BOEC–Exo Addition Promotes In Vitro Maturation of Bovine Oocyte and Enhances the Developmental Competence of Early Embryos
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Aspiration and Collection of Oocytes
2.3. Bovine Primary Cumulus Cells Culture
2.4. In Vitro Maturation of Ooocytes
2.5. DAPI Staining for Oocyte Meiotic Maturation Assessment
2.6. In Vitro Fertilization
2.7. In Vitro Culture
2.8. Supplementation of BOEC–Exo
2.9. RNA Extraction, cDNA Synthesis, and Quantitative Reverse Transcription PCR (q-RT-PCR) Analysis
2.10. Nile Red Staining
2.11. ROS Assay
2.12. Immunofluorescence
2.13. Antibodies
2.14. JC-1 Staining
2.15. Invasion Assay
2.16. Statistical Analysis
3. Results
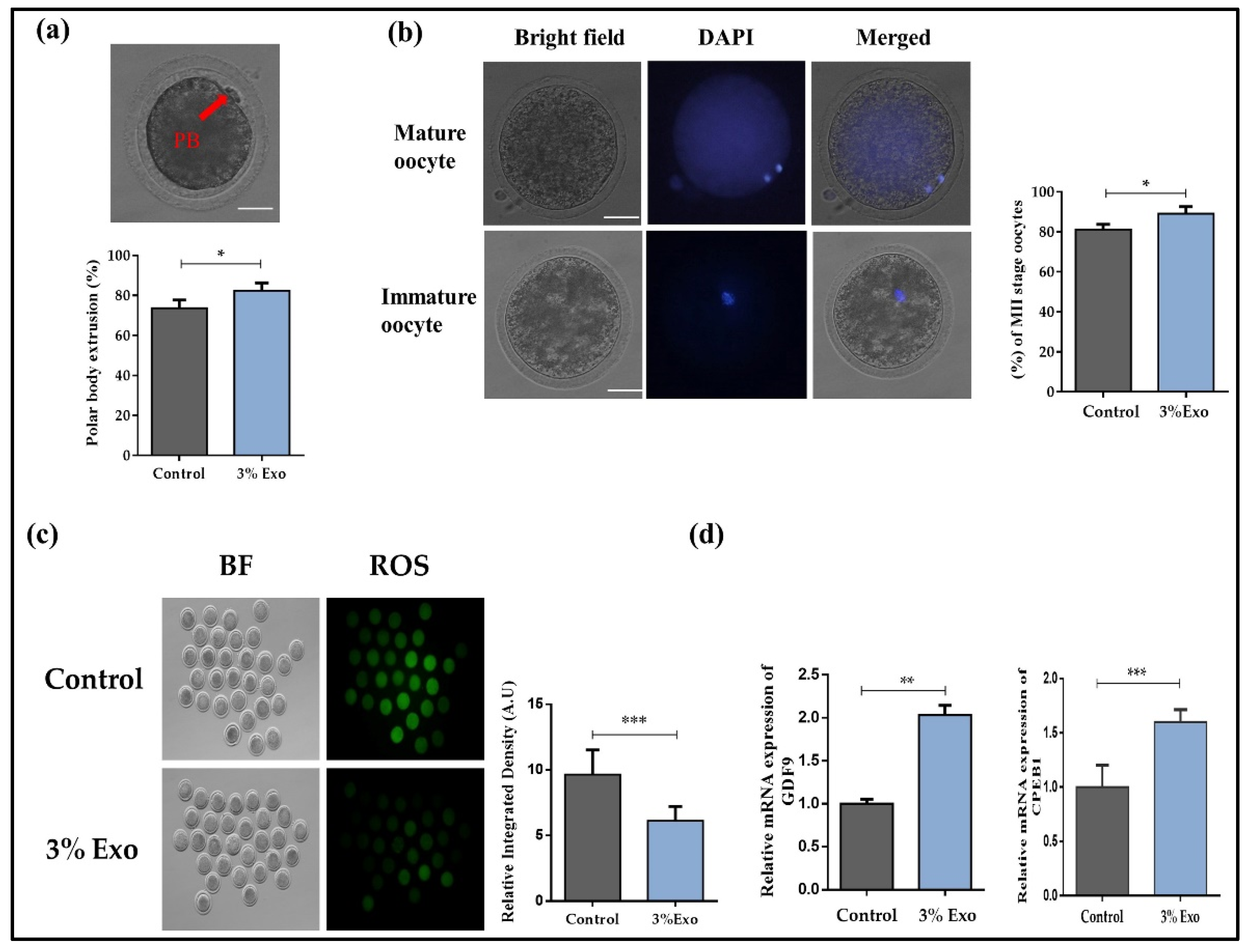
3.1. Developmental Competence of In Vitro Matured Oocytes in the Presence of BOEC–Exo
3.2. Effect of BOEC–Exo on Gap Junction Communication and Cumulus Cell Health
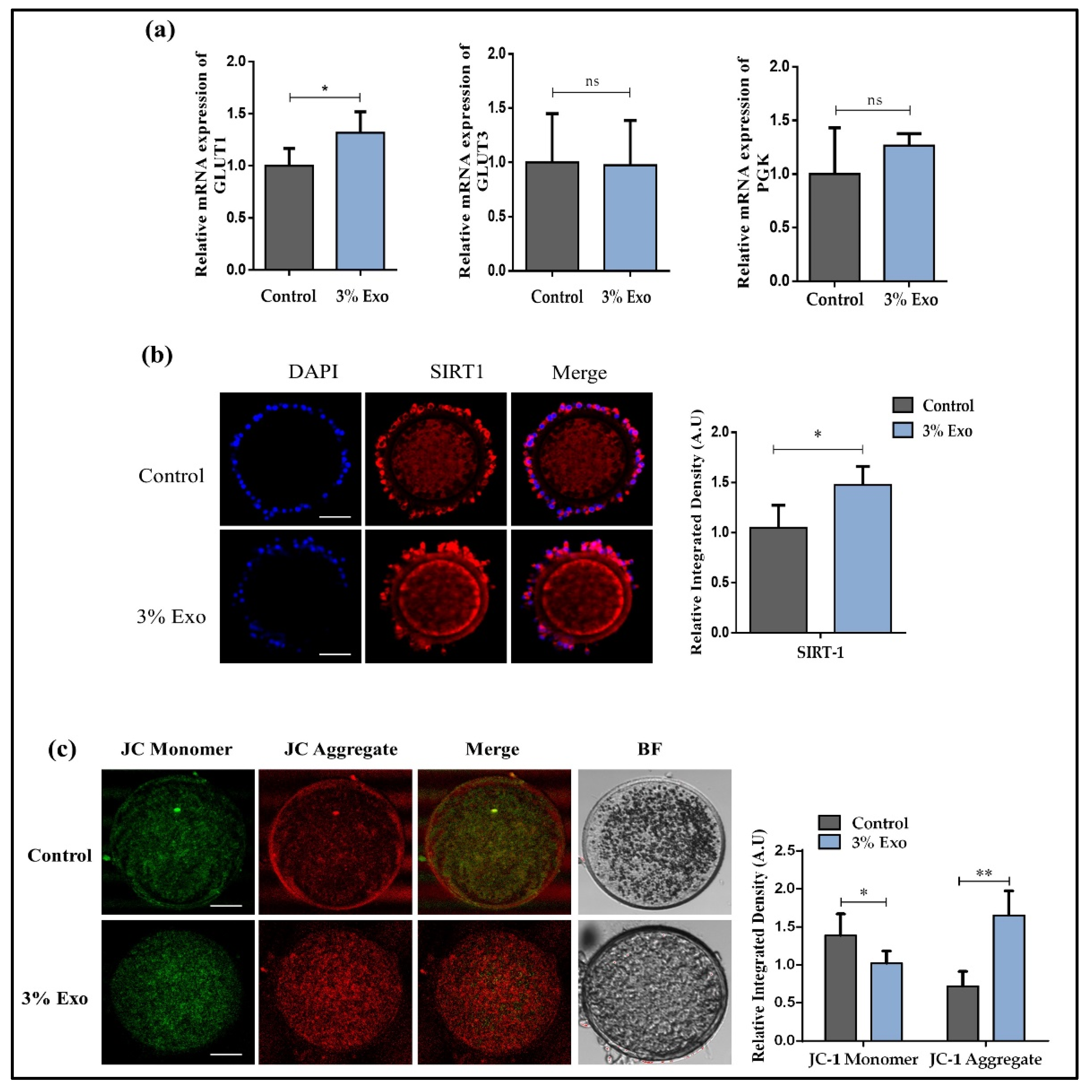
3.3. BOEC–Exo Addition Improves the Glucose Metabolism and Mitochondrial Activity in In Vitro Mature Oocytes
3.4. BOEC–Exo Addition Affects Lipid Level in MII-Stage Bovine Oocytes, Improving Blastocysts’ Development Rate and Implantation Potential
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deb, G.K.; Dey, S.R.; Bang, J.I.; Cho, S.J.; Park, H.C.; Lee, J.G.; Kong, I.K. 9-cis retinoic acid improves developmental competence and embryo quality during in vitro maturation of bovine oocytes through the inhibition of oocyte tumor necrosis factor-α gene expression1. J. Anim. Sci. 2011, 89, 2759–2767. [Google Scholar] [CrossRef]
- Boni, R.; Cuomo, A.; Tosti, E. Developmental Potential in Bovine Oocytes Is Related to Cumulus-Oocyte Complex Grade, Calcium Current Activity, and Calcium Stores. Biol. Reprod. 2002, 66, 836–842. [Google Scholar] [CrossRef]
- Luciano, A.M.; Sirard, M.A. Successful in vitro maturation of oocytes: A matter of follicular differentiation. Biol. Reprod. 2018, 98, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Wrenzycki, C.; Stinshoff, H. Maturation Environment and Impact on Subsequent Developmental Competence of Bovine Oocytes. Reprod. Domest. Anim. 2013, 48 (Suppl. 1), 38–43. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.; De Sousa, P.; Caveney, A.; Barcroft, L.C.; Natale, D.; Urquhart, J.; Westhusin, M. Impact of bovine oocyte maturation media on oocyte transcript levels, blastocyst development, cell number, and apoptosis. Biol. Reprod. 2000, 62, 355–364. [Google Scholar] [CrossRef]
