3D Biomimetic Porous Titanium (Ti6Al4V ELI) Scaffolds for Large Bone Critical Defect Reconstruction: An Experimental Study in Sheep
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement and Animal Care
2.2. Description of the Animal Model
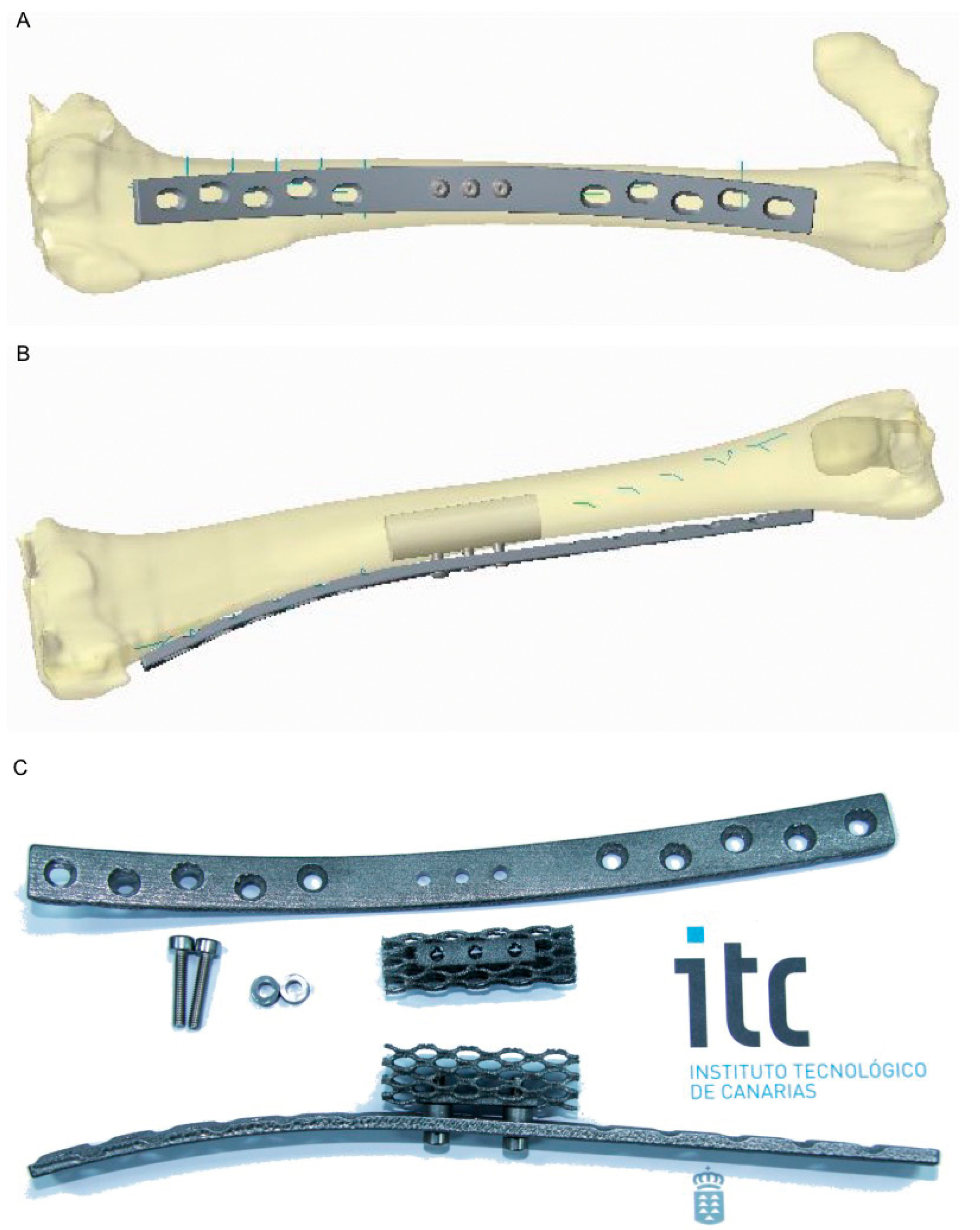
2.3. Titanium Scaffold and Plates
2.4. Porous Ceramic Scaffold
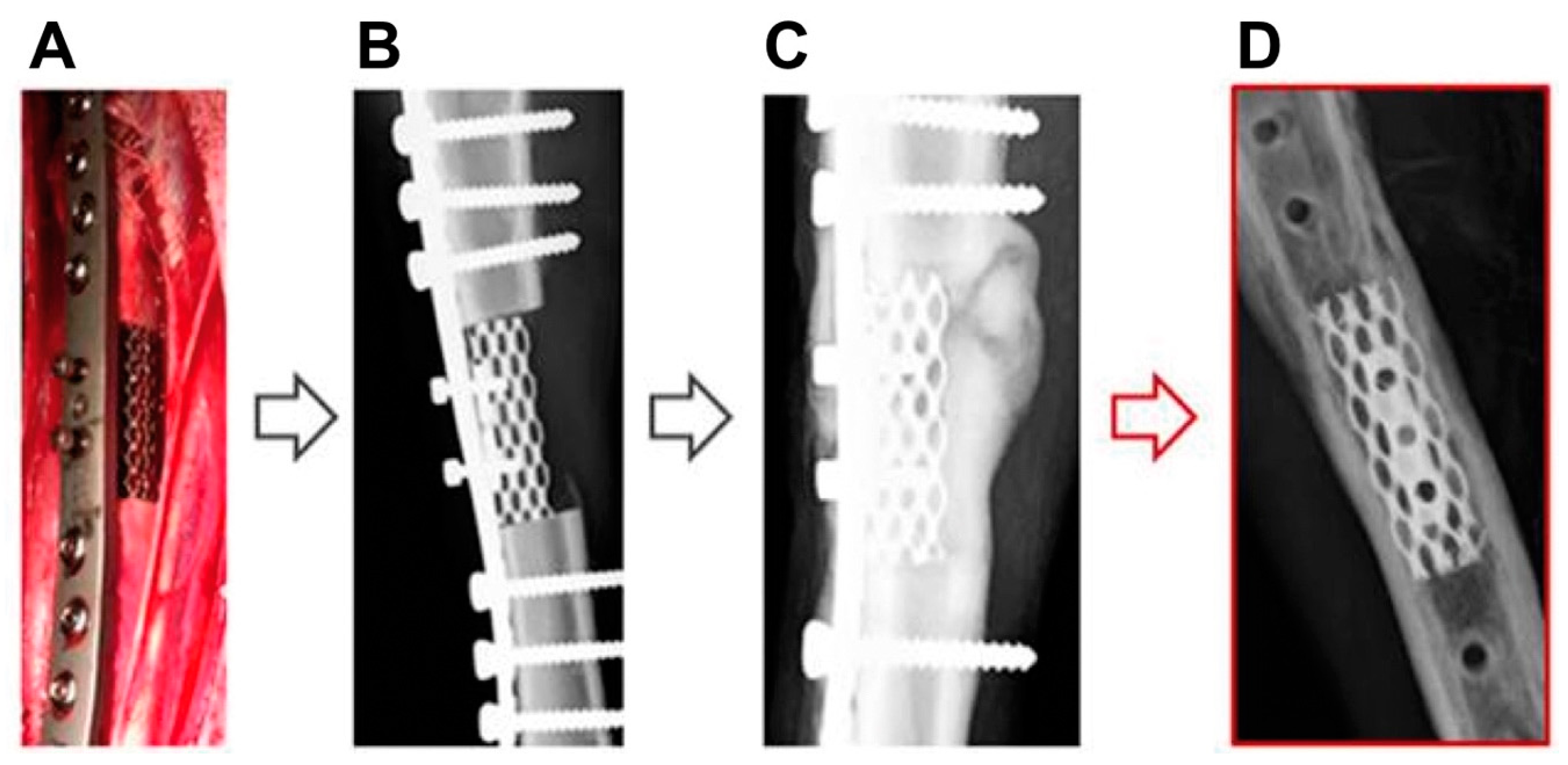
2.5. Surgical Procedure
2.6. Noninvasive Follow-Up
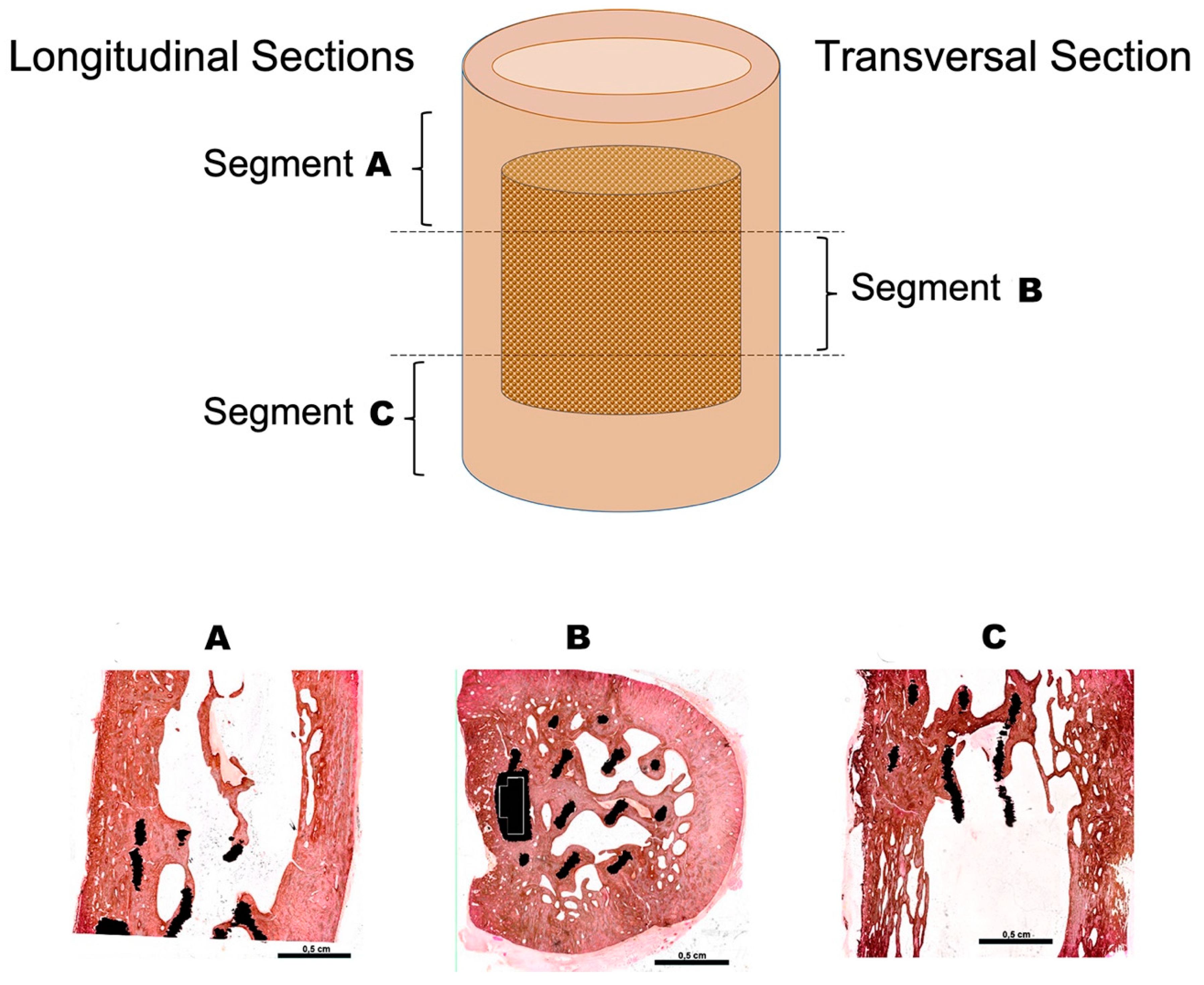
2.7. Histological Analysis
3. Results
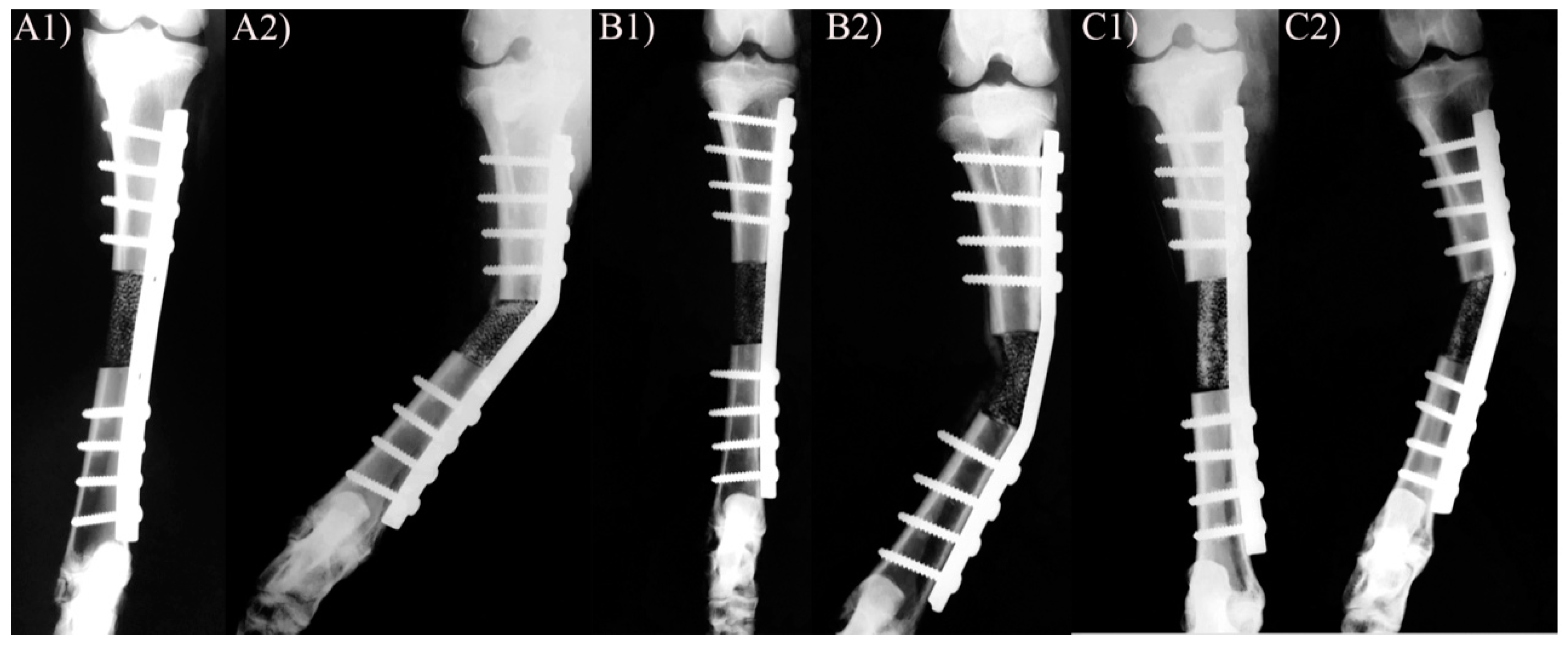
3.1. X-ray and Clinical Follow-Up
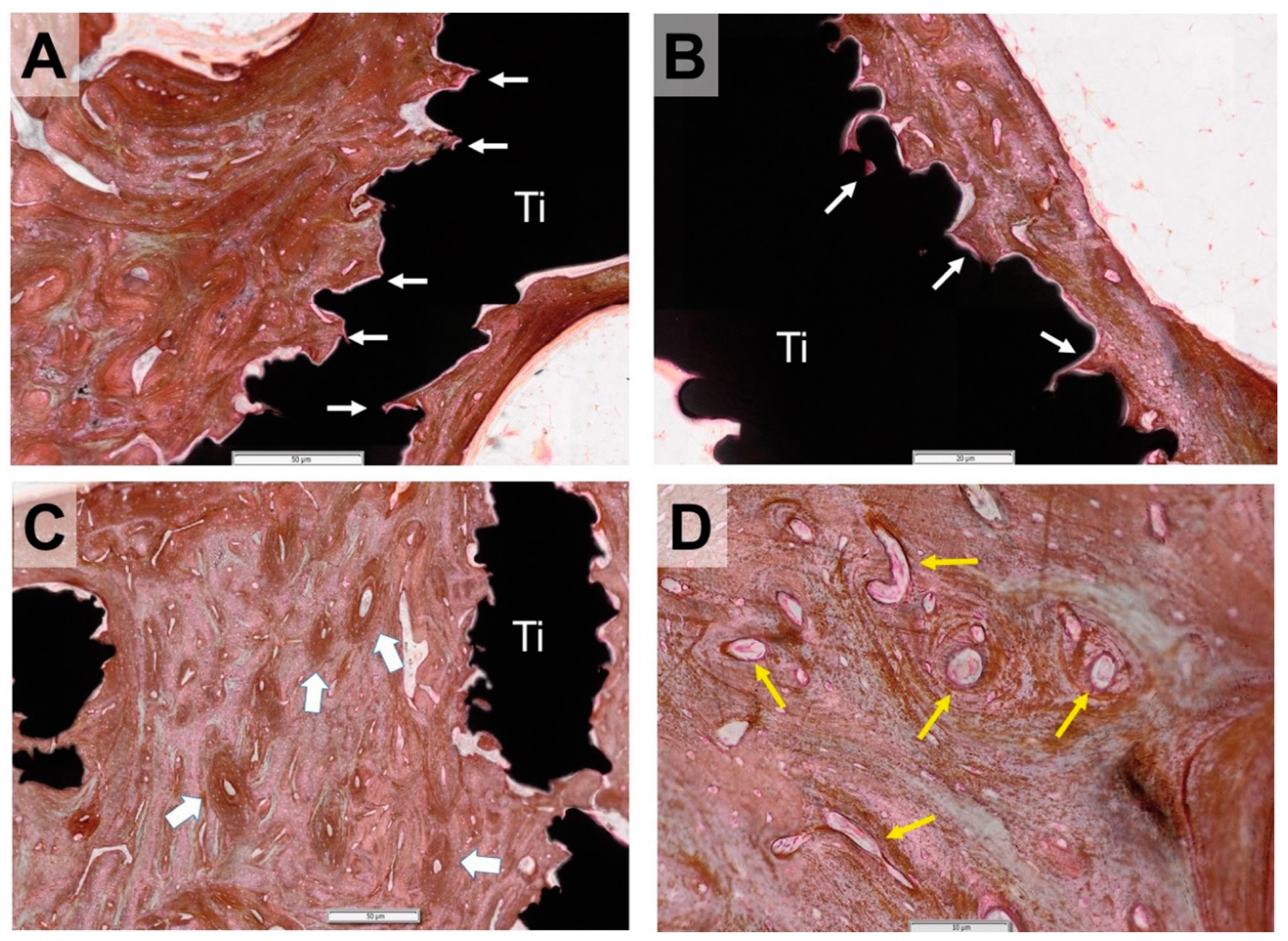
3.2. Histological Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Polyzois, V.D.; Stathopoulos, I.P.; Lampropoulou-Adamidou, K.; Vasiliadis, E.S.; Vlamis, J.; Pneumaticos, S.G. Strategies for managing bone defects of the lower extremity. Clin. Podiatr. Med. Surg. 2014, 31, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Costa Mendes, L.; Sauvigne, T.; Guiol, J. Morbidity of autologous bone harvesting in implantology: Literature review from 1990 to 2015. Rev. Stomatol. Chir. Maxillofac. Chir. Orale 2016, 117, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Arinzeh, T.L.; Tran, T.; McAlary, J.; Daculsi, G. A comparative study of biphasic calcium phosphate ceramics for human mesenchymal stem-cell-induced bone formation. Biomaterials 2005, 26, 3631–3638. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.S.; Bostrom, M.P. Synthetic bone scaffolds and fracture repair. Injury 2007, 38 (Suppl. S1), S33–S37. