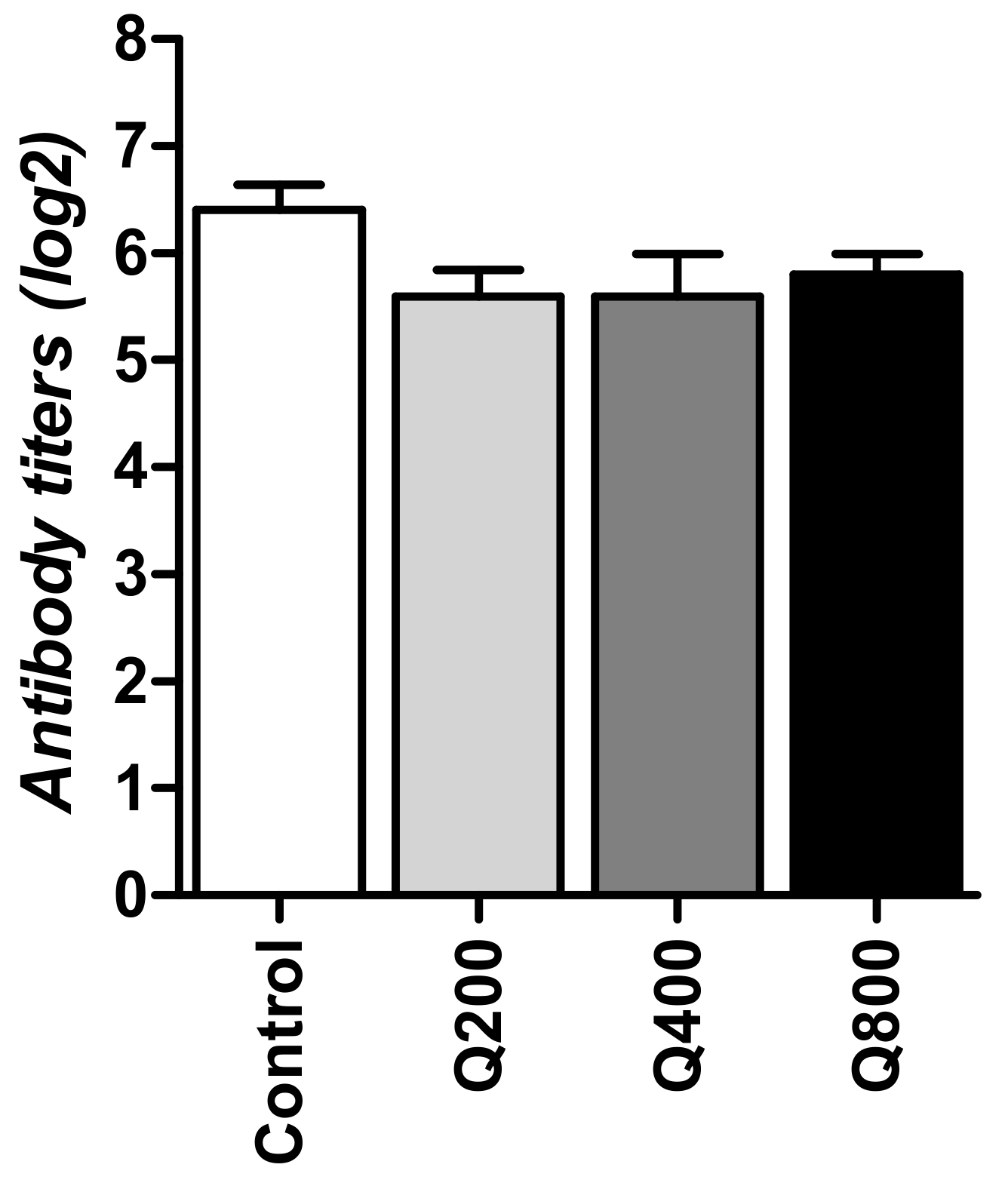
3.3. Hemagglutination Inhibition Test
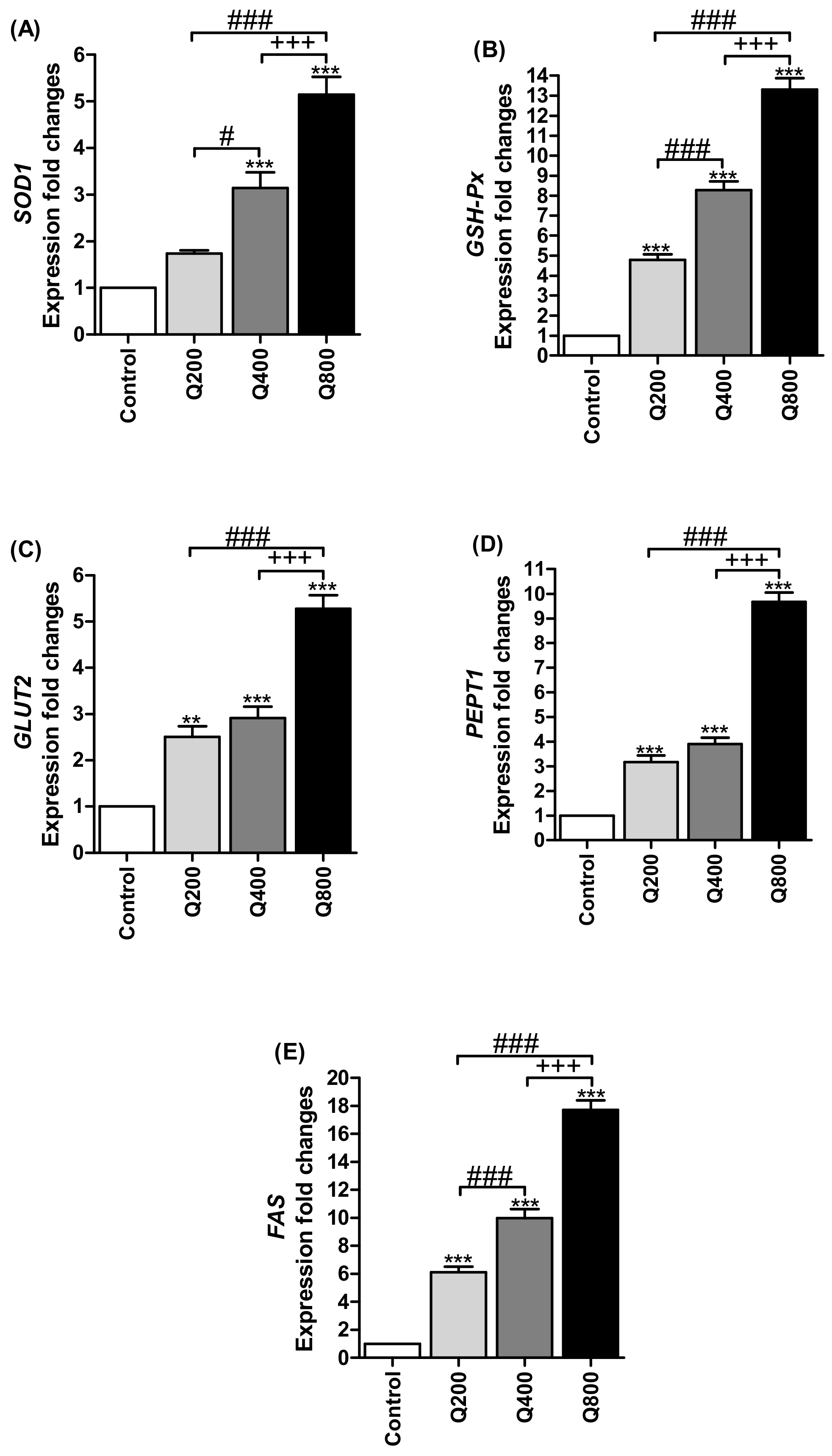
3.4. Antioxidant Enzymes’ Gene Expressions
3.5. Nutrients Transporter Gene Expressions
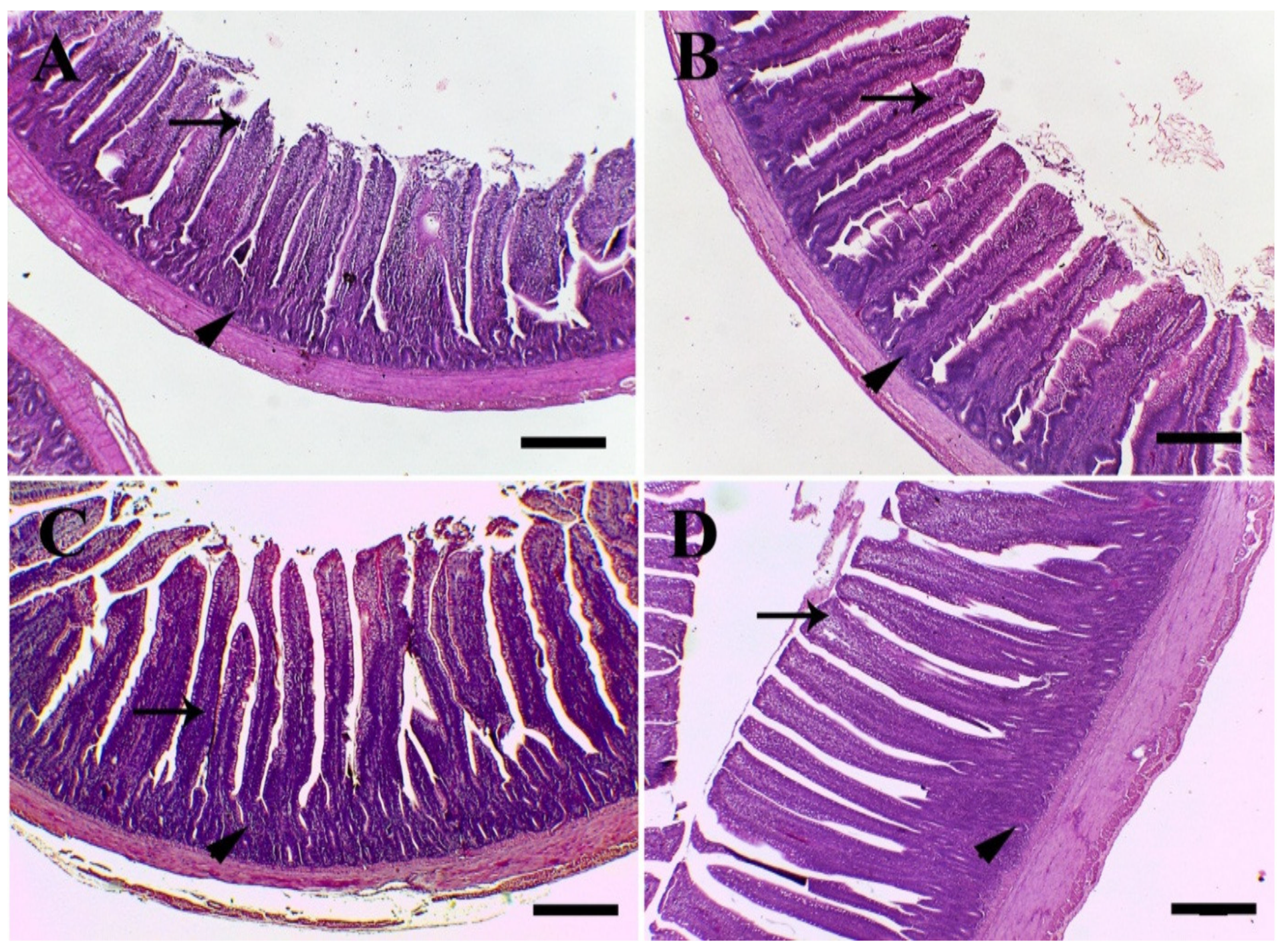
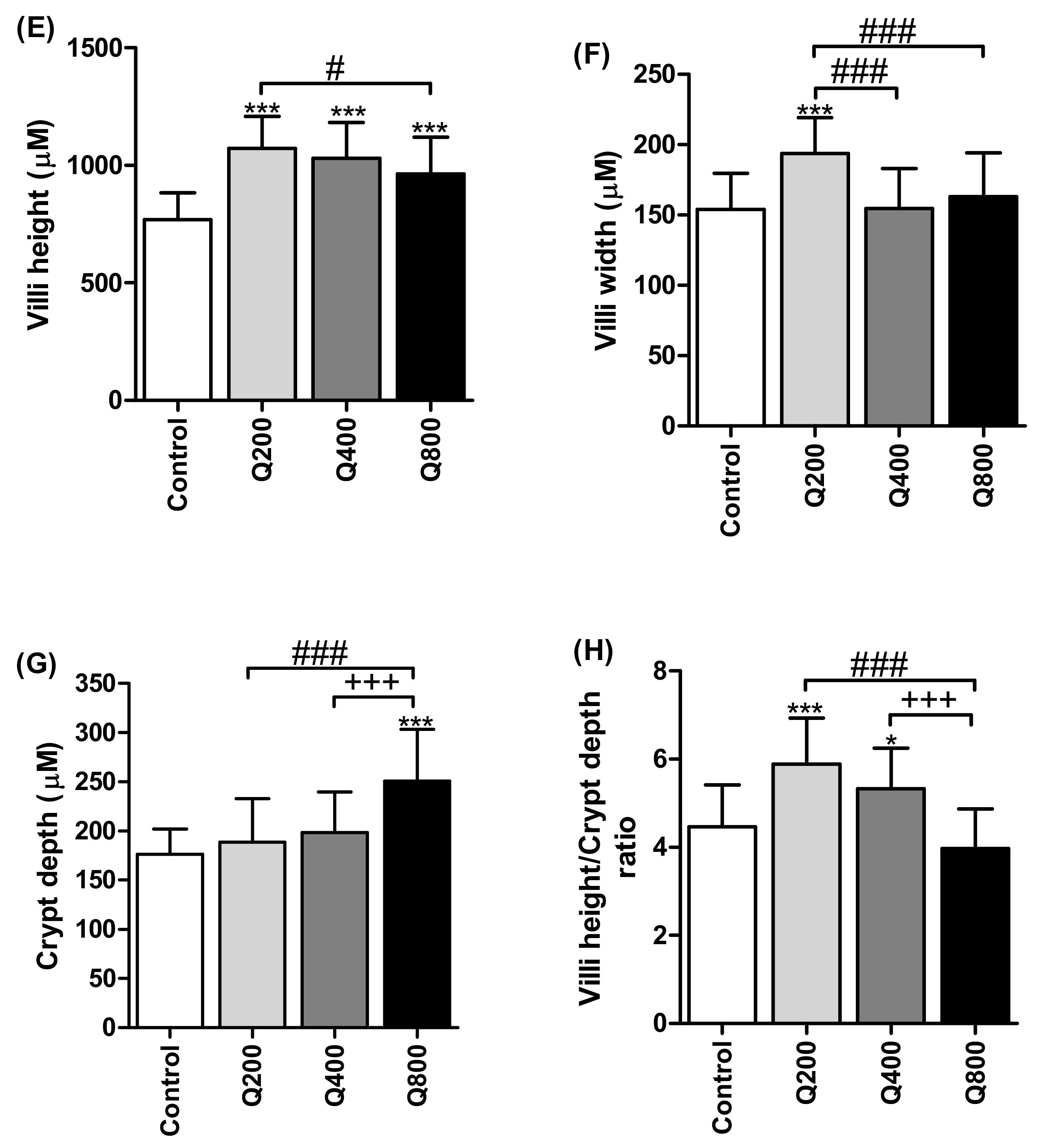
3.6. Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clavijo, V.; Flórez, M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: A review. Poult. Sci. 2018, 97, 1006–1021. [Google Scholar] [CrossRef]
- Schokker, D.; Jansman, A.J.M.; Veninga, G.; De Bruin, N.; Vastenhouw, S.A.; De Bree, F.M.; Bossers, A.; Rebel, J.M.J.; Smits, M.A. Perturbation of microbiota in one-day old broiler chickens with antibiotic for 24 hours negatively affects intestinal immune development. BMC Genom. 2017, 18, 241. [Google Scholar] [CrossRef] [Green Version]
- Ayoub, M.; El-Far, A.; Taha, N.; Korshom, M.; Mandour, A.; Abdel-Hamid, H.; El-Neweshy, M. The biochemical protective role of some herbs against aflatoxicosis in ducklings: II. Nigella sativa. Lucr. Științ. Univ. Științe Agric. Med. Vet. Ser. Zooteh. 2011, 55, 68–77. [Google Scholar]
- Abdel-Latif, M.A.; Abd El-Hack, M.E.; Swelum, A.A.; Saadeldin, I.M.; Elbestawy, A.R.; Shewita, R.S.; Ba-Awadh, H.A.; Alowaimer, A.N.; Abd El-Hamid, H.S. Single and Combined Effects of Clostridium butyricum and Saccharomyces cerevisiae on Growth Indices, Intestinal Health, and Immunity of Broilers. Animals 2018, 8, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Latif, M.A.; El-Far, A.H.; Elbestawy, A.R.; Ghanem, R.; Mousa, S.A.; Abd El-Hamid, H.S. Exogenous dietary lysozyme improves the growth performance and gut microbiota in broiler chickens targeting the antioxidant and non-specific immunity mRNA expression. PLoS ONE 2017, 12, e0185153. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Samak, D.H.; Noreldin, A.E.; El-Naggar, K.; Abdo, M. Probiotics and plant-derived compounds as eco-friendly agents to inhibit microbial toxins in poultry feed: A comprehensive review. Environ. Sci. Pollut. Res. Int. 2018, 25, 31971–31986. