Efficacy of a Semi Automated Commercial Closed System for Autologous Leukocyte- and Platelet-Rich Plasma (l-prp) Production in Dogs: A Preliminary Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Samples Collection and PRP Production
2.3. Platelet and Leukocyte Counts
2.4. Microbiological Evaluation
2.5. Platelet Derived Growth Factor Evaluation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marx, R.E. Platelet-Rich Plasma (PRP): What Is PRP and What Is Not PRP ? What is PRP ? What is PRP in Relation to Recombinant Growth Factors? Implant Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; Woodell, J.E.; Higgins, J. Platelet quantification and growth factor analysis from platelet-rich plasma: Implications for wound healing. Plast. Reconstr. Surg. 2004, 114, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S. Function and clinical significance of platelet-derived microparticles. Int. J. Hematol. 2001, 74, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Kaux, J.; Emonds-Alt, T. The Use of Platelet-Rich Plasma to Treat Chronic Tendinopathies: A Technical Analysis. Platelets 2018, 29, 213–227. [Google Scholar] [CrossRef]
- Bos-Mikich, A.; de Oliveira, R.; Frantz, N. Platelet-rich plasma therapy and reproductive medicine. J. Assist. Reprod. Genet. 2018, 35, 753–756. [Google Scholar] [CrossRef]
- Emer, J. Platelet-Rich Plasma (PRP): Current Applications in Dermatology. Skin Therapy Lett. 2019, 24, 1–6. [Google Scholar]
- Hersant, B.; La Padula, S.; SidAhmed-Mezi, M.; Rodriguez, A.M.; Meningaud, J.P. Use of platelet-rich plasma (PRP) in microsurgery. J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 236–237. [Google Scholar] [CrossRef]
- Wang, D.; Rodeo, S.A. Platelet-Rich Plasma in Orthopaedic Surgery: A Critical Analysis Review. JBJS Rev. 2017, 5, e7. [Google Scholar] [CrossRef]
- Feigin, K.; Shope, B. Use of Platelet-Rich Plasma and Platelet-Rich Fibrin in Dentistry and Oral Surgery: Introduction and Review of the Literature. J. Vet. Dent. 2019, 36, 109–123. [Google Scholar] [CrossRef]
- Tambella, A.; Martin, S.; Cantalamessa, A.; Serri, E.; Attili, A. Platelet-rich Plasma and Other Hemocomponents in Veterinary Regenerative Medicine. Wounds Compend. Clin. Res. Pract. 2018, 30, 329. [Google Scholar]
- Tambella, A.M.; Attili, A.R.; Dupré, G.; Cantalamessa, A.; Martin, S.; Cuteri, V.; Marcazzan, S.; Del Fabbro, M. Platelet-rich plasma to treat experimentally- induced skin wounds in animals: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0191093. [Google Scholar] [CrossRef] [PubMed]
- Arguelles, D.; Carmona, J.U.; Climent, F.; Munoz, E.; Prades, M. Autologous platelet concentrates as a treatment for musculoskeletal lesions in five horses. Vet. Rec. 2008, 162, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.F.; Carmona, J.U.; Rezende, C.M.F. Intra-articular injections of autologous platelet concentrates in dogs with surgical reparation of cranial cruciate ligament rupture: A pilot study. Vet. Comp. Orthop. Traumatol. 2013, 26, 285–290. [Google Scholar] [PubMed]
- Bigliardi, E.; Cantoni, A.M.; De Cesaris, V.; Denti, L.; Conti, V.; Bertocchi, M.; Di Ianni, F.; Parmigiani, E.; Grolli, S. Use of platelet-rich plasma for the treatment of prostatic cysts in dogs. Can. J. Vet. Res. 2018, 82, 264–270. [Google Scholar] [PubMed]
