Simple Summary
The increasing societal concern in animal welfare has changed centuries-old practices in pig breeding. One of the most debatable is the surgical castration of piglets commonly used in many European countries primarily performed to avoid boar taint. Boar taint is an undesirable and unpleasant odor that is released by mature entire male pigs. The two main compounds responsible for boar taint are androstenone and skatole. While androstenone as a steroid is mainly affected genetically, skatole as a product of microbial degradation of the amino acid tryptophan in hind gut can be influenced by nutrition and feeding. Recently, several studies revealed that hydrolysable tannins in the pig diet have the potential to reduce skatole accumulation in fat tissue. Thus, the objective of this study was to determine the effect of tannin administration (1, 2, 3, and 4%—sweet chestnut extract rich in hydrolysable tannins) on skatole as well as androstenone deposition in adipose tissue, and to observe the impact on carcass and meat quality, chemical, amino and fatty acid composition, and boar taint perception. The results showed that tannins in the diet of entire males did not affect chemical composition, androstenone accumulation, amino, and fatty acid profile in subcutaneous tissue. The effect on carcass value was only slight. However, higher doses of tannins (3 and 4%) increased cooking loss, and partially increased (4% dosage) electrical conductivity in semimembranosus muscle. Skatole concentration in fat tissue had a tendency to decline after 2–4% administration compared to control group. Similarly, a tendency to drop in boar taint perception using “hot iron” method was found between control and 2%-supplemented pigs. Higher dietary tannins supplementation (3 and/or 4%) increased several saturated (SFA), monounsaturated (MUFA), and polyunsaturated fatty acids (PUFA) in pork.
Abstract
The slaughtering of entire males increases the probability of incidence of tainted pork due to the presence two main compounds—androstenone and skatole. If a surgical castration of young entire male pigs is stopped in the EU countries, fattening of boars is likely to become one of the most commonly used systems in pig farming. Since skatole production and accumulation in fat tissue can be controlled by dietary approaches, several studies have investigated various feed additives to reduce this compound of boar taint. Ones of the most promising is tannins. The aim of this study was to determine the effect of different dietary tannin level supplementation on carcass, pork quality, chemical, amino and fatty acid composition. as well as perception of boar taint and accumulation of skatole and androstenone in adipose tissue. Eighty entire males were randomly distributed to control (T0) and four experimental groups. Control pigs received standard feed mixture (16.8% CP, 13.9 MJ ME) without any tannin supplementation. Experimental pigs received the same diet with administration of 1% (T1), 2% (T2), 3% (T3) and 4% (T4)—sweet chestnut extract rich in hydrolysable tannins for 40 days (from average live weight of 80 kg until slaughter at average weight 122.28 kg ± 5.63 kg). Dietary tannins supplementation did not show any significant effect on chemical composition, cholesterol content, and amino acid composition of muscle as well as fatty acid composition and androstenone accumulation in adipose tissue. A slight or small effect was observed on carcass and meat quality, respectively. Pigs in groups T4 and/or T3-T4 had higher electrical conductivity in semimembranosus muscle and cooking loss value compared to T1, T2 or T0, T1, and T2 groups (p < 0.05). Tannins in the pig’s diet greatly affected fatty acid profile in meat of entire males. The highest tannin levels (4%) increased concentrations of lauric, myristic, vaccenic, linoleic, total PUFA, and n-6 PUFA in muscle compared to the control. Similar results were found in group T3 except for vaccenic, linoleic, and total PUFA. On the contrary, concentrations of heptadecanoic and oleic acids in groups T3 and T4 were lower than those in T1 and T2 groups. Perception of boar taint using „hot iron“ method (insertion a hot iron tip of soldering iron into adipose tissue) tended to decrease in T2 group compared with control. Skatole accumulation in fat tissue was reduced in groups T2-T4 at significance level (p = 0.052–0.055) compared to the control pigs. In summary, tannins supplementation had no effect on chemical and amino acid composition as well as fatty acid profile in adipose tissue, and only slight on carcass value. However, 4% concentration of tannins significantly increased content of some fatty acids compared to control group.
1. Introduction
Avoiding an undesirable odor, so-called boar taint that is released by sexually maturing young boars, as well as improving the pork and sensory quality of their meat are the main reasons for surgical castration of pigs. This technique has been practiced in European pig farming for several centuries. However, increasing concern in animal welfare in the last decade has caused that surgical castration of piglets seems to be unsustainable and is likely to be abandoned in the near future. In 2010, several European countries agreed to voluntarily abandon of surgical castration and are working on suitable alternatives. European Commission strongly supports these activities.
Currently, there are two animal-welfare and economically acceptable alternatives to common surgical practice: Immunocastration and fattening entire males. Immunocastration is based on vaccination against gonadotropin-releasing hormone (GnRH), which leads to inhibition of luteinising hormone (LH) and subsequently elimination of androstenone—one of the two main compounds responsible for boar taint [,]. The effect of immunocastration on various aspects of entire male production has recently been the subject of extensive research, e.g., on growth intensity, carcass performance and meat quality [,,,,], fatty acid composition in muscle or subcutaneous fat [,,,], sensory characteristics and consumer´s acceptance of meat [,], or boar taint compounds [,]. Fattening entire males might also become a suitable alternative because of some advantages of boars over surgical castrates such as greater growth intensity, better feed conversion, and higher lean meat content in carcass. However, slaughtering the boars increases the risk of higher incidence of boar taint, and thus dissatisfaction of consumers with such meat. Therefore, suitable methods for boar taint detection and reduction of its levels in pork need to be developed.
Boar taint is primarily caused by two compounds: Androstenone [] and skatole []. Androstenone is a steroid synthesized in the Leydig cells in the testicles of uncastrated boars. It acts as pheromone in male mammals. In boars, androstenone is transported to the salivary gland. During sexual excitement, it is excreted into saliva and causes so-called immobility reflex (heating) of sow in estrus. Androstenone has an odor like urine or sweat and is perceived by about two-thirds of the human population. It is mainly affected by genetic background and puberty stage and to a lesser extent by environmental factors such as nutrition and feeding, housing conditions, management, etc. Therefore, it is generally accepted that dietary manipulations have a less importance in the production and accumulation of androstenone. Nevertheless, a few studies also reported positive (though insignificant) effect, of dietary supplementation of feed additives on androstenone levels [,,,,].
