Quality of Death in Fighting Bulls during Bullfights: Neurobiology and Physiological Responses
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Stressors of Psychological Origin
1.2. Stressors of Physical Origin
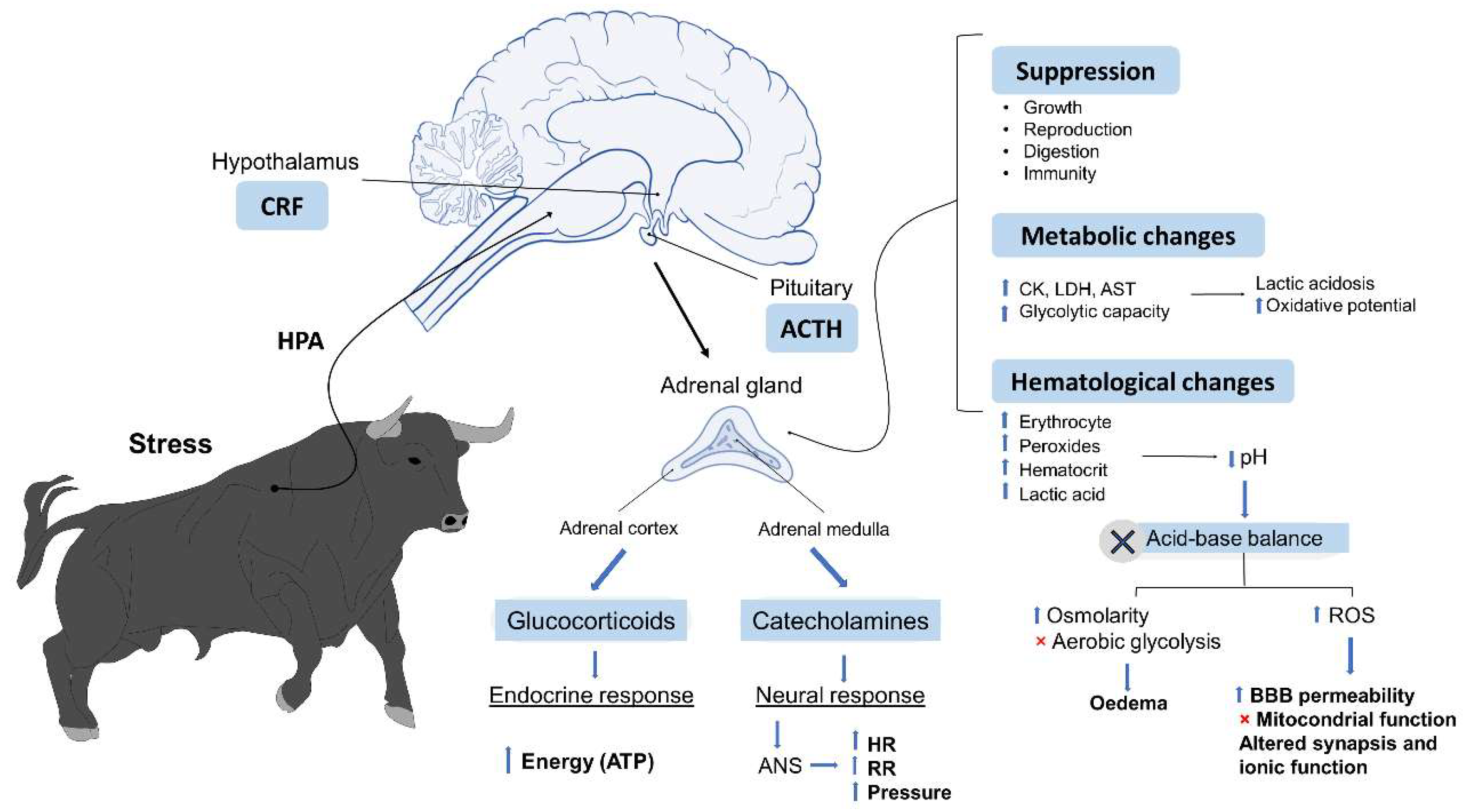
1.3. Physiological Responses to Stressors
1.4. Behavioral Responses to Stressors
1.5. The Aim of the Review
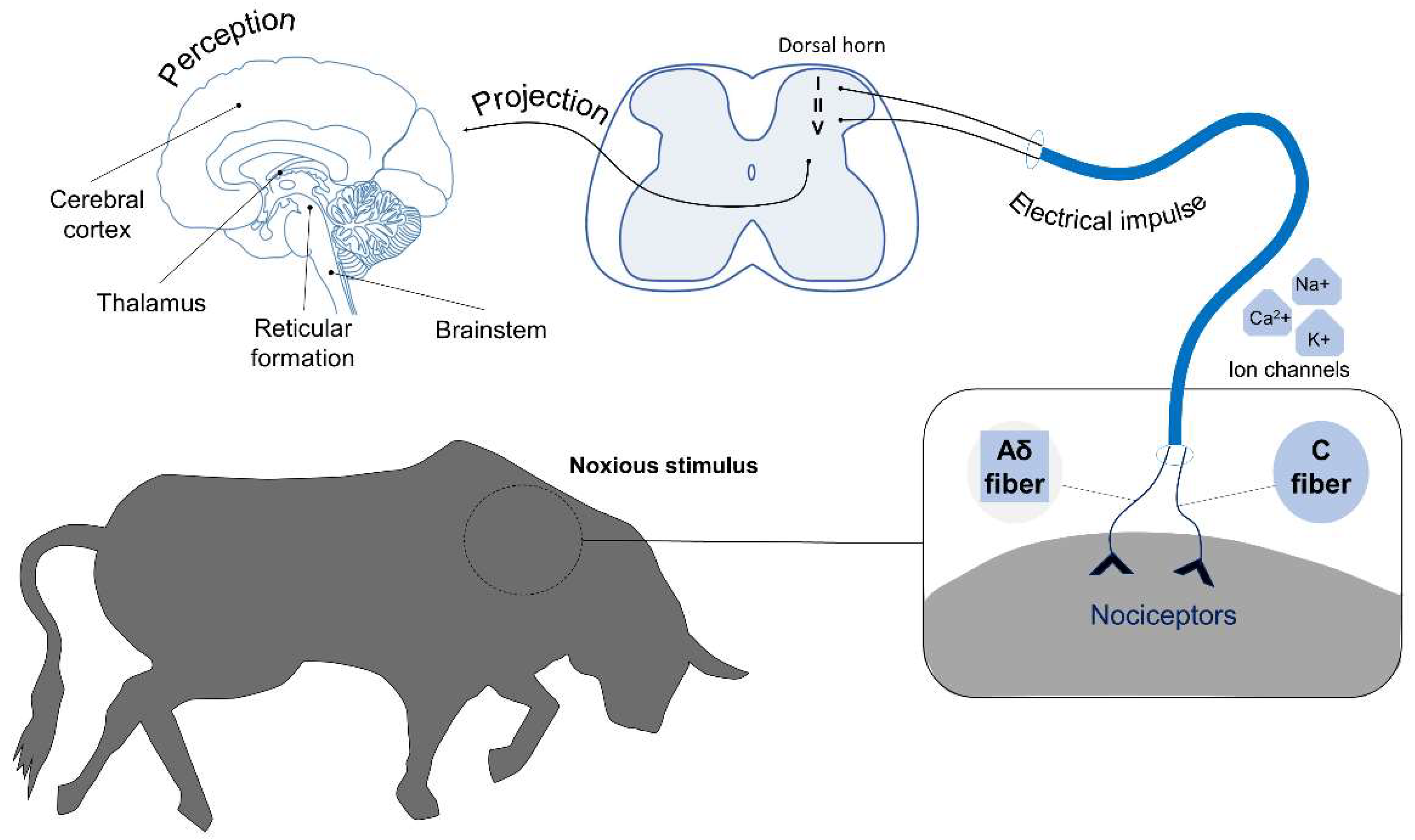
2. Neurobiology of Pain
2.1. Pain Perception
2.2. Emotions and Pain
2.3. Analgesic Effects
3. Muscle-Skeletal Injuries during Bullfights
4. Hypovolemic Shock
5. Metabolic Responses Linked to Psychological Stress and Physical Exercise
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, D.E.; Reeder, A. Mammal Species of the World. A Taxonomic and Geographic Reference; Wilson, D.E., Reeder, A., Eds.; Johns Hopkins University Press: Baltimore, MA, USA, 2005; ISBN 0-8018-8221-4. [Google Scholar]
- Domínguez-Viveros, J.; Rodríguez-Almeida, F.A.; Rafael Núñez-Domínguez, R.; Ramírez-Valverde, R.; Ruiz-Flores, A. Genetic parameters and genetic trends for behavior traits in Mexican bullfighting herds. Rev. Mex. Cienc. Pecu. 2015, 5, 261–271. [Google Scholar] [CrossRef]
- de Boyer des Roches, A.; Faure, M.; Lussert, A.; Herry, V.; Rainard, P.; Durand, D.; Foucras, G. Behavioral and patho-physiological response as possible signs of pain in dairy cows during Escherichia coli mastitis: A pilot study. J. Dairy Sci. 2017, 100, 8385–8397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards-Callaway, L.N.; Cramer, M.C.; Cadaret, C.N.; Bigler, E.J.; Engle, T.E.; Wagner, J.J.; Clark, D.L. Impacts of shade on cattle well-being in the beef supply chain. J. Anim. Sci. 2021, 99, skaa375. [Google Scholar] [CrossRef]
- García-Torres, S.; Cabeza de Vaca, M.; Tejerina, D.; Romero-Fernández, M.P.; Ortiz, A.; Franco, D.; Sentandreu, M.A.; Oliván, M. Assessment of stress by serum biomarkers in calves and their relationship to ultimate pH as an indicator of meat quality. Animals 2021, 11, 2291. [Google Scholar] [CrossRef] [PubMed]
- Kareklas, K.; Kunc, H.P.; Arnott, G. Extrinsic stressors modulate resource evaluations: Insights from territoriality under artificial noise. Front. Zool 2021, 18, 12. [Google Scholar] [CrossRef]
- Gimsa, U.; Tuchscherer, M.; Kanitz, E. Psychosocial stress and immunity—What can we learn from pig studies? Front. Behav. Neurosci. 2018, 12, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, H.D.; Carroll, J.A.; Sanchez, N.C.B.; Richeson, J.T. Natural variations in the stress and acute phase responses of cattle. Innate Immun. 2014, 20, 888–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Stookey, J.; Arsenault, R.; Scruten, E.; Griebel, P.; Napper, S. Investigation of the physiological, behavioral, and biochemical responses of cattle to restraint stress. J. Anim. Sci. 2016, 94, 3240–3254. [Google Scholar] [CrossRef]
- Barona Hernández, L.; Cuesta López, A.; & Montero Agüera, I. Cumplen las puyas su misión? Rev. Estud. Taur. 1999, 9, 95–112. [Google Scholar]
- Fernández, S.J.; Villalón, G.-C. Estudio de las lesiones producidas por la suerte de varas en la segunda parte de la Feria de San Isidro de 1998. Rev. Estud. Taur. 1999, 9, 113–139. [Google Scholar]
- Gomariz, F.M.; Vázquez, J.M.; Gil, F.; Moreno, F.; Ramírez, G.; Latorre, R.; Albors, O.L. Muscle injuries in the fighting bull (Bos taurus ibericus) after the bullfight. An. Vet. Murcia 1999, 15, 17–24. [Google Scholar]