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, E.; Fredholm, Y.C.; Jell, G.; Lotfibakhshaiesh, N.; O’Donnell, M.D.; Hill, R.G.; Stevens, M.M. The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts In Vitro. Biomaterials 2010, 31, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.O. The conflicts between strength and toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef]
- Li, H.; Chang, J. Preparation and characterization of bioactive and biodegradable wollastonite/poly(D,L-lactic acid) composite scaffolds. J. Mater. Sci. Mater. Med. 2004, 15, 1089–1095. [Google Scholar] [CrossRef]
- Blaker, J.J.; Nazhat, S.N.; Maquet, V.; Boccaccini, A.R. Long-term In Vitro degradation of PDLLA/bioglass bone scaffolds in acellular simulated body fluid. Acta Biomater. 2011, 7, 829–840. [Google Scholar] [CrossRef]
- Cai, S.; Xu, G.H.; Yu, X.Z.; Zhang, W.J.; Xiao, Z.Y.; Yao, K.D. Fabrication and biological characteristics of beta-tricalcium phosphate porous ceramic scaffolds reinforced with calcium phosphate glass. J. Mater. Sci. Mater. Med. 2009, 20, 351–358. [Google Scholar] [CrossRef]
- Entezari, A.; Roohani-Esfahani, S.I.; Zhang, Z.; Zreiqat, H.; Dunstan, C.R.; Li, Q. Fracture behaviors of ceramic tissue scaffolds for load bearing applications. Sci. Rep. 2016, 6, 28816. [Google Scholar] [CrossRef]
- Hing, K.A. Bioceramic Bone Graft Substitutes: Influence of Porosity and Chemistry. Int. J. Appl. Ceram. Technol. 2005, 2, 184–199. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiacomo, M.; Corsi, A.; Francioso, E.; Di Comite, M.; Monetti, F.; Scaglione, S.; Favia, A.; Crovace, A.; Bianco, P.; Cancedda, R. Reconstruction of extensive long bone defects in sheep using resorbable bioceramics based on silicon stabilized tricalcium phosphate. Tissue Eng. 2006, 12, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, D.; Loy, B.N.; Lee, B.; Omid, R.; Itamura, J. Mixing implants of differing metallic composition in the treatment of upper-extremity fractures. Orthopedics 2013, 36, e1175–e1179. [Google Scholar] [CrossRef]
- Ponader, S.; Rosenlehner, K.; Vairaktaris, E.; von Wilmowsky, C.; Schlegel, K.A.; Neukam, F.W.; Schmidt, C.D.; Schunk, T.; Hirsch, A.; Nkenke, E. In Vitro behavior of layer-by-layer deposited molecular oligoelectrolyte films on Ti-6Al-4V surfaces. J. Mater. Sci. Mater. Med. 2009, 20, 2455–2463. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.F.; Wang, C.T.; Li, G.C.; Lei, W.; Zhang, Z.Y.; Wang, L. Evaluation of biological properties of electron beam melted Ti6Al4V implant with biomimetic coating In Vitro and In Vivo. PLoS ONE 2012, 7, e52049. [Google Scholar] [CrossRef]
- Niinomi, M. Recent Research and Development in Metallic Materials for Biomedical, Dental and Healthcare Products Applications. Mater. Sci. Forum 2007, 539, 193–200. [Google Scholar] [CrossRef]
- Nouri, A.; Hodgson, P.D.; Wen, C. Biomimetic Porous Titanium Scaffolds for Orthopedic and Dental Applications. In Biomimetics Learning from Nature; Amitava, M., Ed.; Intech: Rijeka, Croatia, 2010; p. 251. [Google Scholar]
- Gugala, Z.; Lindsey, R.W.; Gogolewski, S. New Approaches in the Treatment of Critical-Size Segmental Defects in Long Bones. Macromol. Symp. 2007, 253, 147–161. [Google Scholar] [CrossRef]