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Chaudhry, M.T.; Mahrose, K.M.; Noreldin, A.; Emam, M.; Alagawany, M. The efficacy of using exogenous enzymes cocktail on production, egg quality, egg nutrients and blood metabolites of laying hens fed distiller’s dried grains with solubles. J. Anim. Physiol. Anim. Nutr. 2018, 102, e726–e735. [Google Scholar] [CrossRef] [PubMed]
- El-Far, A.H.; Ahmed, H.A.; Shaheen, H.M. Dietary Supplementation of Phoenix dactylifera Seeds Enhances Performance, Immune Response, and Antioxidant Status in Broilers. Oxid. Med. Cell. Longev. 2016, 2016, 5454963. [Google Scholar] [CrossRef] [Green Version]
- Wenk, C. Herbs and botanicals as feed additives in monogastric animals. Asian Aust. J. Anim. Sci. 2003, 16, 282–289. [Google Scholar] [CrossRef]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules (Basel Switzerland) 2016, 21, 623. [Google Scholar] [CrossRef] [PubMed]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Lakhanpal, P.; Rai, D.K. Quercetin: A versatile flavonoid. Internet J. Med. Update 2007, 2, 22–37. [Google Scholar] [CrossRef] [Green Version]
- Goliomytis, M.; Tsoureki, D.; Simitzis, P.; Charismiadou, M.; Hager-Theodorides, A.; Deligeorgis, S. The effects of quercetin dietary supplementation on broiler growth performance, meat quality, and oxidative stability. Poult. Sci. 2014, 93, 1957–1962. [Google Scholar] [CrossRef]
- Saeed, M.; Naveed, M.; Arain, M.; Arif, M.; El-Hack, M.A.; Alagawany, M.; Siyal, F.; Soomro, R.; Sun, C. Quercetin: Nutritional and beneficial effects in poultry. World’s Poult. Sci. J. 2017, 73, 355–364. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Poultry; National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Analytical Chemists International; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Marcu, A.; Vacaru-Opriş, I.; Dumitrescu, G.; Ciochina, L.P.; Marcu, A.; Nicula, M.; Peţ, I.; Dronca, D.; Kelciov, B. The influence of the genotype on economic efficiency of broiler chickens growth. Sci. Pap. Anim. Sci. Biotechnol. 2013, 46, 339–346. [Google Scholar]