- Fernández-Novo, A.; Lomillos-Pérez, J.M.; García-García, J.A. Lesiones macroscópicas en pulmones en ganado de lidia. Inf. Technol. Econ. Agrar. 2020, 116, 106–115. [Google Scholar] [CrossRef]
- Oquendo, Y.H.E.; Navarro, L.R.; Pereira, B.F.; Martínez, E.D.; Méndez, D.R. Importancia de los conocimientos anatómicos en las lesiones de médula espinal. Rev. Inf. Científica 2004, 43, 1–8. [Google Scholar]
- Restrepo, J.R.M. Sistematización medular. Correlación anatómica y clínica. Med. UPB 2002, 21, 119–135. [Google Scholar]
- Limon, G.; Guitian, J.; Gregory, N.G. An evaluation of the humaneness of puntilla in cattle. Meat. Sci. 2010, 84, 352–355. [Google Scholar] [CrossRef]
- Terlow, E.M.C. The physiology of the brain and determining insensibility and unconsciousness. In The Slaughter of Farmed Animals: Practical Ways of Enhancing Animal Welfare; CABI: Wallingford, CT, USA, 2020; pp. 202–228. [Google Scholar]
- Gregory, N.G.; von Wenzlawowicz, M.; Holleben, K. Blood in the respiratory tract during slaughter with and without stunning in cattle. Meat Sci. 2009, 82, 13–16. [Google Scholar] [CrossRef]
- Terlouw, C.; Bourguet, C.; Deiss, V. Consciousness, unconsciousness and death in the context of slaughter. Part I. Neurobiological mechanisms underlying stunning and killing. Meat Sci. 2016, 118, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Gonzalo, A.; Cañón, J. Genetic parameters of aggressiveness, ferocity and mobility in the fighting bull breed. Anim. Res. 2006, 55, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Menéndez-Buxadera, A.; Cortés, O.; Cañon, J. Genetic (co)variance and plasticity of behavioural traits in Lidia bovine breed. Ital. J. Anim. Sci. 2017, 16, 208–216. [Google Scholar] [CrossRef]
- Lockwood, S.A.; Kattesh, H.G.; Krawczel, P.D.; Kirkpatrick, F.D.; Saxton, A.M.; Rhinehart, J.D.; Wilkerson, J.B. Relationships among temperament, behavior, and growth during performance testing of bulls. J. Anim. Sci. 2015, 93, 5856–5862. [Google Scholar] [CrossRef]
- Parker, A.J.; Hamlin, G.P.; Coleman, C.J.; Fitzpatrick, L.A. Quantitative analysis of acid-base balance in Bos indicus steers subjected to transportation of long duration. J. Anim. Sci. 2003, 81, 1434–1439. [Google Scholar] [CrossRef] [Green Version]
- Tharwat, M.; Al-Sobayil, F. Influence of Transportation on the Serum Concentrations of the Cardiac Biomarkers Troponin I and Creatine Kinase-myocardial Band (CK-MB) and on Cortisol and Lactate in Horses. J. Equine Vet. Sci. 2014, 34, 662–667. [Google Scholar] [CrossRef]
- Becerril-Herrera, M.; Alonso-Spilsbury, M.; Ortega, M.E.T.; Guerrero-Legarreta, I.; Ramírez-Necoechea, R.; Pérez-Sato, M.; Soní-Guillermo, E.; Mota-Rojas, D. Changes in blood constituents of swine transported for 8 or 16h to an Abattoir. Meat Sci. 2010, 86, 945–948. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Becerril-Herrera, M.; Roldan, P.; Alonso-Spilsbury, M.; Flores-Peinado, S.; Ramírez-Necoechea, R.; Ramírez-Telles, J.A.; Mora-Medina, P.; Pérez, M.; Molina, E.; et al. Effects of long distance transportation and CO2 stunning on critical blood values in pigs. Meat Sci. 2012, 90, 893–898. [Google Scholar] [CrossRef]