- Jee, C.-H.; Eom, N.-Y.; Jang, H.-M.; Jung, H.-W.; Choi, E.-S.; Won, J.-H.; Hong, I.-H.; Kang, B.-T.; Jeong, D.W.; Jung, D.-I. Effect of autologous platelet-rich plasma application on cutaneous wound healing in dogs. J. Vet. Sci. 2016, 17, 79–87. [Google Scholar] [CrossRef]
- Franklin, S.P.; Burke, E.E.; Holmes, S.P. The effect of platelet-rich plasma on osseous healing in dogs undergoing high tibial osteotomy. PLoS ONE 2017, 12, e0177597. [Google Scholar] [CrossRef]
- Brossi, P.M.; Moreira, J.J.; Machado, T.S.L.; Baccarin, R.Y.A. Platelet-rich plasma in orthopedic therapy: A comparative systematic review of clinical and experimental data in equine and human musculoskeletal lesions. BMC Vet. Res. 2015, 11, 98. [Google Scholar] [CrossRef]
- Reghini, M.F.S.; Ramires Neto, C.; Segabinazzi, L.G.; Castro Chaves, M.M.B.; Camila de Paula, F.; Bussiere, M.C.C.; Dell’Aqua, J.A.; Papa, F.O.; Alvarenga, M.A. Inflammatory response in chronic degenerative endometritis mares treated with platelet-rich plasma. Theriogenology 2016, 86, 516–522. [Google Scholar] [CrossRef]
- Garbin, L.C.; Olver, C.S. Platelet-Rich Products and Their Application to Osteoarthritis. J. Equine Vet. Sci. 2020, 86, 102820. [Google Scholar] [CrossRef]
- Pereira, R.; De La Côrte, F.; Brass, K.; da Silva Azevedo, M.; Gallio, M.; Cantarelli, C.; Dau, S.; Cezar, A.; Nkelmann, M. Evaluation of Three Methods of Platelet-Rich Plasma for Treatment of Equine Distal Limb Skin Wounds. J. Equine Vet. Sci. 2019, 72, 1–7. [Google Scholar] [CrossRef]
- Ho, L.K.; Baltzer, W.I.; Nemanic, S.; Stieger-Vanegas, S.M. Single ultrasound-guided platelet-rich plasma injection for treatment of supraspinatus tendinopathy in dogs. Can. Vet. J. Rev. Vet. Can. 2015, 56, 845–849. [Google Scholar]
- Marx, R.E. Platelet-Rich Plasma: Evidence to Support Its Use. J. Oral Maxillofac. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, L.; Balbo, V.; Guaschino, R. “Reasonable compromise” to define the quality standards of platelet concentrate for non-transfusion use (CPunT). Transfus. Apher. Sci. 2012, 47, 207–211. [Google Scholar] [CrossRef]
- Anitua, E.; Andia, I.; Ardanza, B.; Nurden, P.; Nurden, A.T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemost. 2004, 91, 4–15. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte-and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Andia, I.; Zumstein, M.A.; Zhang, C.Q.; Pinto, N.R.; Bielecki, T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, platelet-rich fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: Current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014, 4, 3–9. [Google Scholar] [CrossRef]
- McCarrel, T.; Minas, M.; Fortier, L. Optimization of Leukocyte Concentration in Platelet-Rich Plasma for the Treatment of Tendinopathy. J. Bone Jt. Surg. Am. 2012, 94, e143. [Google Scholar] [CrossRef]
- Castillo, T.N.; Pouliot, M.A.; Kim, H.J.; Dragoo, J.L. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am. J. Sports Med. 2011, 39, 266–271. [Google Scholar] [CrossRef]