- Idrees, M.; Xu, L.; Song, S.-H.; Joo, M.-D.; Lee, K.-L.; Muhammad, I.; El Sheikh, M.; Sidrat, T.; Kong, I.-K. PTPN11 (SHP2) Is Indispensable for Growth Factors and Cytokine Signal Transduction During Bovine Oocyte Maturation and Blastocyst Development. Cells 2019, 8, 1272. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, M.; Morris, M.B.; Day, M.L. Amino acid supplementation of a simple inorganic salt solution supports efficient in vitro maturation (IVM) of bovine oocytes. Sci. Rep. 2019, 9, 11739. [Google Scholar] [CrossRef]
- Gandhi, A.; Lane, M.; Gardner, D.; Krisher, R.A. single medium supports development of bovine embryos throughout maturation, fertilization and culture. Hum. Reprod. 2000, 15, 395–401. [Google Scholar] [CrossRef][Green Version]
- Ghersevich, S.; Massa, E.; Zumoffen, C. Oviductal secretion and gamete interaction. Reproduction 2015, 149, R1–R14. [Google Scholar] [CrossRef]
- Saint-Dizier, M.; Schoen, J.; Chen, S.; Banliat, C.; Mermillod, P. Composing the Early Embryonic Microenvironment: Physiology and Regulation of Oviductal Secretions. Int. J. Mol. Sci. 2020, 21, 223. [Google Scholar] [CrossRef]
- Abe, H.; Hoshi, H. Bovine oviductal epithelial cells: Their cell culture and applications in studies for reproductive biology. Cytotechnology 1997, 23, 171–183. [Google Scholar] [CrossRef]
- Ronquist, K.G. Extracellular vesicles and energy metabolism. Clin. Chim. Acta 2019, 488, 116–121. [Google Scholar] [CrossRef]
- Saeed-Zidane, M.; Linden, L.; Salilew-Wondim, D.; Held, E.; Neuhoff, C.; Tholen, E.; Hoelker, M.; Schellander, K.; Tesfaye, D. Cellular and exosome mediated molecular defense mechanism in bovine granulosa cells exposed to oxidative stress. PLoS ONE 2017, 12, e0187569. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Sidrat, T.; Khan, A.; Joo, M.-D.; Wei, Y.; Lee, K.-L.; Xu, L.; Kong, I.-K. Bovine Oviduct Epithelial Cell-Derived Culture Media and Exosomes Improve Mitochondrial Health by Restoring Metabolic Flux during Pre-Implantation Development. Int. J. Mol. Sci. 2020, 21, 7589. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Qing, S.; Liu, R.; Qin, H.; Wang, W.; Qiao, F.; Ge, H.; Liu, J.; Zhang, Y.; Cui, W.; et al. Effects of embryo-derived exosomes on the development of bovine cloned embryos. PLoS ONE 2017, 12, e0174535. [Google Scholar] [CrossRef]
- Jiang, N.-X.; Li, X.-L. The Complicated Effects of Extracellular Vesicles and Their Cargos on Embryo Implantation. Front. Endocrinol. 2021, 12, 681266. [Google Scholar] [CrossRef]