- Takátsy, G. The use of spiral loops in serological and virological micro-methods. Acta Microbiol. Immunol. Hung. 1955, 50, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Brugh, M., Jr. A simple method for recording and analyzing serological data. Avian Dis. 1978, 22, 362–365. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Layton, C. The hematoxylin and eosin, connective and mesenchymal tissues with their stains. In Bancroft s Theory and Practice of Histological Techniques, 7th ed.; Kim suvarna, S., Layton, C., Bancroft, J.D., Eds.; Churchill Livingstone: Philadelphia, PA, USA, 2013; pp. 173–186. [Google Scholar]
- Saeed, M.; Yatao, X.; Hassan, F.U.; Arain, M.A.; Abd El-Hack, M.E.; Noreldin, A.E.; Sun, C. Influence of Graded Levels of l-Theanine Dietary Supplementation on Growth Performance, Carcass Traits, Meat Quality, Organs Histomorphometry, Blood Chemistry and Immune Response of Broiler Chickens. Int. J. Mol. Sci. 2018, 19, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.A.M.; Roushdy, E.M.; Kishawy, A.T.Y.; Zaglool, A.W.; Tukur, H.A.; Saadeldin, I.M. Growth Performance, Antioxidant Capacity, Lipid-Related Transcript Expression and the Economics of Broiler Chickens Fed Different Levels of Rutin. Animals 2018, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Akbarian, A.; Michiels, J.; Golian, A.; Buyse, J.; Wang, Y.; De Smet, S. Gene expression of heat shock protein 70 and antioxidant enzymes, oxidative status, and meat oxidative stability of cyclically heat-challenged finishing broilers fed Origanum compactum and Curcuma xanthorrhiza essential oils. Poult. Sci. 2014, 93, 1930–1941. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Faseleh Jahromi, M.; Liang, J.B.; Soleimani Farjam, A.; Shokryazdan, P.; Idrus, Z. Effect of Dietary Lead on Intestinal Nutrient Transporters mRNA Expression in Broiler Chickens. BioMed Res. Int. 2015, 2015, 149745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Zeng, D.; Ni, X.; Tu, T.; Yin, Z.; Pan, K.; Jing, B. Effects of Bacillus licheniformis on the growth performance and expression of lipid metabolism-related genes in broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Lipids Health Dis. 2016, 15, 48. [Google Scholar] [CrossRef] [Green Version]
- Graefe, E.U.; Wittig, J.; Mueller, S.; Riethling, A.K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H.; Veit, M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2001, 41, 492–499. [Google Scholar] [CrossRef]
- Crespy, V.; Morand, C.; Besson, C.; Manach, C.; Demigne, C.; Remesy, C. Quercetin, but not its glycosides, is absorbed from the rat stomach. J. Agric. Food Chem. 2002, 50, 618–621. [Google Scholar] [CrossRef]
- Day, A.J.; Gee, J.M.; DuPont, M.S.; Johnson, I.T.; Williamson, G. Absorption of quercetin-3-glucoside and quercetin-4′-glucoside in the rat small intestine: The role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochem. Pharmacol. 2003, 65, 1199–1206. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.; Ronalds, C.M.; Rathgeber, B.; Robinson, R.A. Absorption and tissue distribution of dietary quercetin and quercetin glycosides of apple skin in broiler chickens. J. Sci. Food Agric. 2010, 90, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Bieger, J.; Cermak, R.; Blank, R.; de Boer, V.C.; Hollman, P.C.; Kamphues, J.; Wolffram, S. Tissue distribution of quercetin in pigs after long-term dietary supplementation. J. Nutr. 2008, 138, 1417–1420. [Google Scholar] [CrossRef] [PubMed]
- Wein, S.; Wolffram, S. Oral bioavailability of quercetin in horses. J. Equine Vet. Sci. 2013, 33, 441–445. [Google Scholar] [CrossRef]