- Gouveia, A.J.; Orge, L.; Carvalho, P. A dimensão da amígdala cerebral e a agressividade no touro de lide. Arch. Zootec. 2016, 65, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Du, K.; Lu, W.; Sun, Y.; Feng, J.; Wang, J.-H. mRNA and miRNA profiles in the nucleus accumbens are related to fear memory and anxiety induced by physical or psychological stress. J. Psychiatr. Res. 2019, 118, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Hemsworth, P.H.; Coleman, G.J. Human-Livestock Interactions: The Stockperson and the Productivity and Welfare of Intensive Farmed Animals, 2nd ed.; Hemsworth, P.H., Coleman, G.J., Eds.; CAB International: Wallingford, UK, 2010; pp. 1–194. [Google Scholar]
- Hennessy, M.B.; Willen, R.M.; Schiml, P.A. Psychological stress, its reduction, and long-term consequences: What studies with laboratory animals might teach us about life in the dog shelter. Animals 2020, 10, 2061. [Google Scholar] [CrossRef]
- Rostamkhani, F.; Zardooz, H.; Zahediasl, S.; Farrokhi, B. Comparison of the effects of acute and chronic psychological stress on metabolic features in rats. J. Zhejiang Univ. Sci. B 2012, 13, 904–912. [Google Scholar] [CrossRef] [Green Version]
- Grandin, T.; Shivley, C. How farm animals react and perceive stressful situations such as handling, restraint, and transport. Animals 2015, 5, 1233–1251. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, H.; Zhang, L.; Liu, H.; Li, J.; Wang, C.; Zhang, M.; Bao, J. Technical Note: Effects of age and confinement on pupillary light reflex in sows. J. Anim. Sci. 2019, 97, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Arzy, S.; Bertolus, J.B.; Depue, B.; Haas, H.E.; Hofmann, S.G.; Kangas, M.; Kensinger, E.; Lowry, C.A.; Marusak, H.A.; et al. Current understanding of fear learning and memory in humans and animal models and the value of a linguistic approach for analyzing fear learning and memory in humans. Neurosci. Biobehav. Rev. 2019, 105, 136–177. [Google Scholar] [CrossRef]
- Bacher, L.; Prieur, V.; Lardy, R.; Boivin, X. Does the avoidance distance test at the feed barrier have scientific validity for evaluating reactivity to humans in Limousin breeding bulls? Livest Sci. 2021, 249, 104535. [Google Scholar] [CrossRef]
- Daigle, C.L.; Hubbard, A.J.; Grandin, T. The use of traditional fear tests to evaluate different emotional circuits in cattle. J. Vis. Exp. 2020, 158, e60641. [Google Scholar] [CrossRef]
- Cannas, S.; Palestrini, C.; Canali, E.; Cozzi, B.; Ferri, N.; Heinzl, E.; Minero, M.; Chincarini, M.; Vignola, G.; Dalla Costa, E. Thermography as a non-invasive measure of stress and fear of humans in sheep. Animals 2018, 8, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandin, T. Assessment of stress during handling and transport. J. Anim. Sci. 1997, 75, 249. [Google Scholar] [CrossRef] [Green Version]
- Swanson, J.C.; Morrow-Tesch, J. Cattle transport: Historical, research, and future perspectives. J. Anim. Sci. 2001, 79, E102. [Google Scholar] [CrossRef] [Green Version]
- Mpakama, T.; Chulayo, A.Y.; Muchenje, V. Bruising in slaughter cattle and its relationship with creatine kinase levels and beef quality as affected by animal related factors. Asian-Australas. J. Anim. Sci. 2014, 27, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Purroy, A.; García-Belenguer, S.; González, J.; Gascon, M.; Barberan, M. Muscular lesions and enzymatic activities in fighting bulls. Ann. Rech. Vet. 1992, 23, 59–62. [Google Scholar]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; de Lima Umeoka, E.H. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Kassahn, K.S.; Crozier, R.H.; Pörtner, H.O.; Caley, M.J. Animal performance and stress: Responses and tolerance limits at different levels of biological organisation. Biol. Rev. 2009, 84, 277–292. [Google Scholar] [CrossRef]