- Mesalam, A.; Khan, I.; Lee, K.-L.; Song, S.-H.; Chowdhury, M.; Uddin, Z.; Park, K.H.; Kong, I.-K. 2-Methoxystypandrone improves in vitro -produced bovine embryo quality through inhibition of IKBKB. Theriogenology 2017, 99, 10–20. [Google Scholar] [CrossRef]
- Idrees, M.; Kumar, V.; Joo, M.-D.; Ali, N.; Lee, K.-W.; Kong, I.-K. SHP2 Nuclear/Cytoplasmic Trafficking in Granulosa Cells Is Essential for Oocyte Meiotic Resumption and Maturation. Front. Cell Dev. Biol. 2020, 8, 611503. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Idrees, M.; Xu, L.; El Sheikh, M.; Sidrat, T.; Song, S.H.; Joo, M.D.; Lee, K.L.; Kong, I.K. The PPARdelta Agonist GW501516 Improves Lipolytic/Lipogenic Balance through CPT1 and PEPCK during the Development of Pre-Implantation Bovine Embryos. Int. J. Mol. Sci. 2019, 20, 6066. [Google Scholar] [CrossRef] [PubMed]
- Mesalam, A.; Lee, K.-L.; Khan, I.; Chowdhury, M.M.R.; Zhang, S.; Song, S.-H.; Joo, M.-D.; Lee, J.-H.; Jin, J.-I.; Kong, I.-K.A. Combination of bovine serum albumin with insulin–transferrin–sodium selenite and/or epidermal growth factor as alternatives to fetal bovine serum in culture medium improves bovine embryo quality and trophoblast invasion by induction of matrix metalloproteinases. Reprod. Fertil. Dev. 2019, 31, 333–346. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, S.; Wozniak, P.J.; Yang, X.; Godke, R.A. Cumulus cell function during bovine oocyte maturation, fertilization, and embryo development in vitro. Mol. Reprod. Dev. 1995, 40, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Lolicato, F.; Brouwers, J.F.; van de Lest, C.H.; Wubbolts, R.; Aardema, H.; Priore, P.; Roelen, B.A.; Helms, J.B.; Gadella, B.M. The Cumulus Cell Layer Protects the Bovine Maturing Oocyte Against Fatty Acid-Induced Lipotoxicity1. Biol. Reprod. 2015, 92, 16. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lazo, L.; Brisard, D.; Elis, S.; Maillard, V.; Uzbekov, R.; Labas, V.; Desmarchais, A.; Papillier, P.; Monget, P.; Uzbekova, S. Fatty Acid Synthesis and Oxidation in Cumulus Cells Support Oocyte Maturation in Bovine. Mol. Endocrinol. 2014, 28, 1502–1521. [Google Scholar] [CrossRef]
- Yang, E.; Wang, X.; Gong, Z.; Yu, M.; Wu, H.; Zhang, D. Exosome-mediated metabolic reprogramming: The emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct. Target. Ther. 2020, 5, 242. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhu, N.; Yan, T.; Shi, Y.-N.; Chen, J.; Zhang, C.-J.; Xie, X.-J.; Liao, D.-F.; Qin, L. The crosstalk: Exosomes and lipid metabolism. Cell Commun. Signal. 2020, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Jiang, X.; Hameed, U.; Shi, Q. Role of Lipid Metabolism and Signaling in Mammalian Oocyte Maturation, Quality, and Acquisition of Competence. Front. Cell Dev. Biol. 2021, 9, 639704. [Google Scholar] [CrossRef]