- Sundman, E.; Cole, B.; Fortier, L. Growth Factor and Catabolic Cytokine Concentrations Are Influenced by the Cellular Composition of Platelet-Rich Plasma. Am. J. Sports Med. 2011, 39, 2135–2140. [Google Scholar] [CrossRef]
- McCarrel, T.; Mall, N.; Lee, A.; Cole, B.; Butty, D.; Fortier, F. Considerations for the Use of Platelet-Rich Plasma in Orthopedics. Sports Med. 2014, 44, 1025–1036. [Google Scholar] [CrossRef]
- Boswell, S.G.; Schnabel, L.V.; Mohammed, H.O.; Sundman, E.A.; Minas, T.; Fortier, L.A. Increasing Platelet Concentrations in Leukocyte-Reduced Platelet-Rich Plasma Decrease Collagen Gene Synthesis in Tendons. Am. J. Sports Med. 2014, 42, 42–49. [Google Scholar] [CrossRef]
- Zimmermann, R.; Arnold, D.; Strasser, E.; Ringwald, J.; Schlegel, A.; Wiltfang, J.; Eckstein, R. Sample preparation technique and white cell content influence the detectable levels of growth factors in platelet concentrates. Vox Sang. 2003, 85, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Carmona, J.U.; Argüelles, D.; Climent, F.; Prades, M. Autologous Platelet Concentrates as a Treatment of Horses with Osteoarthritis: A Preliminary Pilot Clinical Study. J. Equine Vet. Sci. 2007, 27, 167–170. [Google Scholar] [CrossRef]
- Textor, J.A.; Tablin, F. Activation of equine platelet-rich plasma: Comparison of methods and characterization of equine autologous thrombin. Vet. Surg. 2012, 41, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Textor, J.A.; Norris, J.W.; Tablin, F. Effects of preparation method, shear force, and exposure to collagen on release of growth factors from equine platelet-rich plasma. Am. J. Vet. Res. 2011, 72, 271–278. [Google Scholar] [CrossRef]
- Franklin, S.P.; Birdwhistell, K.E.; Strelchik, A.; Garner, B.C.; Brainard, B.M. Influence of cellular composition and exogenous activation on growth factor and cytokine concentrations in canine platelet-rich plasmas. Front. Vet. Sci. 2017, 4, 40. [Google Scholar] [CrossRef]
- Weibrich, G.; Kleis, W.K.G.; Hafner, G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: Curasan-type PRP kit versus PCCS PRP system. Int. J. Oral Maxillofac. Implants 2002, 17, 184–190. [Google Scholar]
- Weibrich, G.; Kleis, W.K.G.; Streckbein, P.; Moergel, M.; Hitzler, W.E.; Hafner, G. Comparison of point-of-care methods for preparation of platelet concentrate (platelet-rich plasma). Int. J. Oral Maxillofac. Implants 2012, 27, 762–769. [Google Scholar]
- Weibrich, G.; Kleis, W.K.G.; Hitzler, W.E.; Hafner, G. Comparison of the platelet concentrate collection system with the plasma-rich-in-growth-factors kit to produce platelet-rich plasma: A technical report. Int. J. Oral Maxillofac. Implants 2005, 20, 118–123. [Google Scholar]
- Weibrich, G.; Kleis, W. Curasan PRP Kit vs. PCCS PRP System. Collection Efficiency and Platelet Counts of Two Different Methods for the Preparation of Platelet-Rich Plasma. Clin. Oral Implants Res. 2002, 13, 437–443. [Google Scholar] [CrossRef]
