Oocyte Morphometric Assessment and Gene Expression Profiling of Oocytes and Cumulus Cells as Biomarkers of Oocyte Competence in Sheep
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Culture Media and Culture Conditions
2.2. Experimental Design
2.3. Oocyte Collection and In Vitro Maturation (IVM)
2.4. In Vitro Fertilization (IVF)
2.5. In Vitro Embryo Culture (IVC)
2.6. Assessment of Maturation, Fertilization Efficiency, and Blastocyst Quality
2.7. Measure the Oocyte Morphometrics
2.8. Real-Time Quantitative PCR Analysis
2.9. Statistical Analysis
3. Results
3.1. Experiment 1: Assessment of Oocyte Morphometric Parameters as a Biomarker of Oocyte Competence
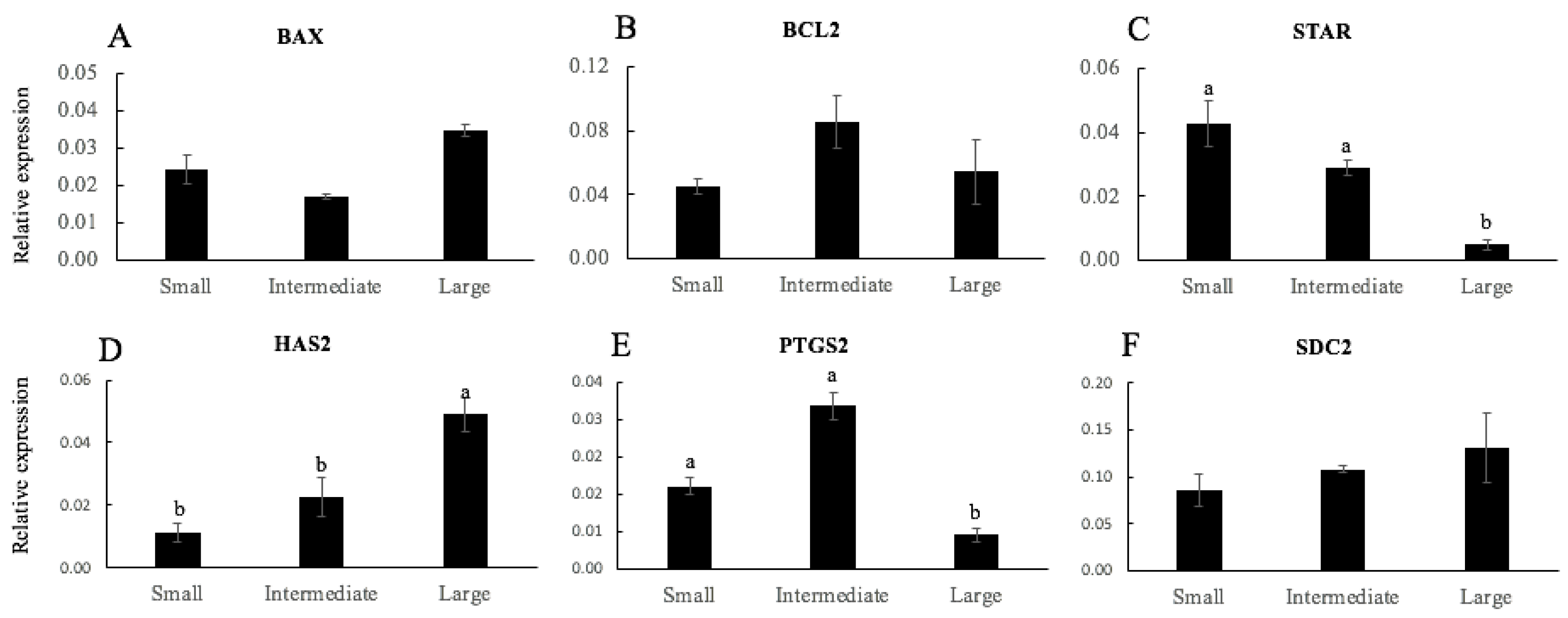
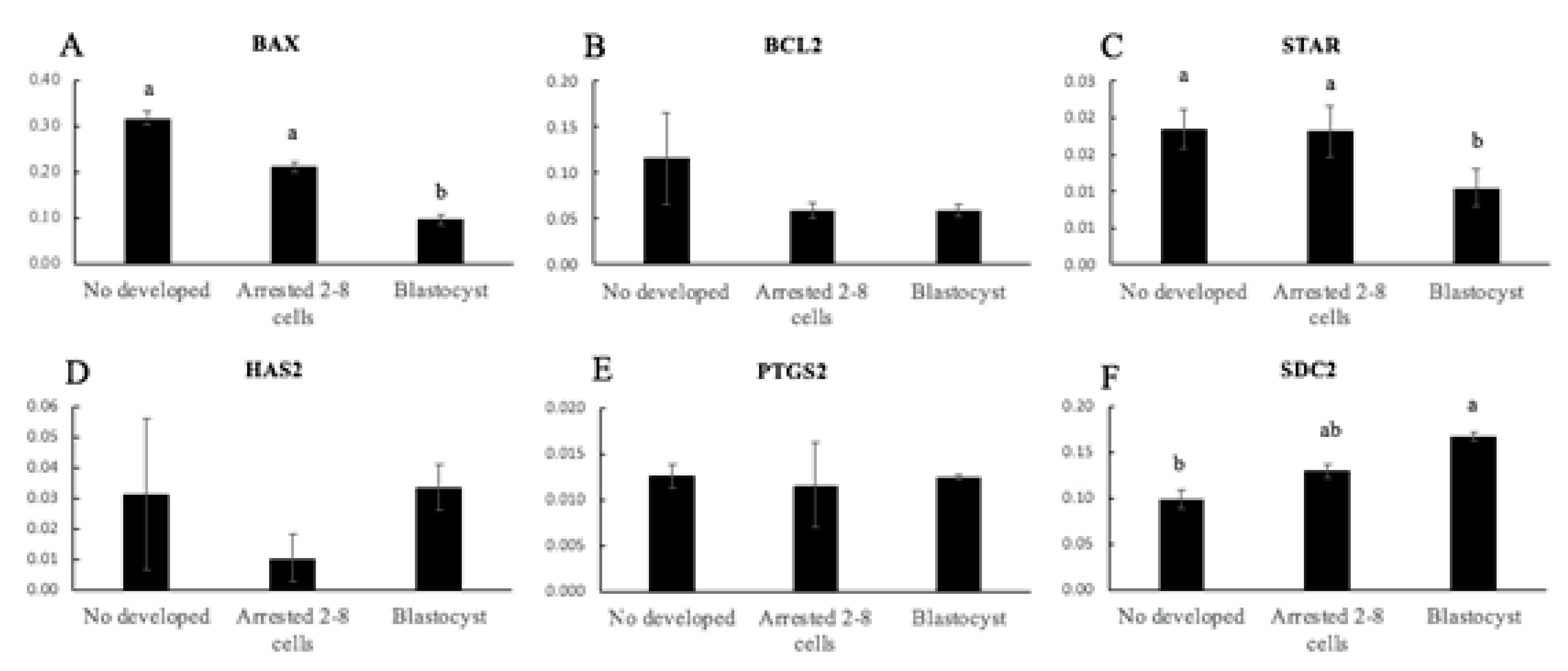
3.2. Experiment 2: Evaluation of Gene Expression of CCs as Biomarker of Oocyte Competence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.; Moawad, A.R.; Wang, C.-Y.; Li, H.-F.; Ren, J.-Y.; Dai, Y.-F. Advances in In Vitro production of sheep embryos. Int. J. Veter. Sci. Med. 2018, 6, S15–S26. [Google Scholar] [CrossRef] [Green Version]
- Ebner, T.; Moser, M.; Sommergruber, M.; Tews, G. Selection based on morphological assessment of oocytes and embryos at different stages of preimplantation development: A review. Hum. Reprod. Updat. 2003, 9, 251–262. [Google Scholar] [CrossRef]
- Rizos, D.; Ward, F.; Duffy, P.; Boland, M.P.; Lonergan, P. Consequences of bovine oocyte maturation, fertilization or early embryo development In Vitro versus In Vivo: Implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 2002, 61, 234–248. [Google Scholar] [CrossRef]
- Gandolfi, F.; Brevini, T.A.L. RFD award lecture 2009. In Vitro maturation of farm animal oocytes: A useful tool for investigating the mechanisms leading to full-term development. Reprod. Fertil. Dev. 2010, 22, 495–507. [Google Scholar] [CrossRef]
- Sirard, M.-A.; Richard, F.; Blondin, P.; Robert, C. Contribution of the oocyte to embryo quality. Theriogenology 2006, 65, 126–136. [Google Scholar] [CrossRef]
- Krisher, R.L. The effect of oocyte quality on development. J. Anim. Sci. 2004, 82, E14–E23. [Google Scholar]
- Lonergan, P.; Rizos, D.; Gutierrez-Adan, A.; Fair, T.; Boland, M.P. Oocyte and embryo quality: Effect of origin, culture conditions and gene expression patterns. Reprod. Domest. Anim. 2003, 38, 259–267. [Google Scholar] [CrossRef]
- Coticchio, G.; Sereni, E.; Serrao, L.; Mazzone, S.; Iadarola, I.; Borini, A. What criteria for the definition of oocyte quality? Ann. N. Y. Acad. Sci. 2004, 1034, 132–144. [Google Scholar] [CrossRef]