- McCobb, E.C.; Patronek, G.J.; Marder, A.; Dinnage, J.D.; Stone, M.S. Assessment of stress levels among cats in four animal shelters. J. Am. Vet. Med. Assoc. 2005, 226, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Bohacek, J.; Mansuy, I.M. Molecular insights into transgenerational non-genetic inheritance of acquired behaviours. Nat. Rev. Genet. 2015, 16, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Andreassi, E.; Watson, W.; Coon, C. Stress and animal health: Physiological mechanisms and ecological consequences. Nat. Educ. Knowl. 2011, 3, 11. [Google Scholar]
- Mota-Rojas, D.; Ghezzi, M.D.; Napolitano, F.; Rosmini, M.R.; Guerrero-Legarreta, I.; Martínez-Burnes, J.; Lezama-García, K.; Miranda-Cortés, A.; de la Vega, L.T.; Mora-Medina, P.; et al. Quality of death in the river buffalo (Bubalus bubalis). J. Anim. Behav. Biometeorol. 2021, 9, 2115. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Napolitano, F.; Strappini, A.; Orihuela, A.; Ghezzi, M.D.; Hernández-Ávalos, I.; Mora-Medina, P.; Whittaker, A.L. Pain at the Slaughterhouse in Ruminants with a Focus on the Neurobiology of Sensitisation. Animals 2021, 11, 1085. [Google Scholar] [CrossRef]
- Stahringer, R.C.; Randel, R.D.; Neuendorff, D.A. Effects of naloxone and animal temperament on serum luteinizing hormone and cortisol concentrations in seasonally anestrous Brahman heifers. Theriogenology 1990, 34, 393–406. [Google Scholar] [CrossRef]
- Curley, K.O.; Paschal, J.C.; Welsh, T.H.; Randel, R.D. Technical note: Exit velocity as a measure of cattle temperament is repeatable and associated with serum concentration of cortisol in Brahman bulls1. J. Anim. Sci. 2006, 84, 3100–3103. [Google Scholar] [CrossRef] [PubMed]
- Escalera-Valente, F.; Ramiro González-Montaña, J.; Alonso de la Varga, M.E.; Lomillos-Pérez, J.M.; Gaudioso-Lacasa, V.R. Influence of intense exercise on acid–base, blood gas and electrolyte status in bulls. Res. Vet. Sci. 2013, 95, 623–628. [Google Scholar] [CrossRef]
- Agüera, E.; Rubio, M.; Vivo, R.; Escribano, B.; Muñoz, A.; Villafuerte, J.; Castejón, F. Adaptaciones fisiológicas a la lidia en el toro bravo. Parámetros plasmáticos y musculares. Vet. Mèxico 1998, 29, 399–403. [Google Scholar]
- Ayo, J.; Oladele, S.; Fayomi, A. Behavioural Reactions of Cattle to Stress Situations: A Review. J. Agri. Technol. 2002, 8, 15–20. [Google Scholar]
- Warriss, P.D. The transport of animals: A long way to go. Vet. J. 2004, 168, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.R.; Stewart, M.; Rogers, A.R.; Verkerk, G.A. Assessment of welfare from physiological and behavioural responses of New Zealand dairy cows exposed to cold and wet conditions. Anim. Welf 2008, 17, 19–26. [Google Scholar]
- Waynert, D.; Stookey, J.; Schwartzkopf-Genswein, K.; Watts, J.; Waltz, C. The response of beef cattle to noise during handling. Appl. Anim. Behav. Sci. 1999, 62, 27–42. [Google Scholar] [CrossRef]
- Adenkola, A.Y.; Ayo, J.O. Physiological and behavioural responses of livestock to road transportation stress: A review. African J. Biotechnol. 2010, 9, 4845–4856. [Google Scholar] [CrossRef]
- Briefer, E.F. Vocal expression of emotions in mammals: Mechanisms of production and evidence. J. Zool 2012, 288, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Yoshihara, Y.; Oya, K. Characterization and assessment of vocalization responses of cows to different physiological states. J. Appl. Anim. Res. 2021, 49, 347–351. [Google Scholar] [CrossRef]