Skatole is a product of microbial degradation of amino acid tryptophan in the large intestine of pigs. It is absorbed from the intestine into blood and through portal vein transported to the liver where is metabolized to a variety of metabolites []. In case of high concentration of skatole in blood stream, it is transported and accumulated in the fatty tissue []. Unlike androstenone, skatole levels are affected mainly by environmental factors. Dietary composition, e.g., amount of tryptophan or energy sources for microbial activity, may influence intestinal skatole synthesis [] due to changing the rate of apoptosis, thus reducing the availability of tryptophan in the colon. Nutrition also can change composition of microbiota in large intestine [], passage of feed through the digestive system, and absorption and metabolism of skatole in the liver.
Recently, several studies have been conducted to reduce skatole levels by nutritional manipulations. Promising results have been achieved by supplementation of boar´s diet with a variety of feed additives such as chicory root or inulin [,,,,,,,,,], raw potato starch [,,,], sugar beet pulp [], Jerusalem artichoke [], oligofructose and fructooligosaccharides [,], and tannins [,,]. The last one is the group of natural astringent polyphenolic compounds widely distributed in many species of plants, where they play a role in protection from predation and help in regulating plant growth [,].
Generally, tannins can be divided into (i) condensed and (ii) hydrolysable. Although they were primarily considered to have an anti-nutritional effect on digestibility of nutrients from feed, and then reduction of growth intensity of pigs [,], recent research has showed that both condensed and hydrolysable may have harmful or beneficial effects depending on their structure and concentrations []. Extract from sweet chestnut wood (Castanea sativa Mill.), consisting mainly of hydrolysable tannins, is commonly used in animal nutrition, especially in piglets, to prevent diarrhea after weaning [,,,,,,].
Recent studies found that hydrolysable tannins can inhibit the activity of bacteria in colon [], cells proliferation, and apoptosis. Reduced microbial activity resulting from a lesser disponibility of tryptophan and cell debris in the hindgut may lead to lowering the intestinal skatole production []. These findings are of considerable interest, especially in the fattening of uncastrated male pigs that might become a suitable alternative to current pork production from castrated males.
The aim of this study was to evaluate the effect of supplementation of hydrolysable tannins on carcass value, chemical composition, and pork quality as well as amino acid and fatty acid profile in uncastrated male pigs. Compared to another work, the present study used shorter time and higher dose of tannins supplementation. Therefore, the effectiveness of this combination in reducing the skatole and/or androstenone has also been the subject of our interest.
2. Material and Methods
2.1. Animals and Diet
The study was performed in accordance with Act on animal veterinary care No. 39/2007 of Slovak Republic and approved by Animal Care Committee of the Research Institute for Animal Production (RIAP) in Nitra, Slovakia. All pigs (n = 80)—progeny of Landrace sows and Yorkshire x Pietrain boars—were housed under conditions of the experimental farm of RIAP. At the average live weight of 65 ± 3.27 kg and age of 123 ± 4.42 days (average ± standard deviation), entire males were housed by pairs/pen and after 2 weeks of transitional period (when fed a control diet) were randomly allocated within litters to the five groups with different levels of tannin supplementation. Control group (T0, n = 16) received feed without supplementation; experimental groups (each of 16) were fed the same diet supplemented with 1% (T1), 2% (T2), 3% (T3) and 4% (T4) of Farmatan (Product Feed a.s., Luzianky, Slovakia—official distributor of Tanin Sevnica d.d., Sevnica, Slovenia)—sweet chestnut extract rich in hydrolysable tannins (Table 1). The content of tannins in this product was 75%.

Table 1.
Ingredients and chemical composition of diets.
After adaptation period (2 weeks), pigs reached average live weight of 80 kg and started dietary supplementation for 40 days until slaughter at the average weight of 122.28 kg ± 5.63 kg. All animals had ad libitum access to feed using automatic feeding system (Schauer s.r.o., Nitra, Slovakia) and drinking water.
2.2. Slaughtering, Carcass and Meat Quality Measurements
Entire males were slaughtered at average live weight of 122.28 kg ± 5.63 kg in one batch at the experimental slaughterhouse of RIAP Nitra by method of electrical stunning (90–100 V, 0.9–1.0 A, 50 Hz) followed by exsanguination. Evisceration was completed about 20 min post mortem. Carcasses were classified by ZP (Zwei-punkte) method for lean meat content, backfat thickness at three points (over the 1st, last thoracic, and 1st sacrum vertebra) was taken and pH (pH1), as well as electrical conductivity (EC1) were measured 45 min post mortem in longissimus dorsi (LD) between 13rd–14th rib and semimembranosus (SM) muscles using METTLER TOLEDO (pH meter FiveGo™, Columbus, USA) device with combined electrode and BIOTECH (USA), respectively. Chilling of the carcasses (air temperature 2–4 °C, velocity 0.5–1.0 m.s−1) started approximately 60 min after slaughter and was continued overnight. After 24-h chilling of the carcasses at 4 °C, measurements of pH (pH24) and electrical conductivity (EC24) at the same points as reported for longissimus dorsi and semimembranosus muscle were performed. Carcass weight (CW) and weight of right-half carcass were determined and subsequently, the detailed dissection of right-half carcass into the valuable primal cuts was performed. Valuable meat cuts (VMC) were calculated as summary of neck, shoulder, loin and ham weight; proportion of VMC (PVMC) as weight of VMC from half carcass weight; proportion of ham as weight of ham from CW; proportion of fatty cuts (PFC) as weight of fatty cuts (fat from loin, neck, shoulder, ham, leaf fat) from CW; and proportion of less valuable cuts (PLC) as weight of less valuable cuts (front and hind feet, front and hind totters, head, sacrum bone). Simultaneously, LD muscle (150 g of sample) was removed from the right side of carcass and sliced into 2.5 cm thick chops for further meat quality and chemical composition analysis. One wrapped sample was stored for 4 days at 4 °C for shear force measurements. Colour (L*—lightness, a*—redness, b*—yellowness) was measured by spectrophotometer MINISCAN XE Plus (Hunter Associates Laboratory, Inc., Reston, VA, USA). Water holding capacity (WHC) was analyzed using method of Grau-Hamm modified by Hašek and Palanská []. The cooking loss value was determined when the core temperature of the sample reached 80 °C. After that, steak was moved from grill, weighed, and the weight was compared with weight of raw steak before grilling. Drip loss was assessed 24 h after slaughter by Honikel method []. Four days after slaughter, the shear force was measured using TEXTURE ANALYSER TA-XT2i device (Stable Micro Systems Ltd., Surrey, UK) reported as tenderness on cooked samples (core temperature 80 °C, time 20 min).