- Mohammadi-Sangcheshmeh, A.; Soleimani, M.; Deldar, H.; Salehi, M.; Soudi, S.; Hashemi, S.M.; Schellander, K.; Hoelker, M. Prediction of oocyte developmental competence in ovine using glucose-6-phosphate dehydrogenase (G6PDH) activity determined at retrieval time. J. Assist. Reprod. Genet. 2012, 29, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Hyttel, P.; Fair, T.; Callesen, H.; Greve, T. Oocyte growth, capacitation and final maturation in cattle. Theriogenology 1997, 47, 23–32. [Google Scholar] [CrossRef]
- Anguita, B.; Jimenez-Macedo, A.R.; Izquierdo, D.; Mogas, T.; Paramio, M.-T. Effect of oocyte diameter on meiotic competence, embryo development, p34 (cdc2) expression and MPF activity in prepubertal goat oocytes. Theriogenology 2007, 67, 526–536. [Google Scholar] [CrossRef]
- Otoi, T.; Yamamoto, K.; Koyama, N.; Tachikawa, S.; Suzuki, T. Bovine oocyte diameter in relation to developmental competence. Theriogenology 1997, 48, 769–774. [Google Scholar] [CrossRef]
- Raghu, H.M.; Nandi, S.; Reddy, S.M. Follicle size and oocyte diameter in relation to developmental competence of buffalo oocytes In Vitro. Reprod. Fertil. Dev. 2002, 14, 55–61. [Google Scholar] [CrossRef]
- Fair, T.; Hyttel, P.; Greve, T. Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Mol. Reprod. Dev. 1995, 42, 437–442. [Google Scholar] [CrossRef]
- Cadenas, J.; Maside, C.; Ferreira, A.; Vieira, L.; Leiva-Revilla, J.; Paes, V.; Alves, B.; Brandão, F.; Rodrigues, A.; Wheeler, M.; et al. Relationship between follicular dynamics and oocyte maturation during In Vitro culture as a non-invasive sign of caprine oocyte meiotic competence. Theriogenology 2018, 107, 95–103. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Meldrum, D.R.; Katz-Jaffe, M.G.; Krisher, R.L.; Schoolcraft, W.B. Oocyte environment: Follicular fluid and cumulus cells are critical for oocyte health. Fertil. Steril. 2015, 103, 303–316. [Google Scholar] [CrossRef]
- Kidder, G.M.; Vanderhyden, B.C. Bidirectional communication between oocytes and follicle cells: Ensuring oocyte developmental competence. Can. J. Physiol. Pharmacol. 2010, 88, 399–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monniaux, D. Driving folliculogenesis by the oocyte-somatic cell dialog: Lessons from genetic models. Theriogenology 2016, 86, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Assidi, M.; Dufort, I.; Ali, A.; Hamel, M.; Algriany, O.; Dielemann, S.; Sirard, M.-A. Identification of potential markers of oocyte competence expressed in bovine cumulus cells matured with follicle-stimulating hormone and/or phorbol myristate acetate In Vitro. Biol. Reprod. 2008, 79, 209–222. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, L.J.; Pangas, S.A.; Carson, S.A.; Kovanci, E.; Cisneros, P.; Buster, J.E.; Amato, P.; Matzuk, M.M. Human cumulus granulosa cell gene expression: A predictor of fertilization and embryo selection in women undergoing IVF. Hum. Reprod. 