- Johnson, C.; Mellor, D.; Hemsworth, P.; Fisher, A. A scientific comment on the welfare of domesticated ruminants slaughtered without stunning. N. Z. Vet. J. 2015, 63, 58–65. [Google Scholar] [CrossRef]
- Gregory, N.G. Animal welfare at markets and during transport and slaughter. Meat Sci. 2008, 80, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Imlan, J.C.; Kaka, U.; Goh, Y.-M.; Idrus, Z.; Awad, E.A.; Abubakar, A.A.; Ahmad, T.; Nizamuddin, H.N.Q.; Sazili, A.Q. Effects of Slaughter Knife Sharpness on Blood Biochemical and Electroencephalogram Changes in Cattle. Animals 2020, 10, 579. [Google Scholar] [CrossRef] [Green Version]
- Gleerup, K.B.; Andersen, P.H.; Munksgaard, L.; Forkman, B. Pain evaluation in dairy cattle. Appl. Anim. Behav. Sci. 2015, 171, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Ellison, D.L. Physiology of Pain. Crit. Care Nurs. Clin. N. Am. 2017, 29, 397–406. [Google Scholar] [CrossRef]
- Bell, A. The neurobiology of acute pain. Vet. J. 2018, 237, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinakar, P.; Stillman, A.M. Pathogenesis of Pain. Semin. Pediatr. Neurol. 2016, 23, 201–208. [Google Scholar] [CrossRef]
- Muir, W. Physiopatology and Pathophysiology of pain. In Handbook of Veterinary Pain Management; Mosby Elsevier: Houston, Texas, USA, 2009; pp. 13–41. [Google Scholar]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaksh, T.L.; Woller, S.A.; Ramachandran, R.; Sorkin, L.S. The search for novel analgesics: Targets and mechanisms. F1000Prime Rep. 2015, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glardon, M.; Schwenk, B.K.; Riva, F.; von Holzen, A.; Ross, S.G.; Kneubuehl, B.P.; Stoffel, M.H. Energy loss and impact of various stunning devices used for the slaughtering of water buffaloes. Meat Sci. 2018, 135, 159–165. [Google Scholar] [CrossRef]
- Battini, M.; Agostini, A.; Mattiello, S. Understanding cows’ emotions on farm: Are eye white and ear posture reliable indicators? Animals 2019, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Core, S.; Widowski, T.; Mason, G.; Miller, S. Eye white percentage as a predictor of temperament in beef cattle. J. Anim. Sci. 2009, 87, 2168–2174. [Google Scholar] [CrossRef]
- Forkman, B.; Boissy, A.; Meunier-Salaün, M.-C.; Canali, E.; Jones, R.B. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 2007, 92, 340–374. [Google Scholar] [CrossRef] [Green Version]
- Rhudy, J.L.; Meagher, M.W. The role of emotion in pain modulation. Curr. Opin. Psychiatry 2001, 14, 241–245. [Google Scholar] [CrossRef]
- Gregory, N. Physiology and Behaviour of Animal Suffering, 1st ed.; Gregory, N.G., Ed.; Blackwell Publishing: Oxford, UK, 2004; ISBN 9780470752494. [Google Scholar]
- Corder, G.; Castro, D.C.; Bruchas, M.R.; Scherrer, G. Endogenous and Exogenous Opioids in Pain. Annu. Rev. Neurosci. 2018, 41, 453–473. [Google Scholar] [CrossRef] [PubMed]
- Centenera, R.L.A. Concentraciones de Hormonas Opiáceas y su Relación con la Respuesta al Dolor en el en el toro de Lidia. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2014; pp. 1–254. [Google Scholar]
- Brooke, M.H.; Kaiser, K.K. Muscle Fiber Types: How Many and What Kind? Arch. Neurol. 1970, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.; Edgerton, V. Motor unit properties and selective involvement in movement. Exerc. Sport Sci. Rev. 1975, 3, 31–80. [Google Scholar] [CrossRef]