2.3. Chemical Composition, Amino and Fatty Acids Profile
Samples from the neck (at level of 5th thoracic vertebra) and from adipose tissue (over the neck, each of approximately 200 g) were taken 24 h postmortem and transported to the Chemical laboratory of the Slovak Agricultural University for basic chemical composition, cholesterol content as well as amino acid and fatty acid composition. Each sample was homogenized and subsequently analyzed by the Fourier Transform Infrared (FTIR) method [] using the device Nicolet 6700 (IET Ltd., Mundelein, IL, USA). Fatty acids were analyzed as percentage of FAME—fatty acid methyl ester.
2.4. Feed Analyses
The samples of feed mixture were taken twice during experiment, at the beginning and at the end. After pooling and milling on a 1 mm sieve, the content of dry mater was determined by drying the samples at 105 °C for 3 h. The N content was analyzed using Kjeldahl procedure (Leco FP-2000, Leco, Mönchengladbach, Germany), and crude protein was calculated as N x 6.25 (STN ISO 20483). Crude fiber was determined after digestion with H2SO4 and KOH, washed with acetone, dried at 130 °C, and then ashed (ČSN ISO 5498). Crude ash content was analysed by combastion at 550 °C (ČSN 467092). Crude fat was determined by acidic hydrolysis as petrol ether extract (ČSN ISO 11085).
2.5. Boar Taint, Skatole and Androstenone Determination
Boar taint level was determined 45 min after slaughter on the half carcass. Backfat on level of the last thoracic vertebra was heated by inserting the hot iron tip (soldering iron) and smell was evaluated by three previously trained persons sensitive to the odor of skatole and androstenone (modificated Aluwé et al., 2012 []). The level of boar taint was evaluated as 0—no, 1—mild or 2—strong. After that, sample of backfat (50 g) was removed from carcass half, moved to the sensory laboratory, inserted into water, heated, and level of boar taint was evaluated at boiling point by the same way. The samples of adipose tissue (100 g) from a part of the belly were taken on the second day after slaughter and stored at −20 °C until analyses. Analysis of androstenone and skatole concentrations in fat tissue was performed in the laboratory of EKOLAB s.r.o. (Košice, Slovakia). The limit for detection was value of 0.02 µg/g for androstenone and 0.01 µg/g for skatole.
2.6. Statistical Analysis
The statistical analysis was performed by the statistical package Statistix 9 []. Differences among groups (T0–T4) were tested using one-way analyses of variance with fixed effects. Comparison was performed with the Bonferroni multiple comparison method []. The values are expressed as mean with standard error of the mean (SEM).
3. Results
3.1. Carcass Value and Meat Quality
No major influence of tannin supplementation on carcass traits with the exception of percentage of ham was observed (Table 2). Entire males in group T3 had significantly lower (p < 0.05) values compared to control group T0.

Table 2.
Carcass value of control (T0) and tannin-supplemented (T1–T4) groups.
The effect of dietary tannins on meat quality traits was limited to the electrical conductivity measured 45 min (EC1) and/or 24 h (EC24) postmortem in semimebranosus muscle, and cooking loss (Table 3). Group T4 had higher EC1 compared to T1 and T2 groups by 0.77 and 0.73 µS, respectively. Greater differences were found in musculus semimembranosus measured 24 h postmortem. Entire males in control T0 and supplemented T4 groups expressed higher values compared to group T1 by 1.69 and 1.54 µS (p < 0.01), and to group T2 by 1.43 and 1.28 µS (p < 0.05). Interestingly, tannin supplementation with 3 and 4% affected negatively cooking loss. Pigs in these two groups (T3, T4) had significantly greater loss (p < 0.01) than T0, T1, and T2 groups in the range 31–47% and 34–50%, respectively.

Table 3.
Meat quality of control (T0) and tannin-supplemented (T1–T4) groups.
3.2. Chemical Composition, Amino and Fatty Acid Profiles
Dietary tannin supplementation had no effect on chemical composition of meat (Table 4). Similarly, amino acid profile of longissimus dorsi muscle was not affected by tannin supplementation (Table 5). In the adipose tissue, the effect of tannin supplementation on fatty acid profile was not observed (p > 0.05), (Table 6). However, significant differences between groups were found in the content of some fatty acids in muscle (Figure 1). The highest tannin dose (T4) increased concentrations of myristic acid compared with control (p < 0.05) and T1 (p < 0.01) groups. Similarly, both higher tannin supplementations (T3–T4) increased (p < 0.01) content of lauric acid compared to the control while lower doses (T1–T2) decreased its content (p < 0.01). Concentration of heptadecanoic acids was greater in T1- and T2-supplemented pigs compared with T3 and T4 (p < 0.05). At the same time, level of oleic acid was increased in T0-, T1-, and T2-supplemented pigs by 7–14% compared to T3 and T4 groups. The concentration of vaccenic acid was the highest in T4-supplemented pigs and significantly different than control (p < 0.05) and T1 pigs (p < 0.01). The highest differences between pigs groups were detected in polyunsaturated fatty acids. The highest tannin supplemented entire males (T4) had 1.13, 1.09 and 1.12 times greater proportions of linoleic, total polyunsaturated fatty acids (PUFA), and n-6 PUFA in comparison to unsupplemented pigs (p < 0.05). In case of n-6 PUFA, significant difference was detected also between T3 and T0 (p < 0.05).

Table 4.
Basic chemical composition and cholesterol content of musculus longissimus dorsi in control (T0) and tannin-supplemented (T1–T4) groups.

Table 5.
Amino acids composition of LD muscle in control (T0) and tannin-supplemented (T1–T4) groups.

Table 6.
Fatty acids 1 composition of adipose tissue in control (T0) and tannin-supplemented (T1–T4) groups.