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal models for bone tissue engineering and modelling disease. Dis. Model. Mech. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.C.; Saifzadeh, S.; Wullschleger, M.E.; Epari, D.R.; Schutz, M.A.; Duda, G.N.; Schell, H.; van Griensven, M.; Redl, H.; Hutmacher, D.W. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials 2009, 30, 2149–2163. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.; Yanez, A.; Martel, O.; Afonso, H.; Monopoli, D. Computational study and experimental validation of porous structures fabricated by electron beam melting: A challenge to avoid stress shielding. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Bemenderfer, T.B.; Harris, J.S.; Condon, K.W.; Kacena, M.A. Tips and techniques for processing and sectioning undecalcified murine bone specimens. Methods Mol. Biol. 2014, 1130, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Marcacci, M.; Kon, E.; Zaffagnini, S.; Giardino, R.; Rocca, M.; Corsi, A.; Benvenuti, A.; Bianco, P.; Quarto, R.; Martin, I.; et al. Reconstruction of extensive long-bone defects in sheep using porous hydroxyapatite sponges. Calcif. Tissue Int. 1999, 64, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.A.; Witte, T.; Arens, D.; Pearce, A.; Pearce, S. Double-plating of ovine critical sized defects of the tibia: A low morbidity model enabling continuous In Vivo monitoring of bone healing. BMC Musculoskelet. Disord. 2011, 12, 214. [Google Scholar] [CrossRef]