- Martínez-Gomariz, F.; Vázquez Autón, J.M.; Moreno Medina, F.; Cano, F.; Ramírez Zarzosa, G.J.; Latorre Reviriego, R.; López Albors, O.M. Muscle fibre types in bullfight (Bos taurus Ibericus). An histochemical and morphometric study. An. Vet. Murcia 1997, 14, 13–14. [Google Scholar]
- Medellín, A.M. El origen de la acidez en la glucólisis anaerobia. Rev. Educ Bioquímica 2008, 27, 111–118. [Google Scholar]
- de Moraes-Bertuzzi, R.C.; Silva, A.E.L.; Abad, C.C.C.; de Oliveira Pires, F. Metabolismo do lactato: Uma revisão sobre a bioenergética e a fadiga muscular. Rev. Bras. Cineantropom. Desempenho Hum. 2009, 11, 226–234. [Google Scholar]
- Agüera, E.I.; Muñoz, A.; Suceso Gómez-Torrico, M.; Villafuerte, J.L.; Escribano, B.M.; Castejón, F. Metabolic characteristics of semitendinosus and gluteus medius muscles in bullfighting bulls at enzymatic level. Ann. Zootech. 2000, 49, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Tripp, M.J.; Schmitz, J.A. Influence of physical exercise on plasma creatine kinase activity in healthy and dystrophic turkeys and sheep. Am. J. Vet. Res. 1982, 43, 2220–2223. [Google Scholar]
- Lomillos-Pérez, J.M.; Alonso de la Varga, M.E. Osteocondrosis en el toro de lidia y evaluación de su efecto sobre la movilidad del animal. Rev. Mex. Cienc. Pecu. 2017, 8, 453. [Google Scholar] [CrossRef] [Green Version]
- Mas, A.; Martínez-Gomariz, F.; Sanes, J.; Sánchez, C.; Reyes, J.; Gutiérrez, C.; Seva, J. Estudio estadístico de la relación de la edad y el peso con la aparición de osteocondrosis carpometacarpiana y síndrome de la caída en el toro de lidia. Symp. Toro Lidia 2011, 12, 189–193. [Google Scholar]
- Dávila, U. Osteocondrosis en el Toro de Lidia; Universidad de Córdoba: Cordoba, Argentina, 2013; pp. 1–247. [Google Scholar]
- Martínez, P. Lesiones Anatómicas Producidas en el Toro por los Trebejos Empleados en la Lidia. Ph.D. Thesis, Universidad de Córdoba, Cordoba, Spain, 1997; pp. 1–156. [Google Scholar]
- Lomillos-Pérez, J.; Alonso-de la Varga, M.; Gaudioso-Lacasa, V. Evolución del síndrome de caída del toro de lidia en los últimos 25 años. Abanico Vet. 2018, 8, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Dávila, U.; Maniscalco, L.; Sierra, M.A.; Biolatti, B.; Méndez, A. Osteochondrosis in Fighting Bulls. J. Comp. Pathol. 2012, 146, 73. [Google Scholar] [CrossRef]
- Morales, C. Monitoring and resuscitation of severely ill and shocked patients. Acta Médica Peru. 2010, 27, 298–301. [Google Scholar]
- Vincent, J.-L.; De Backer, D. Circulatory Shock. N. Engl. J. Med. 2013, 369, 1726–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López Cruz, F.; Rocío, G.D.; De, P.; Barragán, R.; Tapia Ibáñez, E.X.; Christopher, D.; Cordero, P.; Morales, X.O.; Alfredo, A.; Esquivel, C.; et al. Choque hipovolémico. An. Med. (Mex) 2018, 63, 48–54. [Google Scholar]
- Mooring, M.S.; Patton, M.L.; Lance, V.A.; Hall, B.M.; Schaad, E.W.; Fetter, G.A.; Fortin, S.S.; McPeak, K.M. Glucocorticoids of bison bulls in relation to social status. Horm. Behav. 2006, 49, 369–375. [Google Scholar] [CrossRef]
- Abbott, D.; Keverne, E.; Bercovitch, F.; Shively, C.; Mendoza, S.; Saltzman, W.; Snowdon, C.; Ziegler, T.; Banjevic, M.; Garland, T.; et al. Are subordinates always stressed? a comparative analysis of rank differences in cortisol levels among primates. Horm. Behav. 2003, 43, 67–82. [Google Scholar] [CrossRef]