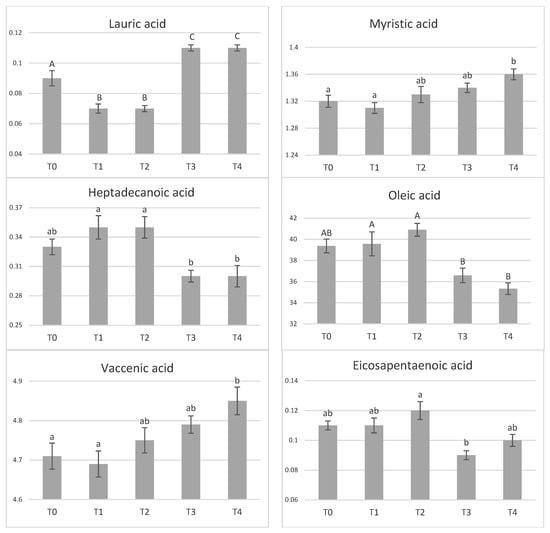
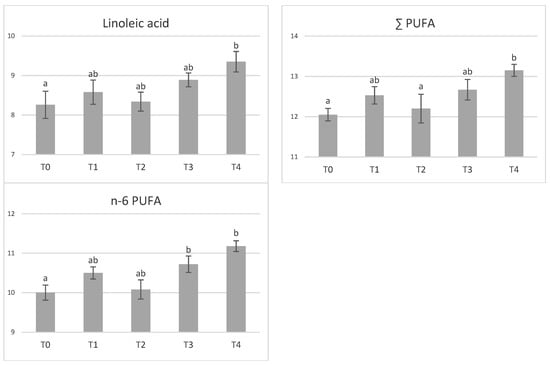
Figure 1.
The effect of tannin supplementation on fatty acid profile of muscle. Fatty acids only significantly influenced (p < 0.05) are shown. T0—control group, T1—1% tannin supplementation, T2—2% tannin supplementation, T3—3% tannin supplementation, T4—4% supplementation, a,b—values with different letters within rows are significantly different at p < 0.05; A,B—values with different letters within rows are significantly different at p < 0.01.
3.3. Boar Taint, Androstenon and Skatole Levels
The supplementation with tannins did not show any significant impact on androstenone concentration in subcutaneous fat of entire males (Table 7). On the contrary, tannin supplementation had a positive effect on reduction of skatole concentration in fatty tissue. This progressive reduction was observed with increasing supplementation of tannins in pig´s diet. A tendency to significant differences (p = 0.052) in supplemented groups T2, T3, and T4 compared to control group (Figure 2) was found. Evaluation of boar taint perception using “hot iron” method also showed a tendency (p = 0.054) to significant reduction in group T2 compared to control pigs (Table 7). In contrast, evaluation of boar taint by “boiling” method was not affected with tannin supplementation.

Table 7.
Effect of tannins on androstenone and skatole accumulation, and perception of boar taint.
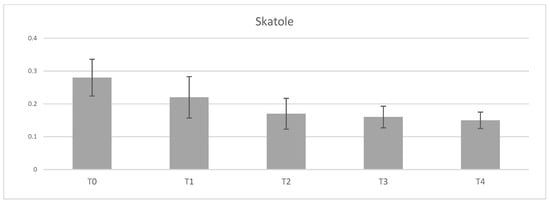
Figure 2.
Effect of tannin supplementation on skatole accumulation in adipose tissue. T0—control group, T1—1% tannin supplementation, T2—2% tannin supplementation, T3—3% tannin supplementation, T4—4% supplementation.
4. Discussion
Traditional pork production system based on diet rich in tannins such as acorns and chestnut are used in some Mediterranean countries [,]. These fruits containing more than 4% of hydrolysable tannins serve as the important energy source for fattening pigs of specialized native breeds in extensive pig farming. That was the reason for deciding to use the dose of up to 4% of tannins per kg of feed in the present study. As known, tannins ability to bind proteins can be considered a harmful effect in monogastric animals including pigs. This property to bind protein is associated with the antinutritional effects of tannins presenting in decreasing feed palatability and nitrogen digestibility [,,]. Tannins in diet are capable of inducing elevated proline rich proteins (PRP) content in the saliva. Binding capacity of this PRP is directly related to its proline content. Pigs, wild and domestic, have developed this defense strategy to prevent intoxication with diets rich in hydrolysable tannins [,]. On the contrary, tannins also show positive properties such as antioxidant, antibacterial, and antidiarrheal, especially important in piglets after weaning [,,,,,].
Carcass value of slaughtered entire males in the present study was not affected by tannin supplementation except for one parameter—percentage of ham. This trait was lower in T3 group compared to control. As other carcass traits did not show any significant differences among tannin-supplemented and control pigs, it can be summarized that dietary tannin administration did not have an adverse impact on carcass quality. No significant effect of tannins on carcass value have been reported in other studies using similar concentrations of tannins (1.5, 3%) in diets and longer period of supplementation (60–98 days) [,,].
In the present study, tannin-supplemented diet did not affect the chemical composition of pork. This finding is in agreement with previous research []. Meat quality in our study was partially influenced by tannin supplementation. However, results are quite inconsistent. It seems that increasing the number of tannins in diet resulted in higher electrical conductivity in semimebranosus but not in longissimus dorsi muscle. Differences between muscles can be explained by different muscle tissue composition, and then resulting different metabolism. Higher cooking loss were also found after 3 and 4% supplementation compared to lower diet doses as well as control group. It seems that higher cooking loss in 3- and 4%-supplemented groups may be related to greater conductivity. However, hypothesis of increasing the electrical conductivity with enhancing tannin supplementation is questioned since a higher value was also found in control group compared to 1- and 2%-supplemented. Therefore, further research is needed to elucidate the mechanism of the effect of tannin supplementation on meat quality traits. The lack of effect of hydrolysable tannins on meat quality has been reported in the other studies with tannins [,] as well as studies with other dietary approaches, e.g., inulin or raw potato starch [,]. On the contrary, Tretola et al. [] observed lower meat color (L*) and WHC as well as greater cooking loss in pigs supplemented with tannins compared to unsupplemented.
To the best of our knowledge, the amino acid composition of meat from entire males supplemented with tannins has not yet been studied. Similar to basic chemical composition in our study, dietary treatment with tannins did not result in any important differences in observed amino acids between the pig groups.