- Giraldo, C.E.; López, C.; Álvarez, M.E.; Samudio, I.J.; Prades, M.; Carmona, J.U. Effects of the breed, sex and age on cellular content and growth factor release from equine pure-platelet rich plasma and pure-platelet rich gel. BMC Vet. Res. 2013, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Weibrich, G.; Kleis, W.K.G.; Hafner, G.; Hitzler, W.E. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J. Craniomaxillofac. Surg. 2002, 30, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.F.; Carmona, J.U.; Rezende, C.M.F. Comparison of the effect of calcium gluconate and batroxobin on the release of transforming growth factor beta 1 in canine platelet concentrates. BMC Vet. Res. 2012, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, M.L.; Mohammed, H.O.; Wakshlag, J.J.; Ledbetter, E.C. Clinical trial of adjunctive autologous platelet-rich plasma treatment following diamond-burr debridement for spontaneous chronic corneal epithelial defects in dogs. J. Am. Vet. Med. Assoc. 2018, 253, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Di Martino, A.; Marcacci, M. Platelet-rich Plasma (PRP) to Treat Sports Injuries: Evidence to Support Its Use. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Rabillard, M.; Grand, J.-G.; Dalibert, E.; Fellah, B.; Gauthier, O.; Niebauer, G.W. Effects of autologous platelet rich plasma gel and calcium phosphate biomaterials on bone healing in an ulnar ostectomy model in dogs. Vet. Comp. Orthop. Traumatol. 2009, 22, 460–466. [Google Scholar]
- Carvalho, M.D.; Suaid, F.F.; Santamaria, M.P.; Casati, M.Z.; Nociti, F.H.; Sallum, A.W.; Sallum, E.A. Platelet-rich plasma plus bioactive glass in the treatment of intra-bony defects: A study in dogs. J. Appl. Oral Sci. 2011, 19, 82–89. [Google Scholar] [CrossRef]
- Streckbein, P.; Kleis, W.; Buch, R.S.R.; Hansen, T.; Weibrich, G. Bone healing with or without platelet-rich plasma around four different dental implant surfaces in Beagle Dogs. Clin. Implant Dent. Relat. Res. 2014, 16, 479–486. [Google Scholar] [CrossRef]
- Mehrjerdi, H.K. Efficacy of Autologous Platelet-Rich Plasma (PRP) Activated By Thromboplastin-D on the Repair and Regeneration of Wounds in Dogs. Iran. J. Vet. Surg. 2008, 3, 19–30. [Google Scholar]
- Kushida, S.; Kakudo, N.; Morimoto, N.; Hara, T.; Ogawa, T.; Mitsui, T.; Kusumoto, K. Platelet and growth factor concentrations in activated platelet-rich plasma: A comparison of seven commercial separation systems. J. Artif. Organs 2014, 17, 186–192. [Google Scholar] [CrossRef]
- Gonshor, A. Technique for producing platelet-rich plasma and platelet concentrate: Background and process. Int. J. Periodontics Restor. Dent. 2002, 22, 547–557. [Google Scholar]
- Amable, P.R.; Carias, R.B.V.; Teixeira, M.V.T.; da Cruz Pacheco, I.; Corrêa do Amaral, R.J.F.; Granjeiro, J.M.; Borojevic, R. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 2013, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Jona, M.; Eto, H.; Aoi, N.; Kato, H.; Suga, H.; Doi, K.; Yatomi, Y.; Yoshimura, K. Optimized preparation method of platelet-concentrated plasma and noncoagulating platelet-derived factor concentrates: Maximization of platelet concentration and removal of fibrinogen. Tissue Eng. Part C Methods 2012, 18, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Argüelles, D.; Carmona, J.U.; Pastor, J.; Iborra, A.; Viñals, L.; Martínez, P.; Bach, E.; Prades, M. Evaluation of single and double centrifugation tube methods for concentrating equine platelets. Res. Vet. Sci. 2006, 81, 237–245. [Google Scholar] [CrossRef]