- Rahal, S.; Teixeira, C.; Volpi, R.; Taga, R.; Cestari, T.; Granjeiro, J.; Vulcano, L.; Corrêa, M. Tibial segmental bone defect treated with bone plate and cage filled with either xenogeneic composite or autologous cortical bone graft. Vet. Comp. Orthop. Traumatol. 2007, 20, 269–276. [Google Scholar] [CrossRef]
- Viateau, V.; Guillemin, G.; Yang, Y.C.; Bensaid, W.; Reviron, T.; Oudina, K.; Meunier, A.; Sedel, L.; Petite, H. A technique for creating critical-size defects in the metatarsus of sheep for use in investigation of healing of long-bone defects. Am. J. Vet. Res. 2004, 65, 1653–1657. [Google Scholar] [CrossRef]
- Daugaard, H.; Elmengaard, B.; Bechtold, J.E.; Jensen, T.; Søballe, K. The effect on bone growth enhancement of implant coatings with hydroxyapatite and collagen deposited electrochemically and by plasma spray. J. Biomed. Mater. Res. Part A 2009, 92, 913–921. [Google Scholar] [CrossRef]
- Goodship, A.E.; Cunningham, J.L.; Kenwright, J. Strain Rate and Timing of Stimulation in Mechanical Modulation of Fracture Healing. Clin. Orthop. Relat. Res. 1998, 355, S105–S115. [Google Scholar] [CrossRef]
- Trisciuzzi, R.; Fracassi, L.; Martin, H.A.; Forleo, D.M.; Amat, D.; Santos-Ruiz, L.; De Palma, E.; Crovace, A. 41 Cases of Treatment of Cranial Cruciate Ligament Rupture with Porous TTA: Three Years of Follow Up. Vet. Sci. 2019, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Aspenberg, P.; Goodman, S.; Toksvig-Larsen, S.; Ryd, L.; Albrektsson, T. Intermittent micromotion inhibits bone ingrowth. Acta Orthop. Scand. 1992, 63, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Wilke, H.-J.; Augat, P.; Rübenacker, S.; Margevicius, K. Effect of dynamization on gap healing of diaphyseal fractures under external fixation. Clin. Biomech. 1995, 10, 227–234. [Google Scholar] [CrossRef]