Related to the fatty acid profile, while no major differences were found in subcutaneous fat, pronounced differences in intramuscular fat were observed. Dietary tannins influenced lauric (C12:0), and myristic (C14:0) acid that were present in a higher proportion in T3 and T4 supplemented groups compared to T0, T1 =, or T2. A similar trend was observed in monounsaturated fatty acids (MUFA) vaccenic acid (C18:1 11c 15t) that was higher in T4 compared to T0 and T1. On the other hand, lower tannin concentrations (T1, T2) resulted in higher accumulation of heptadecanoic (C17:0) and olei acid (C18:1) than those of T3 and T4 supplementation. The most important differences in fatty acid composition were detected in polyunsaturated acids where higher tannin doses, especially T4, resulted in increased linoleic, n-6, and total PUFA amounts compared to the control group. It is debatable if higher accumulation of some SFA and MUFA at T3 and T4 levels were due to direct or indirect impact of tannins in the diet as increasing backfat thickness (even though inconclusive) in the carcass of both two higher-supplemented groups was observed. Interestingly, simultaneous increase in both SFA and PUFA at higher tannin supplementations is unexpected. It is obvious that increase in PUFA (as well as n-6) can be attributed to higher accumulation of linoleic acid. So, it is possible that tannins may affect in some way the metabolism of single fatty acids in pig body. In contrast to our findings, Rezar et al. [] reported great differences in subcutaneous fat and almost none in intramuscular fat. Tretola et al. [] did not find any differences in fatty acid composition either in subcutaneous or intramuscular fat after tannin supplementation.
In general, skatole as one of the two most important compounds responsible for boar taint is mainly affected by environmental factors such as housing conditions (type of floor, temperature, air velocity, and humidity), nutrition, feeding, etc. The concentration of skatole in adipose tissue is a result of variety of processes such as formation, absorption, metabolism, and deposition. The crucial role in accumulation of skatole in fat is associated with the efficiency of hepatic metabolism. The potential impact of tannin supplementation on skatole metabolism has not yet been studied. Lower skatole concentrations are associated with higher activities of digestive enzymes of CYP450 family such as 2E1, 2A19, 1A1, 1A2, etc. [,]. Despite that, results of various studies are controversial as some report reduction of skatole deposition after tannin-rich diet feeding at higher doses (2 and 3% of tannins) [,] while others did not find any positive effect of tannins [,]. The common element of all the above-mentioned studies was the supplementation of 1–3% tannins for a longer time period (60–77 days) compared to the present study where the period of tannins administration was only 40 days. As results showed, there was a tendency for reduced skatole accumulation in adipose tissue in groups supplemented with 2, 3, and 4% tannins compared to the control group. That was partially confirmed by the evaluation of boar taint using „hot iron“ method when group T2 also had tendency to lower perception compared to unsupplemented pigs. Regarding effect of tannins on androstenone, supplementation of these additives did not find any impact, even though the two highest doses had higher (although inconclusive) androstenone concentrations in fat tissue than control group. It is in agreement with other studies [,].
5. Conclusions
Based on the results of this study, dietary tannin supplementation had no effect on chemical and amino acid composition of the muscle, fatty acid profile of subcutaneous tissue and only slight effect on carcass value. However, tannins can affect some pork quality traits. Higher concentrations of tannins (3–4%) increased cooking loss and partially (4%) electrical conductivity of pork. Further, tannins greatly influenced fatty acid composition in muscle. Higher supplementation with 3 and/or 4% of tannins increased several SFA, MUFA, and PUFA in pork. This effect could be beneficial related to increased linoleic, total PUFA and n-6 PUFA but not increased lauric, myristic as well as decreased oleic content. Supplementation with tannins had no effect on androstenone accumulation in fat tissue while there was a tendency to reduction of skatole at higher doses (2–4%). Summarizing, an increase in some polyunsaturated fatty acids and tendency to lower skatole accumulation in adipose tissue can be considered a positive effect of tannin supplementation. In contrast, deterioration of some pork quality traits as well as increasing some saturated fatty acids at higher tannin doses are undesirable from both the meat industry and consumers point of view.
Author Contributions
Conceptualization, I.B.; data curation, I.B.; formal analysis, O.B.; methodology, O.B. and P.F.; writing—original draft, I.B.; writing—review and editing, O.B. and P.F. All authors have read and agreed to the published version of the manuscript.
Funding
The work was created within the COST Action “Innovative approaches in pork production with entire males” (IPEMA–CA 15215) supported by COST (European Cooperation in Science and Technology).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Animal Care Committee of the Research Institute for Animal Production (RIAP) in Nitra, Slovakia (protocol code 257/2019, approved 15 March 2019).
Informed Consent Statement
Not applicable.
Acknowledgments
Authors thank the company EKOLAB, s.r.o. (Košice, Slovakia) for analyses of androstenone and skatole ans Jana Zeleňáková for technical support.
Conflicts of Interest
Authors declare no conflict of interest.
References
- Dunshea, F.R.; Colantoni, C.; Howard, K.; McCauley, I.; Jackson, P.; Long, K.A.; Lopaticki, S.; Nugent, E.A.; Simons, J.A.; Walker, J.; et al. Vaccination of boars with a GnRH vaccine (Improvac) eliminates boar taint and increases growth performance. J. Anim. Sci. 2001, 79, 2524–2535. [Google Scholar] [CrossRef]