2004, 19, 2869–2874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiri, M.V.; Deldar, H.; Pirsaraei, Z.A. Impact of supplementary royal jelly on In Vitro maturation of sheep oocytes: Genes involved in apoptosis and embryonic development. Syst. Biol. Reprod. Med. 2016, 62, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Melo, E.O.; Cordeiro, D.M.; Pellegrino, R.; Wei, Z.; Daye, Z.J.; Nishimura, R.C.; Dode, M.A.N. Identification of molecular markers for oocyte competence in bovine cumulus cells. Anim. Genet. 2016, 48, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Devjak, R.; Tacer, K.F.; Juvan, P.; Klun, I.V.; Rozman, D.; Bokal, E.V. Cumulus cells gene expression profiling in terms of oocyte maturity in controlled ovarian hyperstimulation using GnRH agonist or GnRH antagonist. PLoS ONE 2012, 7, e47106. [Google Scholar] [CrossRef]
- García-Álvarez, O.; Maroto-Morales, A.; Berlinguer, F.; Fernández-Santos, M.; Esteso, M.; Mermillod, P.; Ortiz, J.; Ramon, M.; Pérez-Guzmán, M.; Garde, J.; et al. Effect of storage temperature during transport of ovaries on In Vitro embryo production in Iberian red deer (Cervus elaphus hispanicus). Theriogenology 2011, 75, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; First, N. In Vitro development of bovine ane-cell embryos: Influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology 1992, 37, 963–978. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Goovaerts, I.; Leroy, J.; Jorssen, E.; Bols, P. Noninvasive bovine oocyte quality assessment: Possibilities of a single oocyte culture. Theriogenology 2010, 74, 1509–1520. [Google Scholar] [CrossRef]
- Bunel, A.; Jorssen, E.; Merckx, E.; Leroy, J.; Bols, P.; Sirard, M. Individual bovine In Vitro embryo production and cumulus cell transcriptomic analysis to distinguish cumulus-oocyte complexes with high or low developmental potential. Theriogenology 2015, 83, 228–237. [Google Scholar] [CrossRef]
- Goovaerts, I.; Leroy, J.; Rizos, D.; Bermejo-Álvarez, P.; Gutierrez-Adan, A.; Jorssen, E.; Bols, P. Single In Vitro bovine embryo production: Coculture with autologous cumulus cells, developmental competence, embryo quality and gene expression profiles. Theriogenology 2011, 76, 1293–1303. [Google Scholar] [CrossRef]
- Correia, H.; Vieira, L.; Maside, C.; Paes, V.; Silva, R.; Alves, B.; Santos, F.; Apgar, G.; Rodrigues, A.; Figueiredo, J. Ovarian transport temperature (4 vs. 33 °C) impacts differently the In Vitro development of isolated goat preantral and antral follicles. Small Rumin. Res. 2017, 155, 16–23. [Google Scholar] [CrossRef]