- Mori, T.; Okimoto, N.; Sakai, A.; Okazaki, Y.; Nakura, N.; Notomi, T.; Nakamura, T. Climbing Exercise Increases Bone Mass and Trabecular Bone Turnover Through Transient Regulation of Marrow Osteogenic and Osteoclastogenic Potentials in Mice. J. Bone Miner. Res. 2003, 18, 2002–2009. [Google Scholar] [CrossRef]
- Larson, B.L.; Ylöstalo, J.; Prockop, D.J. Human Multipotent Stromal Cells Undergo Sharp Transition from Division to Development in Culture. Stem Cells 2008, 26, 193–201. [Google Scholar] [CrossRef]
- Notomi, T.; Okimoto, N.; Okazaki, Y.; Tanaka, Y.; Nakamura, T.; Suzuki, M. Effects of Tower Climbing Exercise on Bone Mass, Strength, and Turnover in Growing Rats. J. Bone Miner. Res. 2001, 16, 166–174. [Google Scholar] [CrossRef]
- Rubin, C.T.; Lanyon, L.E. Regulation of bone mass by mechanical strain magnitude. Calcif. Tissue Int. 1985, 37, 411–417. [Google Scholar] [CrossRef]
- Honda, A.; Sogo, N.; Nagasawa, S.; Shimizu, T.; Umemura, Y. High-impact exercise strengthens bone in osteopenic ovariectomized rats with the same outcome as Sham rats. J. Appl. Physiol. 2003, 95, 1032–1037. [Google Scholar] [CrossRef]
- Skerry, T.; Lanyon, L.E.; Bitensky, L.; Chayen, J. Early strain-related changes in enzyme activity in osteocytes following bone loading In Vivo. J. Bone Miner. Res. 2009, 4, 783–788. [Google Scholar] [CrossRef]