- Claus, R.; Lacorn, M.; Danowski, K.; Pearce, M.C.; Bauer, A. Short-term endocrine and metabolic reactions before and after second immunization against GnRH in boars. Vaccine 2007, 25, 4689–4696. [Google Scholar] [CrossRef]
- Pauly, C.; Spring, P.; O’Doherty, J.V.; Ampuero Kragten, S.; Bee, G. Growth performance, carcass characteristics and meat quality of group-penned surgically castrated, immunocastrated (Improvac®) and entire male pigs and individually penned entire male pigs. Animal 2009, 3, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Gispert, M.; Angels Oliver, M.; Velarde, A.; Suarez, P.; Pérez, J.; Font i Furnols, M. Carcass and meat quality characteristics of immunocastrated male, surgically castrated male, entire male and female pigs. Meat Sci. 2010, 85, 664–670. [Google Scholar] [CrossRef]
- Daza, A.; Latorre, M.A.; Olivares, A.; López Bote, C.J. The effects of male and female immunocastration on growth performances and carcass and meat quality of pigs intended for dry-cured ham production: A preliminary study. Livest. Sci. 2016, 190, 20–26. [Google Scholar] [CrossRef]
- Kress, K.; Weiler, U.; Schmucker, S.; Čandek-Potokar, M.; Vrecl, M.; Fazarinc, G.; Škrlep, M.; Batorek-Lukač, N.; Stefanski, V. Influence of housing conditions on reliability of immunocastration and consequences for growth performance of male pigs. Animals 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Dalla Costa, O.A.; de Tavernari, F.C.; dos Lopes, L.S.; Dalla Costa, F.A.; Feddern, V.; de Lima, G.J.M.M. Performance, carcass and meat quality of pigs submitted to immunocastration and different feeding programs. Res. Vet. Sci. 2020, 131, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Minelli, G.; Macchioni, P.; Mezzetti, F.; Belmonte, A.M.; Volpelli, L.A.; Faeti, V.; Lo Fiego, D.P. Characteristics of lipids from immunocastrated medium-heavy pigs fed either a restricted diet or ad libitum. Ital. J. Food Sci. 2019, 31, 98–109. [Google Scholar]
- Škrlep, M.; Poklukar, K.; Kress, K.; Vrecl, M.; Fazarinc, G.; Batorek Lukač, N.; Weiler, U.; Stefanski, V.; Čandek-Potokar, M. Effect of immunocastration and housing conditions on pig carcass and meat quality traits. Transl. Anim. Sci. 2020, 4, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Poklukar, K.; Čandek-Potokar, M.; Vrecl, M.; Batorek-Lukač, N.; Fazarinc, G.; Kress, K.; Weiler, U.; Stefanski, V.; Škrlep, M. The effect of immunocastration on adipose tissue deposition and composition in pigs. Animal 2020, 15, 100118. [Google Scholar] [CrossRef]
- Werner, D.; Baldinger, L.; Bussemas, R.; Büttner, S.; Weissmann, F.; Ciulu, M.; Mörlein, J.; Mörlein, D. Early immunocastration of pigs: From farming to meat quality. Animals 2021, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Font i Furnols, M.; González, J.; Gispert, M.; Oliver, M.A.; Hortós, M.; Pérez, J.; Suárez, P.; Guerrero, L. Sensory characterization of meat from pigs vaccinated against gonadotropin releasing factor compared to meat from surgically castrated, entire male and female pigs. Meat Sci. 2009, 83, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, J.; Nannoni, E.; Sardi, L.; Rubini, G.; Salvatore, R.; Bartoli, L.; Adinolfi, F.; Martelli, G. Towards the abandonment of surgical castration in pigs: How is immunocastration perceived by Italian consumers? Animals 2019, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Jaros, P.; Bürgi, E.; Stärk, K.D.C.; Claus, R.; Hennessy, D.; Thun, R. Effect of active immunization against GnRH on androstenone concentration, growth performance and carcass quality in intact male pigs. Livest. Prod. Sci. 2005, 92, 31–38. [Google Scholar] [CrossRef][Green Version]
- Stupka, R.; Čítek, J.; Vehovský, K.; Zadinová, K.; Okrouhlá, M.; Urbanová, D.; Stádník, L. Effects of immunocastration on growth performance, body composition, meat quality, and boar taint. Czech J. Anim. Sci. 2017, 6, 249–258. [Google Scholar] [CrossRef]
- Patterson, R.L.S. 5α-androst-16-ene-3-one: Compound responsible for taint in boar fat. J. Sci. Food Agric. 1968, 19, 31–38. [Google Scholar] [CrossRef]
- Walstra, P.; Maarse, G. Onderzoek Gestachlengen van Mannelijke Mestvarkens. In IVO-Rapport C-147, Rapport 2; Researchgroep voor Vlees en Vleeswaren TNO: Zeist, The Netherlands, 1970; p. 30. [Google Scholar]
- Čandek-Potokar, M.; Škrlep, M.; Batorek Lukač, N.; Zamaratskaia, G.; Prevolnik Povše, M.; Velikonja Bolta, Š.; Kubale, V.; Bee, G. Hydrolysable tannin fed to entire male pigs affects intestinal production, tissue deposition and hepatic clearance of skatole. Vet. J. 2015, 204, 162–167. [Google Scholar] [CrossRef]
- Bee, G.; Silacci, P.; Ampuero-Kragten, S.; Čandek-Potokar, M.; Wealleans, A.L.; Litten-Brown, J.; Salminen, J.-P.; Mueller-Harvey, I. Hydrolysable tannin-based diet rich gallotannins has a minimal impact on pig performance but significantly reduces salivary and bulbourethral gland size. Animal 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fredriksen, B.; Nafstad, O.; Lium, B.; Marka, C.; Heier, B.; Almaas, C. The effect of group composition on the levels of androstenone and skatole in entire male pigs. In Proceedings of the EAAP Working Group on Production and Utilisation of Meat from Entire Male Pigs, Dublin, Ireland, 13–14 November 2003. [Google Scholar]
- Øverland, M.; Berg, J.; Matre, T. The effect of feed and feeding regime on skatole and androstenone levels and on sensory attributes of entire male and female pigs. In Proceedings of the EAAP Working Group Production and Utilisation of Meat from Entire Male Pigs, Milton Keynes, UK, 27–29 September 1995. [Google Scholar]
- Bonneau, M. Factors affecting the level of androstenone. Acta Vet. Scand. 2006, 48 (Suppl. 1), S1–S7. [Google Scholar] [CrossRef]
- Diaz, G.J.; Skordos, K.; Yost, G.S.; Squires, E.J. Identification of phase I metabolites of 3-methylindole produced by pig liver microsomes. Drug Metab. Dispos. 1999, 27, 1150–1156. [Google Scholar] [PubMed]