- Mohammadi-Sangcheshmeh, A.; Held, E.; Rings, F.; Ghanem, N.; Salilew-Wondim, D.; Tesfaye, D.; Sieme, H.; Schellander, K.; Hoelker, M. Developmental competence of equine oocytes: Impacts of zona pellucida birefringence and maternally derived transcript expression. Reprod. Fertil. Dev. 2014, 26, 441–452. [Google Scholar] [CrossRef]
- Ebner, T.; Balaban, B.; Moser, M.; Shebl, O.; Urman, B.; Ata, B.; Tews, G. Automatic user-independent zona pellucida imaging at the oocyte stage allows for the prediction of preimplantation development. Fertil. Steril. 2010, 94, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Stalf, T.; Mehnert, C.; Eichenlaub-Ritter, U.; Tinneberg, H.-R. High magnitude of light retardation by the zona pellucida is associated with conception cycles. Hum. Reprod. 2005, 20, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Van Soom, A.; Vandaele, L.; Goossens, K.; de Kruif, A.; Peelman, L. Gamete origin in relation to early embryo development. Theriogenology 2007, 68, S131–S137. [Google Scholar] [CrossRef]
- Opiela, J.; Kątska-Książkiewicz, L.; Lipiński, D.; Słomski, R.; Bzowska, M.; Ryńska, B. Interactions among activity of glucose-6-phosphate dehydrogenase in immature oocytes, expression of apoptosis-related genes Bcl-2 and Bax, and developmental competence following IVP in cattle. Theriogenology 2008, 69, 546–555. [Google Scholar] [CrossRef]
- Anchamparuthy, V.M.; Pearson, R.E.; Gwazdauskas, F.C. Expression pattern of apoptotic genes in vitrified-thawed bovine oocytes. Reprod. Domest. Anim. 2009, 45, e83–e90. [Google Scholar] [CrossRef]
- Li, Y.; Li, R.Q.; Ou, S.B.; Zhang, N.F.; Ren, L.; Wei, L.N.; Zhang, Q.X.; Yang, D.Z. Increased GDF9 and BMP15 mRNA levels in cumulus granulosa cells correlate with oocyte maturation, fertilization, and embryo quality in humans. Reprod. Biol. Endocrinol. 2014, 12, 81. [Google Scholar] [CrossRef] [Green Version]
- Reyes, M.D.L.; Rojas, C.; Parraguez, V.; Palomino, J. Expression of growth differentiation factor 9 (GDF-9) during In Vitro maturation in canine oocytes. Theriogenology 2013, 80, 587–596. [Google Scholar] [CrossRef]
- Gittens, J.E.I.; Kidder, G.M. Differential contributions of connexin37 and connexin43 to oogenesis revealed in chimeric reaggregated mouse ovaries. J. Cell Sci. 2005, 118, 5071–5078. [Google Scholar] [CrossRef] [Green Version]