- de Andrade Melo-Sterza, F.; Poehland, R. Lipid Metabolism in Bovine Oocytes and Early Embryos under In Vivo, In Vitro, and Stress Conditions. Int. J. Mol. Sci. 2021, 22, 3421. [Google Scholar] [CrossRef]
- Suwik, K.; Sinderewicz, E.; Boruszewska, D.; Kowalczyk-Zięba, I.; Staszkiewicz-Chodor, J.; Łukaszuk, K.; Wocławek-Potocka, I. mRNA Expression and Role of PPARgamma and PPARdelta in Bovine Preimplantation Embryos Depending on the Quality and Developmental Stage. Animals 2020, 10, 2358. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef] [PubMed]
- Pangas, S.; Jorgez, C.J.; Matzuk, M.M. Growth Differentiation Factor 9 Regulates Expression of the Bone Morphogenetic Protein Antagonist Gremlin. J. Biol. Chem. 2004, 279, 32281–32286. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, C.; Salustri, A.; Hascall, V.C. Coding Sequence of a Hyaluronan Synthase Homologue Expressed during Expansion of the Mouse Cumulus–Oocyte Complex. Arch. Biochem. Biophys. 1997, 337, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.M.; Vandevoort, C.A. Maturation and fertilization of nonhuman primate oocytes are compromised by oral administration of a cyclooxygenase-2 inhibitor. Fertil. Steril. 2011, 95, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Novais, A.A.; Chuffa, L.G.D.A.; Zuccari, D.A.P.D.C.; Reiter, R.J. Exosomes and Melatonin: Where Their Destinies Intersect. Front. Immunol. 2021, 12, 692022. [Google Scholar] [CrossRef] [PubMed]
- Krämer-Albers, E.-M. Exosomes deliver ROS for regeneration. Nat. Cell Biol. 2018, 20, 225–226. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Maheshwari, S.; Singh, A.K.; Arya, R.K.; Pandey, D.; Singh, A.; Datta, D. Exosomes: Emerging Players of Intercellular Communication in Tumor Microenvironment. Discoveries 2014, 2, e26. [Google Scholar] [CrossRef]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent advancements in the use of exosomes as drug delivery systems. J. Nanobiotechnology 2018, 16, 81. [Google Scholar] [CrossRef]
- Uhde, K.; Van Tol, H.T.A.; Stout, T.A.E.; Roelen, B.A.J. Metabolomic profiles of bovine cumulus cells and cumulus-oocyte-complex-conditioned medium during maturation in vitro. Sci. Rep. 2018, 8, 9477. [Google Scholar] [CrossRef]