- Zamaratskaia, G.; Babol, J.; Andersson, H.K.; Anderson, K.; Lundström, K. Effect of liveweight and dietary supplement of raw potato starch on the levels of skatole, androstenone, testosterone and oestrone sulphate in entire male pigs. Livest. Sci. 2005, 93, 235–243. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Zamaratskaia, G. Regulation of porcine hepatic cytochrome p450-implication of boar taint. Comput. Struct. Biotechnol. J. 2014, 11, 106–112. [Google Scholar] [CrossRef]
- Wesoly, R.; Weiler, U. Nutritional influences on skatole formation and skatole metabolism in the pig. Animals 2012, 2, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Aluwé, M.; Langedries, K.C.M.; Bekaert, K.M.; Tuyttens, F.A.M.; De Brabander, D.L.; De Smet, S.; Millet, S. Effect of surgical castration, immunocastration and chicory-diet on the meat quality and palatability of boars. Meat Sci. 2013, 94, 402–407. [Google Scholar] [CrossRef]
- Aluwé, M.; Millet, S.; Nijs, G.; Tuyttens, F.A.M.; Verheyden, K.; De Brabander, H.F.; De Brabander, D.L.; Van Oeckel, M.J. Absence of an effect of dietary fibre or clinoptilolite on boar taint in entire male pigs fed practical diets. Meat Sci. 2009, 82, 346–352. [Google Scholar] [CrossRef]
- Kjos, N.P.; Overland, M.; Fauske, A.K.; Sorum, H. Feeding chicory inulin to entire male pigs during the last period before slaughter reduces skatole in digesta and backfat. Livest. Sci. 2010, 134, 143–145. [Google Scholar] [CrossRef]
- Zammerini, D.; Whittington, F.; Nute, G. Effect of dietary chicory on boar taint. In Proceedings of the British Society of Animal Science Annual Konference, Belfast, UK, 12–14 April 2010; p. 181. [Google Scholar]
- Aluwé, M.; Heyrman, E.; Theis, S.; Sieland, C.; Thurman, K.; Millet, S. Chicory fructans in pig diet reduce skatole in back fat of entire male pigs. Res. Vet. Sci. 2017, 115, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jensen, B.B.; Canibe, N. The mode of action of chicory roots on skatole production in entire male pigs is neither via reducing the population of skatole-producing bacteria nor via increased butyrate production in the hindgut. Appl. Environ. Microbiol. 2019, 85, e02327-18. [Google Scholar] [CrossRef]
- Maribo, H.; Claudi-Magnussen, C.; Jensen, B.B. Effect of 15 % dried chicory root in feed for male pigs. Meddelelse 2010, 876, 14. [Google Scholar]
- Maribo, H.; Jensen, B.B.; Thorning, H. Fibres reduces skatole in male pigs. Meddelelse 2015, 1055, 13. [Google Scholar]
- Hansen, L.L.; Stolzenbach, S.; Jensen, J.A.; Henckel, P.; Hansen-Møller, J.; Syriopoulos, K.; Byrne, D. Effect of feeding fermentable fibre-rich feedstuffs on meat quality with emphasis on chemical and sensory boar taint in entire male and female pigs. Meat Sci. 2008, 80, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Zammerini, D.; Wood, J.D.; Whittington, F.M.; Nute, G.R.; Hughes, S.I.; Hazzledine, M.; Matthews, K. Effect of dietary chicory on boar taint. Meat Sci. 2012, 91, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fang, L.; Sun, Y.; Su, Y.; Zhu, W. Effects of a diet high in resistant starch on fermentation end-products of protein and mucin secretion in the colons of pigs. Starch 2016, 69, 1600032. [Google Scholar] [CrossRef]
- Lösel, D.; Claus, R. Dose-dependent effects of resistant potato starch in the diet on intestinal skatole formation and adipose tissue accumulation in the pig. J. Vet. Med. A 2005, 52, 209–212. [Google Scholar] [CrossRef]
- Lösel, D.; Lacorn, M.; Büttner, D.; Claus, R. Flavor improvement in pork from barrows and gilts via inhibition of intestinal skatole formation with resistant potato starch. J. Agr. Food Chem. 2006, 54, 5990–5995. [Google Scholar] [CrossRef]
- Pieper, R.; Boudry, C.; Bindelle, J.; Vahjen, W.; Zentek, J. Interaction between dietary protein content and the source of carbohydrates along the gastrointestinal tract of weaned piglets. Arch. Anim. Nutr. 2014, 68, 263–280. [Google Scholar] [CrossRef]
- Vhile, S.G.; Kjos, N.P.; Sørum, H.; Øverland, M. Feeding Jerusalem artichoke reduced skatole level and changed intestinal microbiota in the gut of entire male pigs. In Proceedings of the Symposium “Sustainable Animal Production in the Tropics” (SAPT), Guadeloupe, France, 15–18 November 2010; Volume 6, pp. 807–814. [Google Scholar] [CrossRef]
- Salmon, L.; Edwards, S.A. The effects of dietary fructo-oligosaccharide addition on boar taint compounds and performance in heavy slaughter weight boars and gilts. Anim. Feed Sci. Tech. 2015, 207, 130–139. [Google Scholar] [CrossRef][Green Version]
- Tretola, M.; Maghin, F.; Silacci, P.; Ampuero, S.; Bee, G. Effect of supplementing hydrolysable tannins to a grower-finisher diet containing divergent PUFA levels on growth performance, boar taint levels in back fat and intestinal microbiota of entire males. Animals 2019, 9, 1063. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Constabel, C.P. Tannins in plant–herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef]
- Furlan, C.M.; Motta, L.B.; Santos, D.Y.A.C. Tannins: What do they represent in plant life? In Tannins: Types, Foods Containing, and Nutrition; Chapter, 10; Petridis, G.K., Ed.; Nova Science Publishers, Inc.: Harpak, NY, USA, 2010; ISBN 978-1-61761-127-8. [Google Scholar]
- Ferguson, N.S.; Gous, R.M.; Iji, P.A. Determining the source of anti-nutritional factor(s) found in two species of lupin (L. albus and L. angustifolius) fed to growing pigs. Livest. Sci. 2003, 84, 83–91. [Google Scholar] [CrossRef]
- Mariscal-Landín, G.; Lebreton, Y.; Sève, B. Apparent and standardised true ileal digestibility of protein and amino acids from faba bean, lupin and pea, provided as whole seeds, dehulled or extruded in pig diets. Anim. Feed Sci. Tech. 2002, 97, 183–198. [Google Scholar] [CrossRef]
- Mennen, L.I.; Bennetau-Pellisero, C.; Scalbert, A. Risks and safety of polyphenol consumption1-3. Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 326S–329S. [Google Scholar] [CrossRef] [PubMed]