- Muller, M.N.; Wrangham, R.W. Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii). Behav. Ecol. Sociobiol. 2004, 55, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Escribano, B.; Tunez, I.; Requena, F.; Rubio, M.; De Miguel, R.; Montilla, P.; Tovar, P.; Aguera, E. Effects of an aerobic training program on oxidative stress biomarkers in bulls. Vet. Med. (Praha) 2010, 55, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, J.; Harvey, J.; Bruss, M. Clinical Biochemistry of Domestic Animals; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Klein, B.G. Cunningham. Fisiología Veterinaria; Bradley, G.K., Ed.; Elsevier-Saunders: Barcelona, España, 2014; ISBN 9781437723618. pp. 1–607. [Google Scholar]
- Castejón, F.; Muñoz, A.; Agüera, E.; Gómez-Torrico, M.; Essén-Gustavsson, B. Diferencias en la Respuesta Metabólica del Músculo del toro Bravo a la Lidia. Resúmenes del II Congreso Mundial Taurino de Veterinaria; Consejo General de Colegios de Veterinarios de España: Cordoba, Spain, 1997; pp. 207–210. [Google Scholar]
- Lacourt, A.; Tarrant, P.V. Glycogen depletion patterns in myofibres of cattle during stress. Meat Sci. 1985, 15, 85–100. [Google Scholar] [CrossRef]
- Agüera, E.I.; Munoz, A.; Castejon, F.M.; Essen-Gustavsson, B. Skeletal Muscle Fibre Characteristics in Young and Old Bulls and Metabolic Response after a Bullfight. J. Vet. Med. Ser. A 2001, 48, 313–319. [Google Scholar] [CrossRef]
- Hanna, P.E.; Bellamy, J.E.; Donald, A. Postmortem eyefluid analysis in dogs, cats and cattle as an estimate of antemortem serum chemistry profiles. Can. J. Vet. Res. 1990, 54, 487–494. [Google Scholar]
- González-Montaña, J.-R.; Escalera-Valente, F.; Alonso, M.E.; Lomillos, J.M.; Gaudioso, V. Relationships Between Concentrations of Biological Variables in Eye Fluids and Blood After Exercise in Lidia Cattle. Acta Vet. Brno 2018, 68, 420–433. [Google Scholar] [CrossRef] [Green Version]
- Rivero, J.L.L.; Galisteo, A.M.; Agüera, E.; Miró, F. Skeletal muscle histochemistry in male and female Andalusian and Arabian horses of different ages. Res. Vet. Sci. 1993, 54, 160–169. [Google Scholar] [CrossRef]
- Essén-Gustavsson, B.; Lindholm, A. Fiber types and metabolic characteristics in muscles of wild boars, normal and halothane sensitive swedish landrace pigs. Comp. Biochem. Physiol. Part A Physiol. 1984, 78, 67–71. [Google Scholar] [CrossRef]
- Essén-Gustavsson, B.; Karlström, K.; Lindholm, A. Fibre types, enzyme activities and substrate utilisation in skeletal muscles of horses competing in endurance rides. Equine Vet. J. 1984, 16, 197–202. [Google Scholar] [CrossRef]
- Essén-Gustavsson, B.; Lindholm, A. Muscle fibre characteristics of active and inactive Standardbred horses. Equine Vet. J. 1985, 17, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Purroy, A.; Buitrago, J. Etude des enzymes plasmatiques des taureaux de combat tués en corridas. Reprod. Nutr. Dévelop. 1985, 25, 599–603. [Google Scholar]
- Muñoz Juzado, A.; Castejón, F.M.; Agüera, E.I. Diferencias en el perfil enzimático muscular y respuesta metabólica a la lidia en toros de uno a tres años de edad. Arch. Med. Vet. 2007, 39, 35–41. [Google Scholar]
- Antonelli, M.; Levy, M.; Andrews, P.J.D.; Chastre, J.; Hudson, L.D.; Manthous, C.; Meduri, G.U.; Moreno, R.P.; Putensen, C.; Stewart, T.; et al. Hemodynamic monitoring in shock and implications for management. Intensive Care Med. 2007, 33, 575–590. [Google Scholar] [CrossRef]
- Rivers, E.; Nguyen, B. Early Goal-Directed Therapy in the treatment of Severe Sepsis and Septic Shock. N. Engl. J. Med. 2001, 345, 1368–1377. [Google Scholar] [CrossRef] [Green Version]