- Feuerstein, P.; Cadoret, V.; Dalbies-Tran, R.; Guerif, F.; Bidault, R.; Royere, D. Gene expression in human cumulus cells: One approach to oocyte competence. Hum. Reprod. 2007, 22, 3069–3077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, J.; Yanaihara, A.; Iwasaki, S.; Mitsukawa, K.; Negishi, M.; Okai, T. Reduction of connexin 43 in human cumulus cells yields good embryo competence during ICSI. J. Assist. Reprod. Genet. 2007, 24, 463–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edry, I.; Sela-Abramovich, S.; Dekel, N. Meiotic arrest of oocytes depends on cell-to-cell communication in the ovarian follicle. Mol. Cell. Endocrinol. 2006, 252, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Chian, R.-C.; Xu, Y.; Yan, Z.; Zhang, Y.; Gao, C.; Gao, L.; Liu, J.; Cui, Y. Genomic expression profiles in cumulus cells derived from germinal vesicle and MII mouse oocytes. Reprod. Fertil. Dev. 2016, 28, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Joo, B.S.; Na, Y.J.; Yoon, M.S.; Choi, O.H.; Kim, W.W. Cumulus cells apoptosis as an indicator to predict the quality of oocytes and the outcome of IVF-ET. J. Assist. Reprod. Genet. 2001, 18, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, G.; Bosco, L.; Pane, A.; Morici, G.; Cittadini, E.; Roccheri, M.C. Lower apoptosis rate in human cumulus cells after administration of recombinant luteinizing hormone to women undergoing ovarian stimulation for In Vitro fertilization procedures. Fertil. Steril. 2007, 87, 542–546. [Google Scholar] [CrossRef] [Green Version]
- Corn, C.M.; Hauser-Kronberger, C.; Moser, M.; Tews, G.; Ebner, T. Predictive value of cumulus cell apoptosis with regard to blastocyst development of corresponding gametes. Fertil. Steril. 2005, 84, 627–633. [Google Scholar] [CrossRef]
- Salhab, M.; Dhorne-Pollet, S.; Auclair, S.; Guyader-Joly, C.; Brisard, D.; Dalbiès-Tran, R.; Dupont, J.; Ponsart, C.; Mermillod, P.; Uzbekova, S. In Vitro maturation of oocytes alters gene expression and signaling pathways in bovine cumulus cells. Mol. Reprod. Dev. 2012, 80, 166–182. [Google Scholar] [CrossRef]
- Sasson, R.; Rimon, E.; Dantes, A.; Cohen, T.; Shinder, V.; Land-Bracha, A.; Amsterdam, A. Gonadotrophin-induced gene regulation in human granulosa cells obtained from IVF patients. Modulation of steroidogenic genes, cytoskeletal genes and genes coding for apoptotic signalling and protein kinases. Mol. Hum. Reprod. 2004, 10, 299–311. [Google Scholar] [CrossRef]
- Davis, B.J.; Lennard, D.E.; Lee, C.A.; Tiano, H.F.; Morham, S.G.; Wetsel, W.C.; Langenbach, R. Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1beta. Endocrinology 1999, 140, 2685–2695. [Google Scholar] [CrossRef]
- Kang, J.-T.; Atikuzzaman, M.; Kwon, D.-K.; Park, S.-J.; Kim, S.-J.; Moon, J.-H.; Koo, O.-J.; Jang, G.; Lee, B.-C. Developmental competence of porcine oocytes after In Vitro maturation and In Vitro culture under different oxygen concentrations. Zygote 2011, 20, 1–8. [Google Scholar] [CrossRef]