- Sallam, K.I.; Ishioroshi, M.; Samejima, K. Antioxidant and antimicrobial effects of garlic in chicken sausage. Lebensm. Wiss. Technol. 2004, 37, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Willis, W.L.; Goktepe, I.; Isikhuemhen, O.S.; Reed, M.; King, K.; Murray, C. The effect of mushroom and pokeweed extract on salmonella, egg production, and weight loss in molting hens. Poult. Sci. 2008, 87, 2451–2457. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.N.; Liu, Y.; Hu, L.L.; Suo, Y.L.; Zhang, L.; Jin, F.; Feng, X.A.; Teng, N.; Li, Y. Effects of dietary supplementation of quercetin on performance, egg quality, cecal microflora populations, and antioxidant status in laying hens. Poult. Sci. 2014, 93, 347–353. [Google Scholar] [CrossRef]
- Ohemeng, K.; Schwender, C.; Fu, K.; Barrett, J. DNA gyrase inhibitory and antibacterial activity of some flavones (1). Bioorga. Med. Chem. Lett. 1993, 3, 225–230. [Google Scholar] [CrossRef]
- Mirzoeva, O.; Grishanin, R.; Calder, P. Antimicrobial action of propolis and some of its components: The effects on growth, membrane potential and motility of bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef]
- Tasdemir, D.; Lack, G.; Brun, R.; Rüedi, P.; Scapozza, L.; Perozzo, R. Inhibition of Plasmodium f alciparum Fatty Acid Biosynthesis: Evaluation of FabG, FabZ, and FabI as Drug Targets for Flavonoids. J. Med. Chem. 2006, 49, 3345–3353. [Google Scholar] [CrossRef]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. D-Alanine: D-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef]
- Rychlik, I. Composition and Function of Chicken Gut Microbiota. Animals 2020, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Teirlynck, E.; Bjerrum, L.; Eeckhaut, V.; Huygebaert, G.; Pasmans, F.; Haesebrouck, F.; Dewulf, J.; Ducatelle, R.; Van Immerseel, F. The cereal type in feed influences gut wall morphology and intestinal immune cell infiltration in broiler chickens. Br. J. Nutr. 2009, 102, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Sukhotnik, I.; Moati, D.; Shaoul, R.; Loberman, B.; Pollak, Y.; Schwartz, B. Quercetin prevents small intestinal damage and enhances intestinal recovery during methotrexate-induced intestinal mucositis of rats. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Y.; Liu, S.; Xie, X.N.; Tan, Z.R. Regulation profile of the intestinal peptide transporter 1 (PepT1). Drug Des. Dev. Ther. 2017, 11, 3511–3517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Yang, Z.; Rey, F.E.; Ridaura, V.K.; Davidson, N.O.; Gordon, J.I.; Semenkovich, C.F. Fatty acid synthase modulates intestinal barrier function through palmitoylation of mucin 2. Cell Host Microbe 2012, 11, 140–152. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Nagababu, E.; Chrest, F.J.; Rifkind, J.M. Hydrogen-peroxide-induced heme degradation in red blood cells: The protective roles of catalase and glutathione peroxidase. Biochim. Biophys. Acta 2003, 1620, 211–217. [Google Scholar] [CrossRef]
- Iskender, H.; Yenice, G.; Dokumacioglu, E.; Kaynar, O.; Hayirli, A.; Kaya, A. The effects of dietary flavonoid supplementation on the antioxidant status of laying hens. Braz. J. Poult. Sci. 2016, 18, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Korver, D. Implications of changing immune function through nutrition in poultry. Anim. Feed Sci. Technol. 2012, 173, 54–64. [Google Scholar] [CrossRef]

| Ingredients % | Starter (0–10 d) | Grower (11–21 d) | Finisher (22–35 d) |
|---|---|---|---|
| Yellow corn | 54.78 | 58.88 | 63.90 |
| Soybean meal (44%) | 33.5 | 29.4 | 24 |
| Corn gluten (60%) | 5 | 5 | 5 |
| Corn oil | 2 | 2.65 | 3.15 |
| Dicalcium phosphate | 1.73 | 1.6 | 1.5 |
| Lime stone | 1.35 | 1 | 1 |
| Salt | 0.4 | 0.4 | 0.4 |
| DL-methionine * | 0.15 | 0.12 | 0.1 |
| HCl-lysine ** | 0.35 | 0.3 | 0.3 |
| Vitamins and minerals premix *** | 0.3 | 0.3 | 0.3 |
| Antimycotoxin | 0.2 | 0.2 | 0.2 |
| Sodium bicarbonate | 0.1 | 0.1 | 0.1 |
| Choline chloride | 0.05 | 0.05 | 0.05 |
| Calculated composition | |||
| ME, Kcal/Kg diet | 3005 | 3100 | 3195 |
| CP% | 23 | 21.5 | 19.5 |
| Ca% | 1 | 0.87 | 0.82 |
| Avail. P% | 0.47 | 0.44 | 0.41 |
| Methionine% | 0.56 | 0.51 | 0.47 |
| Lysine% | 1.44 | 1.29 | 1.14 |
| Meth. + Cyst.% | 0.93 | 0.86 | 0.78 |