- Giraldo, C.E.; Álvarez, M.E.; Carmona, J.U. Effects of sodium citrate and acid citrate dextrose solutions on cell counts and growth factor release from equine pure-platelet rich plasma and pure-platelet rich gel. BMC Vet. Res. 2015, 11, 60. [Google Scholar] [CrossRef]
- Giraldo, C.; Álvarez, M.; Carmona, J.U. Influence of Calcium Salts and Bovine Thrombin on Growth Factor Release From Equine Platelet-Rich Gel Supernatants. Vet. Comp. Orthop. Traumatol. 2017, 30, 1–7. [Google Scholar] [CrossRef]
- Fontenot, R.L.; Sink, C.A.; Werre, S.R.; Weinstein, N.M.; Dahlgren, L.A. Simple tube centrifugation for processing platelet-rich plasma in the horse. Can. Vet. J. 2012, 53, 1266–1272. [Google Scholar]
- Perazzi, A.; Busetto, R.; Martinello, T.; Drigo, M.; Pasotto, D.; Cian, F.; Patruno, M.; Iacopetti, I. Description of a double centrifugation tube method for concentrating canine platelets. BMC Vet. Res. 2013, 9, 146. [Google Scholar] [CrossRef]
- Franklin, S.P.; Garner, B.C.; Cook, J.L. Characteristics of canine platelet-rich plasma prepared with five commercially available systems. Am. J. Vet. Res. 2015, 76, 822–827. [Google Scholar] [CrossRef]
- Shin, H.S.; Woo, H.M.; Kang, B.J. Optimisation of a double-centrifugation method for preparation of canine platelet-rich plasma. BMC Vet. Res. 2017, 13, 198. [Google Scholar] [CrossRef]
- Carr, B.J.; Canapp, S.O.; Mason, D.R.; Cox, C.; Hess, T. Canine Platelet-Rich Plasma Systems: A Prospective Analysis. Front. Vet. Sci. 2016, 2, 73. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.F.B.; Andrade, A.L.; Ferrreira, G.T.N.M.; Sakamoto, S.S.; Albuquerque, V.B.; Bonfim, S.R.M.; Luvizotto, M.C.R.; Louzada, M.J.Q. Healing and expression of growth factors (TGF-β and PDGF) in canine radial ostectomy gap containing platelet-rich plasma. Vet. Comp. Orthop. Traumatol. 2012, 25, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Frye, C.W.; Enders, A.; Brooks, M.B.; Struble, A.M.; Wakshlag, J.J. Assessment of canine autologous platelet-rich plasma produced with a commercial centrifugation and platelet recovery kit. Vet. Comp. Orthop. Traumatol. 2016, 29, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Perego, R.; Proverbio, D.; Baggiani, L.; Roggero, N.; Bagnagatti De Giorgi, G.S.E. In House Double Centrifugation Method for Preparation of Homologous Platelet-Rich Plasma (Prp) in Dogs Perego. ECronicon 2016, 126–132. [Google Scholar]
- Stief, M.; Gottschalk, J.; Ionita, J.C.; Einspanier, A.; Oechtering, G.; Böttcher, P. Concentration of platelets and growth factors in canine autologous conditioned plasma. Vet. Comp. Orthop. Traumatol. 2011, 24, 122–125. [Google Scholar] [CrossRef]
- Markey, B.; Leonard, F.; Archambault, M.; Cullinane, A.; Maguire, D. Clinical Veterinary Microbiology. In Clinical Veterinary Microbiology, 2nd ed.; Mosby Elsevier: St. Louis, MO, USA, 2013; pp. 3–47. [Google Scholar]
- Birdwhistell, K.; Basinger, R.; Hayes, B.; Norton, N.; Hurley, D.J.; Franklin, S.P. Validation of commercial ELISAs for quantifying anabolic growth factors and cytokines in canine ACD-A anticoagulated plasma. J. Vet. Diagn. Investig. 2017, 29, 143–147. [Google Scholar] [CrossRef]