- Richard, S.; Baltz, J.M. Prophase I Arrest of Mouse Oocytes Mediated by Natriuretic Peptide Precursor C Requires GJA1 (connexin-43) and GJA4 (connexin-37) Gap Junctions in the Antral Follicle and Cumulus-Oocyte Complex1. Biol. Reprod. 2014, 90, 137. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.E.; Armstrong, D.T.; Gilchrist, R.B. Bovine cumulus cell-oocyte gap junctional communication during in vitro maturation in response to manipulation of cell-specific cyclic adenosine 3′,5′-monophosophate levels. Biol. Reprod. 2004, 70, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Leese, H.J. Metabolism of the preimplantation embryo: 40 years on. Reproduction 2012, 143, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Leese, H.J. Quiet please, do not disturb: A hypothesis of embryo metabolism and viability. BioEssays 2002, 24, 845–849. [Google Scholar] [CrossRef]
- Chang, H.-C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef]
- Babayev, E.; Seli, E. Oocyte mitochondrial function and reproduction. Curr. Opin. Obstet. Gynecol. 2015, 27, 175–181. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, J.; Miao, Y.; Zhang, Q. The effect of extracellular vesicles on the regulation of mitochondria under hypoxia. Cell Death Dis. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Sansone, P.; Savini, C.; Kurelac, I.; Chang, Q.; Amato, L.B.; Strillacci, A.; Stepanova, A.; Iommarini, L.; Mastroleo, C.; Daly, L.; et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E9066–E9075. [Google Scholar] [CrossRef]
- Luquet, S.; Gaudel, C.; Holst, D.; Lopez-Soriano, J.; Jehl-Pietri, C.; Fredenrich, A.; Grimaldi, P.A. Roles of PPAR delta in lipid absorption and metabolism: A new target for the treatment of type 2 diabetes. Biochim. Biophys. 2005, 1740, 313–317. [Google Scholar] [CrossRef]
- Borini, A.; Lagalla, C.; Cattoli, M.; Sereni, E.; Sciajno, R.; Flamigni, C.; Coticchio, G. Predictive factors for embryo implantation potential. Reprod. Biomed. 2005, 10, 653–668. [Google Scholar] [CrossRef]
- Xu, L.; Idrees, M.; Joo, M.-D.; Sidrat, T.; Wei, Y.; Song, S.-H.; Lee, K.-L.; Kong, I.-K. Constitutive Expression of TERT Enhances β-Klotho Expression and Improves Age-Related Deterioration in Early Bovine Embryos. Int. J. Mol. Sci. 2021, 22, 5327. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Primer Sequences | Accession Number |
|---|---|---|
| GAPDH | F: TTCAACGGCACAGTCAAGG R: ACATACTCAGCACCAGCATCAC | NM_001034034 |
| GREM1 | F: CAGTGCAACTCCTTCTACATCC R: GAGTTCAGGACAGTTGAGAGTGAC | XM_024997728.1 |
| HAS2 | F: TCTCTAGAAACCCCCATTAAGTTG R: ATCTTCCGAGTTTCCATCTATGAC | NM_174079.3 |