- Creel, S. Social dominance and stress hormones. Trends Ecol. Evol. 2001, 16, 491–497. [Google Scholar] [CrossRef]
- Sands, J.; Creel, S. Social dominance, aggression and faecal glucocorticoid levels in a wild population of wolves, Canis lupus. Anim. Behav. 2004, 67, 387–396. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Miranda-Cortes, A.; Casas-Alvarado, A.; Mora-Medina, P.; Boscato-Funes, L.; Hernández-Avalos, I. Neurobiology and modulation of stress-induced hyperthermia and fever in animal. Abanico Vet. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Schwartzkopf-Genswein, K.S.; Stookey, J.M.; de Passillé, A.; Rushen, J. Comparison of hot-iron and freeze branding on cortisol levels and pain sensitivity in beef cattle. Can. J. Anim. Sci. 1997, 77, 369–374. [Google Scholar] [CrossRef]
- García-Belenguer, S.; Gascón, M.; Purroy, A.; Aceña, M. Distrofia muscular nutricional por deficiencia de selenio y/o vitamina E en rumiantes. Med. Vet. 1992, 9, 84–92. [Google Scholar]
- Carpintero, C.; Fernández, C.; Gómez, J.; Gómez, S.; Gómez, J.; Hebrero, C. Estudio de las variaciones de ciertos parámetros hematológicos y bioquímicos sanguíneos del toro bravo tras la lidia. Vet Madrid S/V:22–26. Douglas CB (1991) The ‘“fiesta”’ cycle of ‘“Spain”’. Anthropol. Q 1996, 64, 126–142. [Google Scholar]
- González-Montaña, J.R.; Escalera-Valent, F.; Lomillos, J.; Alonso, A.; Gaudioso, V.; Alonso, M. Relationships between eye fluids and blood values after exercise in lidia cattle: Mineral parameters. Pol. J. Vet. Sci. 2019, 22, 445–455. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
| Muscle Group | Function |
|---|---|
| Common digital extensor, gluteobiceps, and long digital extensor | Support in extending and retracting extremities |
| Long thorax | Fixing and righting action of the rachis; dorsal flexor agent of the thoracic-lumbar rachis; regulating mechanical influences in the protraction–retraction of pelvic members |
| Latissimus dorsi | When contracted, once the protraction of the thoracic member is culminated (support in extension); drags body mass while retraction of the member lasts |
| Ventral thoracic serrate | Constitutes the principal suspensor agent of the trunk. |
| From Gomariz et al. [12] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota-Rojas, D.; Napolitano, F.; Strappini, A.; Orihuela, A.; Martínez-Burnes, J.; Hernández-Ávalos, I.; Mora-Medina, P.; Velarde, A. Quality of Death in Fighting Bulls during Bullfights: Neurobiology and Physiological Responses. Animals 2021, 11, 2820. https://doi.org/10.3390/ani11102820
Mota-Rojas D, Napolitano F, Strappini A, Orihuela A, Martínez-Burnes J, Hernández-Ávalos I, Mora-Medina P, Velarde A. Quality of Death in Fighting Bulls during Bullfights: Neurobiology and Physiological Responses. Animals. 2021; 11(10):2820. https://doi.org/10.3390/ani11102820
Chicago/Turabian StyleMota-Rojas, Daniel, Fabio Napolitano, Ana Strappini, Agustín Orihuela, Julio Martínez-Burnes, Ismael Hernández-Ávalos, Patricia Mora-Medina, and Antonio Velarde. 2021. "Quality of Death in Fighting Bulls during Bullfights: Neurobiology and Physiological Responses" Animals 11, no. 10: 2820. https://doi.org/10.3390/ani11102820
APA StyleMota-Rojas, D., Napolitano, F., Strappini, A., Orihuela, A., Martínez-Burnes, J., Hernández-Ávalos, I., Mora-Medina, P., & Velarde, A. (2021). Quality of Death in Fighting Bulls during Bullfights: Neurobiology and Physiological Responses. Animals, 11(10), 2820. https://doi.org/10.3390/ani11102820