- Silva, R.F.; Alvarez, M.E.; Rios, D.L.; Catalina, L.; Carmona, J.U.; Rezende, C.M.F. Evaluation of the effect of calcium gluconate and bovine thrombin on the temporal release of transforming growth factor beta 1 and platelet-derived growth factor isoform BB from feline platelet concentrates. BMC Vet. Res. 2012, 8, 212. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, C.; Park, H.M. Curative effect of autologous platelet-rich plasma on a large cutaneous lesion in a dog. Vet. Dermatol. 2009, 20, 123–126. [Google Scholar] [CrossRef]
- Karayannopoulou, M.; Psalla, D.; Kazakos, G.; Loukopoulos, P.; Giannakas, N.; Savvas, I.; Kritsepi-Konstantinou, M.; Chantes, A.; Papazoglou, L.G. Effect of locally injected autologous platelet-rich plasma on second intention wound healing of acute full-thickness skin defects in dogs. Vet. Comp. Orthop. Traumatol. 2015, 28, 172–178. [Google Scholar]
- Farghali, H.A.; AbdElKader, N.A.; Khattab, M.S.; AbuBakr, H.O. Evaluation of subcutaneous infiltration of autologous platelet-rich plasma on skin-wound healing in dogs. Biosci. Rep. 2017, 37, BSR20160503. [Google Scholar] [CrossRef]
- Farghali, H.A.; AbdElKader, N.A.; AbuBakr, H.O.; Aljuaydi, S.H.; Khattab, M.S.; Elhelw, R.; Elhariri, M. Antimicrobial action of autologous platelet-rich plasma on MRSA-infected skin wounds in dogs. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, B.; Rubio, M.; Chicharro, D.; Damiá, E.; Santana, A.; Carrillo, J.M.; Del Romero, A.; Vilar, J.M.; Cerón, J.J.; Sopena, J.J. Objective comparison between platelet rich plasma alone and in combination with physical therapy in dogs with osteoarthritis caused by hip dysplasia. Animals 2020, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.H.; Baek, D.S.; Kim, N.; Park, J.H.; Park, C. Topical allogeneic platelet-rich plasma treatment for a massive cutaneous lesion induced by disseminated intravascular coagulation in a toy breed dog. Ir. Vet. J. 2015, 68, 4. [Google Scholar] [CrossRef] [PubMed]
- Wróbel-Dudzińska, D.; Alio, J.; Rodriguez, A.; Suchodoła-Ratajewicz, E.; Kosior-Jarecka, E.; Rymgayłło-Jankowska, B.; Ćwiklińska-Haszcz, A.; Zarnowski, T. Clinical Efficacy of Platelet-Rich Plasma in the Treatment of Neurotrophic Corneal Ulcer. J. Ophthalmol. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Del Pino-Sedeño, T.; Trujillo-Martín, M.; Andia, I.; Aragón-Sánchez, J.; Herrera-Ramos, E.; Iruzubieta Barragán, F.; Serrano-Aguilar, P. Platelet-rich Plasma for the Treatment of Diabetic Foot Ulcers: A Meta-Analysis. Wound Repair Regen. 2019, 27, 170–182. [Google Scholar] [CrossRef]
- McClain, A.K.; McCarrel, T.M. The effect of four different freezing conditions and time in frozen storage on the concentration of commonly measured growth factors and enzymes in equine platelet-rich plasma over six months. BMC Vet. Res. 2019, 15, 292. [Google Scholar] [CrossRef]
- Do Amaral Kwirant, L.A.; De La Corte, F.D.; Brass, K.E.; Rubin, M.I.B.; França, R.T.; Vieira, P.S.; Cocco, M. Cryopreservation protocol for equine platelet-rich plasma. Semin. Agrar. 2016, 37, 1389–1396. [Google Scholar] [CrossRef]