| PTX3 | F: CACAGGTCATGTTGTTCCTGAG R: CAGATATTGAAGCCTGTGAGTCTG | NM_001076259.2 |
| GDF9 | F: TGTTTAACCTGGATCGTGTTACTG R: AAACTCTGGCTCTTTTATCACCAG | NM_174681.2 |
| CX43 | F: CTTTCGTTGTAACACTCAACAACC R: GTAGAACACATGAGCCAGGTACAG | J05535.1 |
| CX37 | F: AGCCCGTGTTTGTGTGCCAG R: ACCAGGGAGATGAGTCCGACCA | NM_001083738.1 |
| GLUT1 | F: ATCCTCATTGCCGTGGTGCT R: ACGATGCCAGAGCCGATGGT | M60448 |
| GLUT3 | F: CGCCTTTGGCACTCTCAAC R: GCACTGGATGATGGCTGGTAA | AF308829 |
| PGK1 | F: CCTGTGGGTGTATTTGAATGG R: AGCACTTTACCTTCCAGGAG | BT021601.1 |
| CPT1 | F: GAGGGAGACTTTACACGGATGA R: AGATGTATTCCTCCCACCAGTC | NM_001304989 |
| FABP3 | F: GACAGCAAGAATTTCGATGACTAC R: CTGATCTCTGTGTTCTTGAAGGTG | NM_174313.2 |
| PPARγ | F: ATATTTCCCTCTTTGTGGCTGC R: ATGGTTGTTCTGTAGGTGGAGT | XM_0249913 |
| CPEB1 | F: GTGTGGAGTGGCCTGGTAAG R: GAGAGCAAGCCTGAAGCAAG | XM_864691 |
| SLC2A1 | F: CCCCAGAAGGTGATTGAAGA R: GCCGAAACGGTTAACAAAAA | NM_174602.2 |
| CD36 | F: ACTGAGGATGACACGTTCA R: AATGGATCCGTATAGCCC | NM_174010 |
| ATGL | F: CTGCTGACCACACTCTCCAA R: GGCGCGTATCATCAGGTACT | FJ798978.1 |
| Groups | Oocytes, n | Presumed Zygotes, n | ≥8-cell Embryos, n % | Total Blastocysts, n % | Hatched Blastocysts, n % |
|---|---|---|---|---|---|
| Control | 314 | 294 | 164 (55.8 ± 3.40) b | 83 (28.2± 1.08) b | 18 (20.5 ± 4.24) a |
| 3% Exo | 312 | 288 | 191 (66.3 ± 2.90) a | 104 (36.1 ± 0.67) a | 32 (27.9 ± 3.83) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Idrees, M.; Sidrat, T.; Joo, M.; Xu, L.; Ko, J.; Kong, I. BOEC–Exo Addition Promotes In Vitro Maturation of Bovine Oocyte and Enhances the Developmental Competence of Early Embryos. Animals 2022, 12, 424. https://doi.org/10.3390/ani12040424
Wei Y, Idrees M, Sidrat T, Joo M, Xu L, Ko J, Kong I. BOEC–Exo Addition Promotes In Vitro Maturation of Bovine Oocyte and Enhances the Developmental Competence of Early Embryos. Animals. 2022; 12(4):424. https://doi.org/10.3390/ani12040424
Chicago/Turabian StyleWei, Yiran, Muhammad Idrees, Tabinda Sidrat, Myeondon Joo, Lianguang Xu, Jonghyeok Ko, and Ilkeun Kong. 2022. "BOEC–Exo Addition Promotes In Vitro Maturation of Bovine Oocyte and Enhances the Developmental Competence of Early Embryos" Animals 12, no. 4: 424. https://doi.org/10.3390/ani12040424
APA StyleWei, Y., Idrees, M., Sidrat, T., Joo, M., Xu, L., Ko, J., & Kong, I. (2022). BOEC–Exo Addition Promotes In Vitro Maturation of Bovine Oocyte and Enhances the Developmental Competence of Early Embryos. Animals, 12(4), 424. https://doi.org/10.3390/ani12040424