| Na% | 0.20 | 0.20 | 0.20 |
| Ingredients | Amount (g) |
|---|---|
| Tryptose | 15.0 |
| Soya peptone | 5.0 |
| Yeast extract | 5.0 |
| Sodium metabisulphite | 1.0 |
| Ferric ammonium citrate | 1.0 |
| Agar | 19.0 |
| Distilled water added to make 1 liter | |
| Ingredients | Amount (g) |
|---|---|
| Tryptone | 10.0 |
| Yeast extract | 5.0 |
| Glucose | 20.0 |
| Sodium acetate, anhydrous | 17.0 |
| Ammonium citrate | 2.0 |
| Potassium dihydrogen phosphate | 6.0 |
| Magnesium sulfate | 0.575 |
| Manganese sulfate | 0.120 |
| Ferrous sulfate | 0.034 |
| Bacteriological Agar | 20 |
| Tween 80 | 1 |
| Distilled water added to make 1 liter | |
| Target Gene | Primers Sequences | Reverse Transcription | Primary Denaturation | Amplification (40 Cycles) | Dissociation Curve (1 Cycle) | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Secondary Denaturation | Annealing (Optics on) | Extension | Secondary Denaturation | Annealing | Final Denaturation | |||||
| β. actin | F: ATTGTCCACCGCAA ATGCTTC | 50 °C 30 min | 94 °C 5 min | 94 °C 15 s | 60 °C 30 s | 72 °C 30 s | 94 °C 1 min | 60 °C 1 min | 94 °C 1 min | [23] |
| R: AAATAAAGCCATGCCAATCTCGTC | ||||||||||
| SOD1 | F: AGGGGGTCATCCACTTCC | 60 °C 30 s | 60 °C 1 min | [24] | ||||||
| R: CCCATTTGTGTTGTCTCCAA | ||||||||||
| GSH-PX | F: TTGTAAACATCAGGGGCAAA | |||||||||
| R: ATGGGCCAAGATCTTTCTGTAA | ||||||||||
| GLUT2 | F: CACACTATGGGCGCATGCT | 60 °C 30 s | 60 °C 1 min | [25] | ||||||
| R: ATTGTCCCTGGAGGTGTTGGTG | ||||||||||
| PEPT1 | F: CCCCTGAGGAGGATCACTGTT | |||||||||
| R: CAAAAGAGCAGCAGCAACGA | ||||||||||
| FAS | F: CTATCGACACAGCCTGCTCCT | 62 °C 30 s | 62 °C 1 min | [26] | ||||||
| R: CAGAATGTTGACCCCTCCTACC | ||||||||||
| Control | Quercetin Supplementation | ||||
|---|---|---|---|---|---|
| Q200 | Q400 | Q800 | p-Value | ||
| Initial weight, g | 41.46 ± 0.47 | 41.67 ± 0.49 | 41.67 ± 0.49 | 41.67 ± 0.49 | 0.99 |
| 1 fBwt, g | 1768.10 ± 31.56 | 1826.59 ± 35.88 | 1824.29± 31.97 | 1809.13 ± 25.77 | 0.54 |