- Arias-Álvarez, M.; Garcia, R.; López-Tello, J.; Rebollar, P.G.; Gutierrez-Adan, A.; Lorenzo, P.L. In Vivo and In Vitro maturation of rabbit oocytes differently affects the gene expression profile, mitochondrial distribution, apoptosis and early embryo development. Reprod. Fertil. Dev. 2017, 29, 1667–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenfelder, M.; Einspanier, R. Expression of hyaluronan synthases and corresponding hyaluronan receptors is differentially regulated during oocyte maturation in cattle. Biol. Reprod. 2003, 69, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Dhali, A.; Javvaji, P.K.; Kolte, A.P.; Francis, J.R.; Roy, S.C.; Sejian, V. Temporal expression of cumulus cell marker genes during In Vitro maturation and oocyte developmental competence. J. Assist. Reprod. Genet. 2017, 34, 1493–1500. [Google Scholar] [CrossRef] [PubMed]


| Target Gene | Gene Function | Primer Sequence (5′→3′) | Product Size | GenBank Accession No. |
|---|---|---|---|---|
| PPIA | Reference gene | F-TCAACCCCACCGTGTTCTTC R-GTCACCACCCTGGCACATAA | 194 | NM_001308578.1 |
| BAX | Apoptosis | F-GTTGTCGCCCTTTTCTACTTTGC R-CAGCCCATGATGGTCCTGATC | 89 | NM_173894.1 |
| BCL2 | Apoptosis | F-GGAGCTGGTGGTTGACTTTC R-CTAGGTGGTCATTCAGGTAAG | 518 | NM_001077486.2 |
| BMP15 | Oocyte maturation | F-CTACGACTCCGCTTCGTGTGT R-AGTGCCATGCCACCAGAAC | 69 | NM_001031752.1 |
| GDF9 | Oocyte maturation | F-GAAGTGGGACAACTGGATTGTG R-CCCTGGGACAGTCCCCTTTA | 71 | NM_174681.2 |
| GJA3 | Gap junctions | F-TGCCTTTCGTTGTAACACTCA R-AGAACACATGAGCCAGGTACA | 143 | NM_174068.2 |
| HAS2 | Oocyte competence | F-CCTCATCATCCAAAGCCTG R-ACATTTCCGCAAATAGTCTG | 138 | NM_174079.2 |
| IGF2R | Growth factor | FGCTGCGGTGTGCCAAGTGAAAAAG R-AGCCCCTCTGCCGTTGTTACCT | 201 | NM_174352.2 |
| STAR | Oocyte competence | F-GCCAGGTGTTGAAGGCCA R-TCTTTAACAGACTTGGAGGCTTCC | 104 | NM_001009243.1 |
| PTGS2 | Oocyte competence | F-AGGAGGTCTTTGGTCTGGTG R-TCTGGAACAACTGCTCATCG | 126 | NM_001009432.1 |
| Total | Maturation % | Fertilization Efficiency % | Cleavage % | Blastocyst % | Number of Cells/Blastocysts | |
|---|---|---|---|---|---|---|
| Individual | 199 | 81.0 ± 5.2 | 56.4 ± 1.2 | 78.2 ± 6.7 | 29.9 ± 1.3 | 108.9 ± 3.5 |
| Control | 167 | 81.9 ± 7.1 | 52.5 ± 3.1 | 77.9 ± 6.9 | 30.5 ± 1.3 | 88.9 ± 16.2 |
| Cluster | Total Diameter µm | Oocyte Diameter µm | ZP Thickness µm |
|---|---|---|---|
| 1 (small) | 88.4 | 78.3 | 9.7 |
| 2 (intermediate) | 127.5 | 104.4 | 14.9 |
| 3 (large) | 141.9 | 116.8 | 18.8 |
| Cluster Total Diameter | Developmental Stages | |||
|---|---|---|---|---|
| Undeveloped % | Total Embryos at 2–8-Cell Stage % | Embryos Arrested at 2–8-Cell Stage % | Blastocysts % | |