- Mikuni-Takagaki, Y. Mechanical responses and signal transduction pathways in stretched osteocytes. J. Bone Miner. Metab. 1999, 17, 57–60. [Google Scholar] [CrossRef]
- Weiss, S.; Baumgart, R.; Jochum, M.; Strasburger, C.J.; Bidlingmaier, M. Systemic Regulation of Distraction Osteogenesis: A Cascade of Biochemical Factors. J. Bone Miner. Res. 2002, 17, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Lewinson, D.; Rachmiel, A.; Rihani-Bisharat, S.; Kraiem, Z.; Schenzer, P.; Korem, S.; Rabinovich, Y. Stimulation of Fos- and Jun-related genes during distraction osteogenesis. J. Histochem. Cytochem. 2003, 51, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F.; Johnson, M.L. Osteocytes, mechanosensing and Wnt signaling. Bone 2008, 42, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.-H.W.; Baylink, D.J.; Zhou, X.-D.; Rodriguez, D.; Bonewald, L.F.; Li, Z.; Ruffoni, D.; Müller, R.-A.; Kesavan, C.; Sheng, M.H.-C. Osteocyte-derived insulin-like growth factor I is essential for determining bone mechanosensitivity. Am. J. Physiol. Metab. 2013, 305, E271–E281. [Google Scholar] [CrossRef]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.; Harris, S.E.; et al. Mechanical Stimulation of Bonein VivoReduces Osteocyte Expression of Sost/Sclerostin. J. Biol. Chem. 2007, 283, 5866–5875. [Google Scholar] [CrossRef]
- Tu, X.; Rhee, Y.; Condon, K.W.; Bivi, N.; Allen, M.R.; Dwyer, D.; Stolina, M.; Turner, C.H.; Robling, A.G.; Plotkin, L.I.; et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 2012, 50, 209–217. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Pan, W.; Yang, F.; Jiang, W.; Wu, X.; Kong, X.; Dai, K.; Hao, Y. In Vitro and In Vivo study of additive manufactured porous Ti6Al4V scaffolds for repairing bone defects. Sci. Rep. 2016, 6, 34072. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crovace, A.M.; Lacitignola, L.; Forleo, D.M.; Staffieri, F.; Francioso, E.; Di Meo, A.; Becerra, J.; Crovace, A.; Santos-Ruiz, L. 3D Biomimetic Porous Titanium (Ti6Al4V ELI) Scaffolds for Large Bone Critical Defect Reconstruction: An Experimental Study in Sheep. Animals 2020, 10, 1389. https://doi.org/10.3390/ani10081389
Crovace AM, Lacitignola L, Forleo DM, Staffieri F, Francioso E, Di Meo A, Becerra J, Crovace A, Santos-Ruiz L. 3D Biomimetic Porous Titanium (Ti6Al4V ELI) Scaffolds for Large Bone Critical Defect Reconstruction: An Experimental Study in Sheep. Animals. 2020; 10(8):1389. https://doi.org/10.3390/ani10081389
Chicago/Turabian StyleCrovace, Alberto Maria, Luca Lacitignola, Donato Monopoli Forleo, Francesco Staffieri, Edda Francioso, Antonio Di Meo, José Becerra, Antonio Crovace, and Leonor Santos-Ruiz. 2020. "3D Biomimetic Porous Titanium (Ti6Al4V ELI) Scaffolds for Large Bone Critical Defect Reconstruction: An Experimental Study in Sheep" Animals 10, no. 8: 1389. https://doi.org/10.3390/ani10081389
APA StyleCrovace, A. M., Lacitignola, L., Forleo, D. M., Staffieri, F., Francioso, E., Di Meo, A., Becerra, J., Crovace, A., & Santos-Ruiz, L. (2020). 3D Biomimetic Porous Titanium (Ti6Al4V ELI) Scaffolds for Large Bone Critical Defect Reconstruction: An Experimental Study in Sheep. Animals, 10(8), 1389. https://doi.org/10.3390/ani10081389