| 2 BWG, g | 1727.14 ± 31.36 | 1785 ± 35.58 | 1782.86 ± 31.60 | 1767.61 ± 25.45 | 0.54 |
| BWG/day, g | 49.35 ± 0.90 | 51 ± 1.02 | 50.94 ± 0.90 | 50.50 ± 0.73 | 0.54 |
| 3 FI, g | 2887.24± 25.32 ab | 2848.31 ± 28.45 b | 2894.72 ± 6.11 ab | 2926.58 ± 11.29 a | 0.05 |
| 4 FCR | 1.67 ± 0.03 | 1.60 ± 0.033 | 1.63 ± 0.03 | 1.66 ± 0.02 | 0.32 |
| 5 PER | 2.80 ± 0.05 | 2.93 ± 0.06 | 2.87 ± 0.05 | 2.81 ± 0.04 | 0.27 |
| 6 EPEF | 296.54 ± 10.12 b | 331.06 ± 13.03 a | 323.29 ± 11.54 ab | 313 ± 8.32 ab | 0.01 |
| Mortality% | 3.13 | 0 | 0 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Latif, M.A.; Elbestawy, A.R.; El-Far, A.H.; Noreldin, A.E.; Emam, M.; Baty, R.S.; Albadrani, G.M.; Abdel-Daim, M.M.; Abd El-Hamid, H.S. Quercetin Dietary Supplementation Advances Growth Performance, Gut Microbiota, and Intestinal mRNA Expression Genes in Broiler Chickens. Animals 2021, 11, 2302. https://doi.org/10.3390/ani11082302
Abdel-Latif MA, Elbestawy AR, El-Far AH, Noreldin AE, Emam M, Baty RS, Albadrani GM, Abdel-Daim MM, Abd El-Hamid HS. Quercetin Dietary Supplementation Advances Growth Performance, Gut Microbiota, and Intestinal mRNA Expression Genes in Broiler Chickens. Animals. 2021; 11(8):2302. https://doi.org/10.3390/ani11082302
Chicago/Turabian StyleAbdel-Latif, Mervat A., Ahmed R. Elbestawy, Ali H. El-Far, Ahmed E. Noreldin, Mohamed Emam, Roua S. Baty, Ghadeer M. Albadrani, Mohamed M. Abdel-Daim, and Hatem S. Abd El-Hamid. 2021. "Quercetin Dietary Supplementation Advances Growth Performance, Gut Microbiota, and Intestinal mRNA Expression Genes in Broiler Chickens" Animals 11, no. 8: 2302. https://doi.org/10.3390/ani11082302
APA StyleAbdel-Latif, M. A., Elbestawy, A. R., El-Far, A. H., Noreldin, A. E., Emam, M., Baty, R. S., Albadrani, G. M., Abdel-Daim, M. M., & Abd El-Hamid, H. S. (2021). Quercetin Dietary Supplementation Advances Growth Performance, Gut Microbiota, and Intestinal mRNA Expression Genes in Broiler Chickens. Animals, 11(8), 2302. https://doi.org/10.3390/ani11082302