- Do Amaral Kwirant, L.A.; De La Corte, F.D.; Cantarelli, C.; Cargnelutti, J.F.; Martins, M.; Cabral, M.W.; Maciel, N.; Rubin, M.I.B. Cooling and Cryopreservation of Equine Platelet-Rich Plasma With Dimethyl Sulfoxide and Trehalose. J. Equine Vet. Sci. 2019, 72, 112–116. [Google Scholar] [CrossRef]
- Huber, S.C.; Junior, J.L.R.C.; Silva, L.Q.; Montalvão, S.A.L.; Annichino-Bizzacchi, J.M. Freeze-dried versus fresh platelet-rich plasma in acute wound healing of an animal model. Regen. Med. 2019, 14, 525–534. [Google Scholar] [CrossRef]
- Fahie, M.A.; Ortolano, G.A.; Guercio, V.; Schaffer, J.A.; Johnston, G.; Au, J.; Hettlich, B.A.; Phillips, T.; Allen, M.J.; Bertone, A.L. A randomized controlled trial of the efficacy of autologous platelet therapy for the treatment of osteoarthritis in dogs. J. Am. Vet. Med. Assoc. 2013, 243, 1291–1297. [Google Scholar] [CrossRef]
- Bordeau, P.; Bruet, V.; Gremillet, C. Evaluation of phytosphingosine- containing shampoo and microemulsion spray in the clinical control of allergic dermatoses in dogs: Preliminary results of a multicentre study. Vet. Dermatol. 2007, 18, 177–178. [Google Scholar]
- Russell, R.P.; Apostolakos, J.; Hirose, T.; Cote, M.P.; Mazzocca, A.D. Variability of Platelet-rich Plasma Preparations. Sports Med. Arthrosc. 2013, 21, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, A.; Vavken, P.; Fleming, B.; Harrison, S.; Murray, M. Reduced Platelet Concentration Does Not Harm PRP Effectiveness for ACL Repair in a Porcine in Vivo Model. J. Orthop. Res. 2011, 29, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Cei, S.; Ducci, F.; Giuca, M.R.; Donos, N.; Gabriele, M. In vitro effects of different concentration of PRP on primary bone and gingival cell lines. Preliminary results. Minerva Stomatol. 2005, 54, 15–22. [Google Scholar]
- Braun, H.J.; Kim, H.J.; Chu, C.R.; Dragoo, J.L. The effect of platelet-rich plasma formulations and blood products on human synoviocytes: Implications for intra-articular injury and therapy. Am. J. Sports Med. 2014, 42, 1204–1210. [Google Scholar] [CrossRef]
- Anitua, E.; Sánchez, M.; Orive, G.; Andía, I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials 2007, 28, 4551–4560. [Google Scholar] [CrossRef]
- McLellan, J.; Plevin, S. Does it matter which platelet-rich plasma we use? Equine Vet. Educ. 2011, 23, 101–104. [Google Scholar] [CrossRef]
- Dragoo, J.L.; Braun, H.J.; Durham, J.L.; Ridley, B.A.; Odegaard, J.I.; Luong, R.; Arnoczky, S.P. Comparison of the acute inflammatory response of two commercial platelet-rich plasma systems in healthy rabbit tendons. Am. J. Sports Med. 2012, 40, 1274–1281. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Pereira Ruiz, M.; Vaccaro, F.; Guitaldi, R.; Di Martino, A.; Cenacchi, A.; Fornasari, P.; Marcacci, M. Platelet-rich Plasma Intra-Articular Injections for Cartilage Degeneration and Osteoarthritis: Single-Versus Double-Spinning Approach. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 2082–2091. [Google Scholar] [CrossRef]
- Zimmermann, R.; Reske, S.; Metzler, P.; Schlegel, A.; Ringwald, J.; Eckstein, R. Preparation of Highly Concentrated and White Cell-Poor Platelet-Rich Plasma by Plateletpheresis. Vox Sang. 2008, 95, 20–25. [Google Scholar] [CrossRef]