| 1 | 54.8 ± 11.4 a | 45.1 ± 11.4 a | 42.0 ± 8.9 | 3.1 ± 3.1 a |
| (small) | (13/24) | (11/24) | (10/24) | (1/24) |
| 2 | 20.9 ± 9.3 b | 79.0 ± 9.3 b | 49.6 ± 5.3 | 29.4 ± 5.2 b |
| (intermediate) | (12/76) | (64/76) | (40/76) | (24/76) |
| 3 | 4.5 ± 4.5 b | 95.4 ± 4.5 b | 41.2 ± 13.3 | 54.2 ± 13.5 b |
| (arge) | (2/33) | (31/33) | (12/33) | (19/33) |
| Cluster Oocyte Diameter | Developmental Stages | |||
|---|---|---|---|---|
| Undeveloped % | Total Embryos at 2–8-Cell Stage % | Embryos Arrested at 2–8-Cell Stage % | Blastocysts % | |
| 1 | 50.8 ± 11.0 a | 49.2 ± 11.0 a | 35.0 ± 1.6 | 14.1 ± 9.4 a |
| (small) | (12/28) | (16/28) | (10/28) | (6/28) |
| 2 | 17.2 ± 7.8 b | 82.8 ± 7.8 b | 52.3 ± 10.1 | 30.5 ± 10.1 ab |
| (intermediate) | (9/71) | (62/71) | (41/71) | (21/71) |
| 3 | 17.1 ± 5.7 b | 82.8 ± 5.7 b | 38.4 ± 9.6 | 44.4 ± 3.9 b |
| (large) | (7/34) | (27/34) | (11/34) | (16/34) |
| Cluster ZP Thickness | Developmental Stages | |||
|---|---|---|---|---|
| Undeveloped % | Total Embryos at 2–8-Cell Stage % | Embryos Arrested at 2–8-Cell Stage % | Blastocysts % | |
| 1 | 41.8 ± 10.3 a | 58.2 ± 10.3 a | 31.5 ± 12.4 | 26.7 ± 3.9 a |
| (small) | (24/60) | (45/60) | (21/60) | (15/60) |
| 2 | 6.6 ± 4.1 b | 93.3 ± 4.1 b | 64.7 ± 6.3 | 28.6 ± 9.6 a |
| (intermediate) | (3/56) | (53/56) | (34/56) | (19/56) |
| 3 | 0.0 ± 0.0 b | 100 ± 0.0 b | 32.4 ± 12.4 | 67.6 ± 12.4 b |
| (large) | (0/17) | (17/17) | (6/17) | (11/17) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maside, C.; Sánchez-Ajofrín, I.; Medina-Chávez, D.; Alves, B.; Garde, J.J.; Soler, A.J. Oocyte Morphometric Assessment and Gene Expression Profiling of Oocytes and Cumulus Cells as Biomarkers of Oocyte Competence in Sheep. Animals 2021, 11, 2818. https://doi.org/10.3390/ani11102818
Maside C, Sánchez-Ajofrín I, Medina-Chávez D, Alves B, Garde JJ, Soler AJ. Oocyte Morphometric Assessment and Gene Expression Profiling of Oocytes and Cumulus Cells as Biomarkers of Oocyte Competence in Sheep. Animals. 2021; 11(10):2818. https://doi.org/10.3390/ani11102818
Chicago/Turabian StyleMaside, Carolina, Irene Sánchez-Ajofrín, Daniela Medina-Chávez, Benner Alves, José Julián Garde, and Ana Josefa Soler. 2021. "Oocyte Morphometric Assessment and Gene Expression Profiling of Oocytes and Cumulus Cells as Biomarkers of Oocyte Competence in Sheep" Animals 11, no. 10: 2818. https://doi.org/10.3390/ani11102818
APA StyleMaside, C., Sánchez-Ajofrín, I., Medina-Chávez, D., Alves, B., Garde, J. J., & Soler, A. J. (2021). Oocyte Morphometric Assessment and Gene Expression Profiling of Oocytes and Cumulus Cells as Biomarkers of Oocyte Competence in Sheep. Animals, 11(10), 2818. https://doi.org/10.3390/ani11102818