- Biagi, G.; Cipollini, I.; Paulicks, B.R.; Roth, F.X. Effect on tannins on growth performance and intestinal ekosystem in weaned piglets. Arch. Anim. Nutr. 2010, 64, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Brus, M.; Dolinšek, J.; Cencič, A.; Škorjanc, D. Effect of chestnut (Castanea sativa Mill.) wood tannins and organic acids on growth performance and faecal microbiota of pigs from 23 to 127 days of age. Bulg. J. Agric. Sci. 2013, 19, 841–847. [Google Scholar]
- Girard, M.; Thanner, S.; Pradervand, N.; Hu, D.; Ollagnier, C.; Bee, G. Hydrolysable chestnut tannins for reduction of postwening diarrhea: Efficacy on an experimental ETEC F4 model. PLoS ONE 2018, 13, e0197878. [Google Scholar]
- Girard, M.; Hu, D.; Pradervand, N.; Neuenschwander, S.; Bee, G. Chestnut extract but not sodium salicylate decrease the severity of diarrhea and enterotoxigenic Escherichia coli F4 shedding in artificially infected piglets. PLoS ONE 2020, 15, e0214267. [Google Scholar] [CrossRef]
- Girard, M.; Bee, G. Invited review: Tannins as a potential alternative to antibiotics to prevent coliform diarrhea in weaned pigs. Animal 2020, 14, 95–107. [Google Scholar] [CrossRef]
- Caprarulo, V.; Hejna, M.; Giromini, C.; Liu, Y.; Dell´ Anno, M.; Sotira, S.; Reggi, S.; Sgoifo-Rossi, C.A.; Callegari, M.L.; Rossi, L. Evaluation of dietary administration of chestnut and quebracho tannins on growth, serum metabolites and fecal parameters of weaned piglets. Animals 2020, 10, 1945. [Google Scholar] [CrossRef] [PubMed]
- Caprarulo, V.; Giromini, C.; Rossi, L. Review: Chestnut and quebracho tannins in pig nutrition: The effects on performance and intestinal health. Animal 2021, 15, 100064. [Google Scholar] [CrossRef]
- Bilić-Šobot, D.; Kubale, V.; Škrlep, M.; Čandek-Potokar, M.; Prevolnik Povše, M.; Fazarinc, G.; Škorjanc, D. Effect of hydrolysable tannins on intestinal morphology, proliferation and apoptosis in entire male pigs. Arch. Anim. Nutr. 2016, 70, 378–388. [Google Scholar] [CrossRef]
- Bilić-Šobot, D.; Zamaratskaia, G.; Kroyer Rasmussen, M.; Čandek-Potokar, M.; Škrlep, M.; Prevolnik Povše, M.; Škorjanc, D. Chestnut wood extract in boar diet reduces intestinal skatole production, a boar taint. Agron. Sustain. Dev. 2016, 36, 36–62. [Google Scholar] [CrossRef]
- Hašek, O.; Palanská, O. Determination of water holding capacity in meat by instrument at constant pressure. [in SLOVAK: Stanovenie obsahu “voľne” viazanej vody v mäse prístrojom za konštantného tlaku]. Hydinársky Priemysel 1976, 18, 228–233. [Google Scholar]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Carbonaro, M.; Nucara, A. Secondary structure of food proteins by Fourier transform spectroscopy in the mid-infrared region. Amino Acids 2010, 38, 679–690. [Google Scholar] [CrossRef]
- Aluwé, M.; Tuyttens, F.A.M.; Bekaert, K.M.; De Smet, S.; De Brabander, D.L.; Millet, S. Evaluation of various boar taint detection methods. Animal 2012, 6, 1868–1877. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anonymous. Statistix for Windows. 2000, Version 8.0. and 9.0. User’s Manual; Analytical Software: Tallahassee, FL, USA, 2000. [Google Scholar]
- Grofík, R.; Fľak, P. Štatistické Metódy v poľNohospodárstve (Statistical Methods for Agriculture); Príroda: Bratislava, Slovakia, 1990; p. 344. ISBN 80-07-00018-6. [Google Scholar]
- Rodríguez-Estévez, V.; Sánchez-Rodríguez, M.; García, A.R.; Gómez-Castro, A.G. Average daily weight gain of Iberian fattening pigs when grazing natural resources. Livest. Sci. 2011, 137, 292–295. [Google Scholar] [CrossRef]
- Cantos, E.; Espí, J.C.; López-Bote, C.; de la Hoz, L.; Ordóñez, J.A.; Tomás-Barberán, F.A. Phenolic compounds and fatty acids from acorns (Quercus spp.), the main dietary constituent of free- ranged Iberian pigs. J. Agric. Food Chem. 2003, 51, 6248–6255. [Google Scholar] [CrossRef] [PubMed]
- Antongiovanni, M.; Minieri, S.; Petacchi, F. Effect of tannin supplementation on nitrogen digestibility and retention in growing pigs. Ital. J. Anim. Sci. 2007, 6, 245–247. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Mariscal-Landín, G.; Avellaneda, J.H.; Reis de Souza, T.C.; Aguilera, A.; Borbolla, G.A.; Mar, B. Effect of tannins in sorghum on amino acid ileal digestibility and on trypsin (E.C.2.4.21.4) and chymotrypsin (E.C.2.4.21.1) activity of growing pigs. Anim. Feed Sci. Tech. 2004, 117, 245–264. [Google Scholar] [CrossRef]
- Cappai, M.G.; Wolf, P.; Pinna, W.; Kamphues, J. Pigs use endogenous proline to cope with acorn (Quercus pubescens Willd.) combined diet high in hydrolysable tannins. Livest. Sci. 2013, 155, 316–322. [Google Scholar] [CrossRef]
- Cappai, M.G.; Wolf, P.; Dimauro, C.; Pinna, W.; Kamphues, J. The bilateral parotidomegaly (hypertrophy) induced by acorn consumption in pigs is dependent on individual´s age but not on intake duration. Livest. Sci. 2014, 167, 263–268. [Google Scholar] [CrossRef]
- Prevolnik, M.; Škrlep, M.; Brus, M.; Pugliese, C.; Čandek-Potokar, M.; Škorjanc, D. Supplementing pig diet with 0.2 % sweet chestnut (Castanea sativa Mill.) wood extract had no effect on growth, carcass or meat quality. Acta Agric. Slov. 2012, 3 (Suppl. 3), 83–88. [Google Scholar]
- Galassi, G.; Mason, F.; Rapetti, L.; Crovetto, G.M.; Spanghero, M. Digestibility and metabolic utilisation of diets containing chestnut tannins and their effects on growth and slaughter traits of heavy pigs. Ital. J. Anim. Sci. 2019, 18, 746–753. [Google Scholar] [CrossRef]
- Rezar, V.; Salobir, J.; Levart, A.; Tomažin, U.; Škrlep, M.; Batorek Lukač, N.; Čandek-Potokar, M. Supplementing entire male pig diet with hydrolysable tannins: Effect on carcass traits, meat quality and oxidative stability. Meat Sci. 2017, 133, 95–102. [Google Scholar] [CrossRef]
- Pauly, C.; Spring, P.; Doherty, J.V.; Ampuero Kragten, S.; Bee, G. Performances, meat quality and boar taint of castrates and entire male pigs fed a standard and a raw potato starch-enriched diet. Animal 2008, 2, 1707–1715. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Squires, E. Biochemical, nutritional and genetic effects on boar taint in entire male pigs. Animal 2009, 2, 1508–1521. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).