- Bielecki, T.; Dohan, M.; Ehrenfest, D. Leukocyte- and Platelet-Rich Plasma (L-PRP)/fibrin (L-PRF) in Medicine-Past, Present, Future. Curr. Pharm. Biotechnol. 2012, 13, i–ii. [Google Scholar] [PubMed]
- Álvarez, M.; López, C.; Giraldo, C.; Samudio, I.; Carmona, J. In vitro bactericidal activity of equine platelet concentrates, platelet poor plasma, and plasma against methicillin-resistant Staphylococcus aureus. Arch. Med. Vet. 2011, 43, 155–161. [Google Scholar] [CrossRef]
- Burnouf, T.; Chou, M.L.; Wu, Y.W.; Su, C.Y.; Lee, L.W. Antimicrobial activity of platelet (PLT)-poor plasma, PLT-rich plasma, PLT gel, and solvent/detergent-treated PLT lysate biomaterials against wound bacteria. Transfusion 2013, 53, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Sutter, W.W.; Kaneps, A.J.; Bertone, A.L. Comparison of hematologic values and transforming growth factor- and insulin-like growth factor concentrations in platelet concentrates obtained by use of buffy coat and apheresis methods from equine blood. Am. J. Vet. Res. 2004, 65, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Weibrich, G.; Kleis, W.K.; Kunz-Kostomanolakis, M.; Loos, A.H.; Wagner, W. Correlation of platelet concentration in platelet-rich plasma to the extraction method, age, sex, and platelet count of the donor. Int. J. Oral Maxillofac. Implant. 2001, 16, 693–699. [Google Scholar]
- Dugrillon, A.; Eichler, H.; Kern, S.; Klüter, H. Autologous Concentrated Platelet-Rich Plasma (cPRP) for Local Application in Bone Regeneration. Int. J. Oral Maxillofac. Surg. 2002, 31, 615–619. [Google Scholar] [CrossRef]
- Weibrich, G.; Kleis, W.K.G.; Hafner, G.; Hitzler, W.E.; Wagner, W. Comparison of platelet, leukocyte, and growth factor levels in point-of-care platelet-enriched plasma, prepared using a modified Curasan kit, with preparations received from a local blood bank. Clin. Oral Implant. Res. 2003, 14, 357–362. [Google Scholar] [CrossRef]
- Hessel, L.N.; Bosch, G.; van Weeren, P.R.; Ionita, J.C. Equine autologous platelet concentrates: A comparative study between different available systems. Equine Vet. J. 2015, 47, 319–325. [Google Scholar] [CrossRef]
- Ortel, T.; Mercer, M.; Thames, E.; Moore, K.; Lawson, J. Immunologic Impact and Clinical Outcomes after Surgical Exposure to Bovine Thrombin. Ann. Surg. 2001, 233, 88. [Google Scholar] [CrossRef]
- González, J.C.; López, C.; Carmona, J.U. Implications of anticoagulants and gender on cell counts and growth factor concentration in platelet-rich plasma and platelet-rich gel supernatants from rabbits. Vet. Comp. Orthop. Traumatol. 2016, 29, 115–124. [Google Scholar] [CrossRef]
- Mazzucco, L.; Balbo, V.; Cattana, E.; Guaschino, R.; Borzini, P. Not Every PRP-gel Is Born Equal. Evaluation of Growth Factor Availability for Tissues Through Four PRP-gel Preparations: Fibrinet, RegenPRP-Kit, Plateltex and One Manual Procedure. Vox Sang. 2009, 97, 110–118. [Google Scholar] [CrossRef] [PubMed]
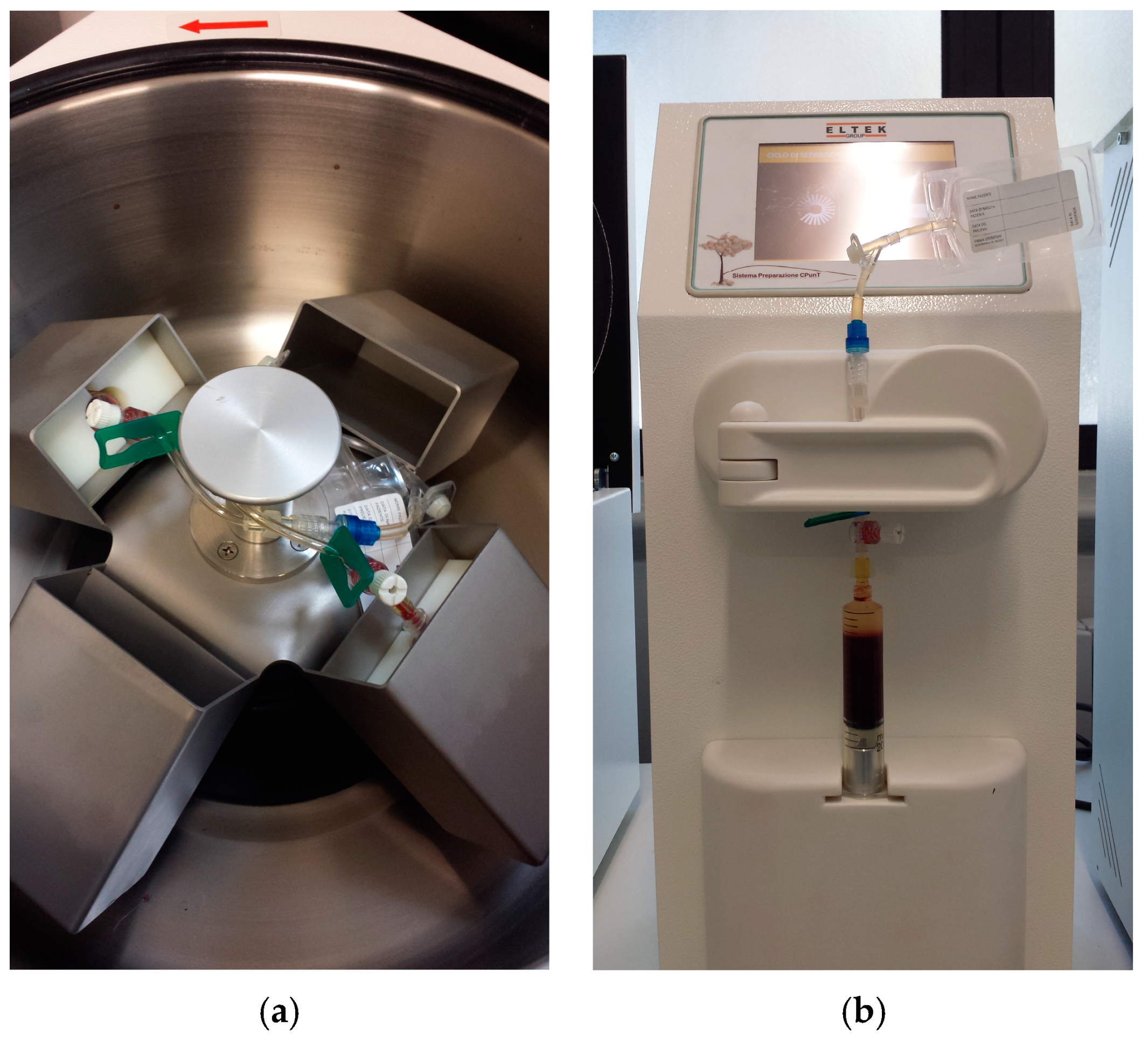
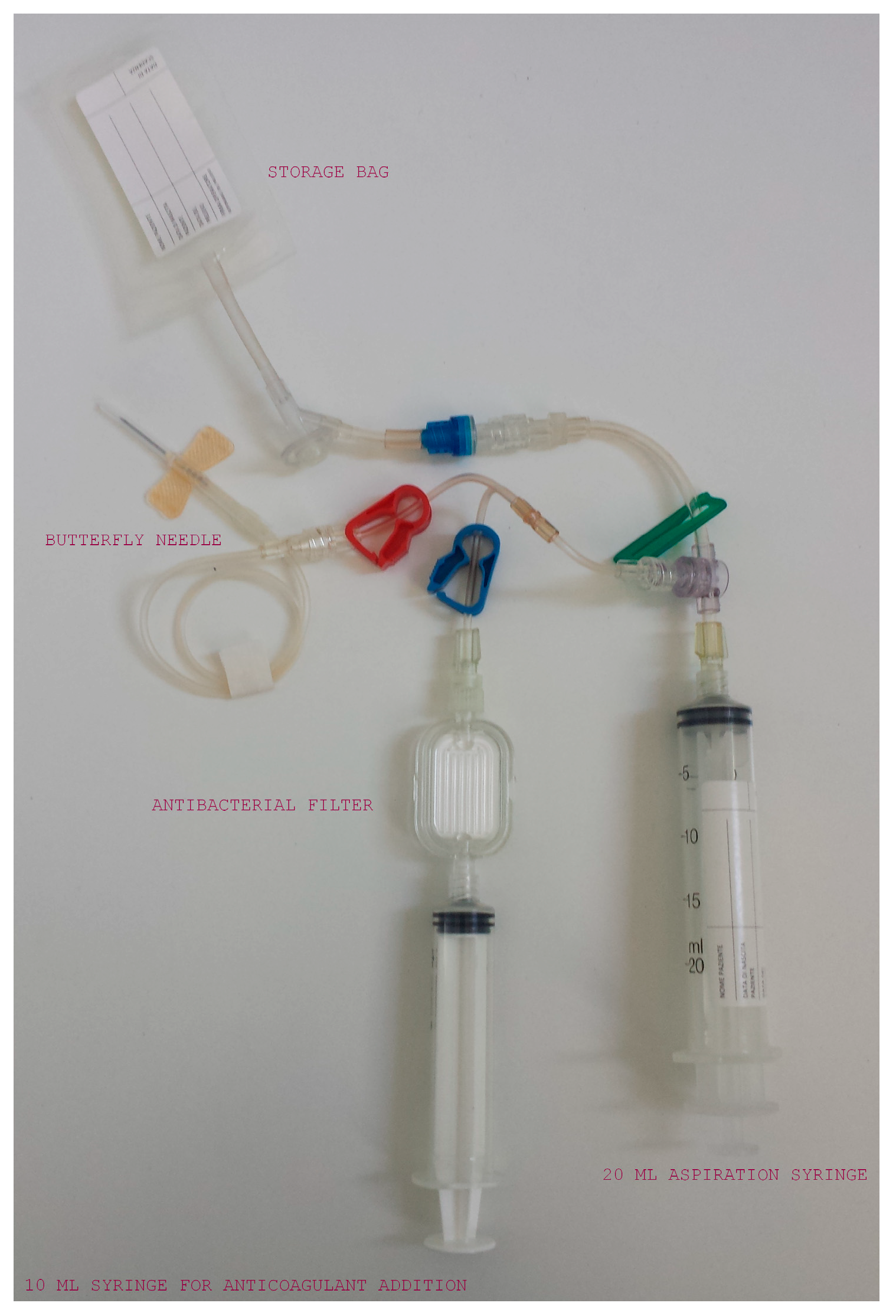
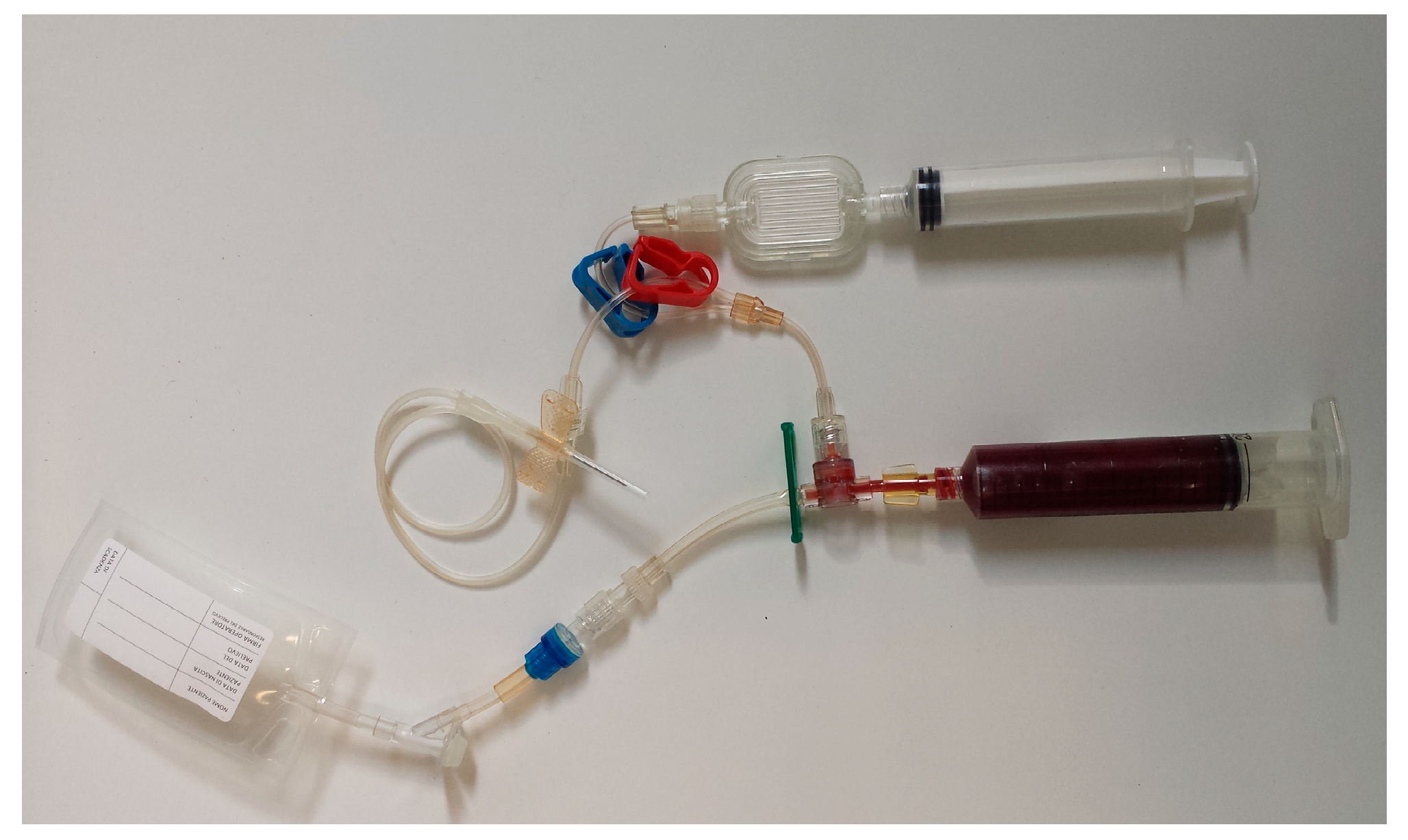
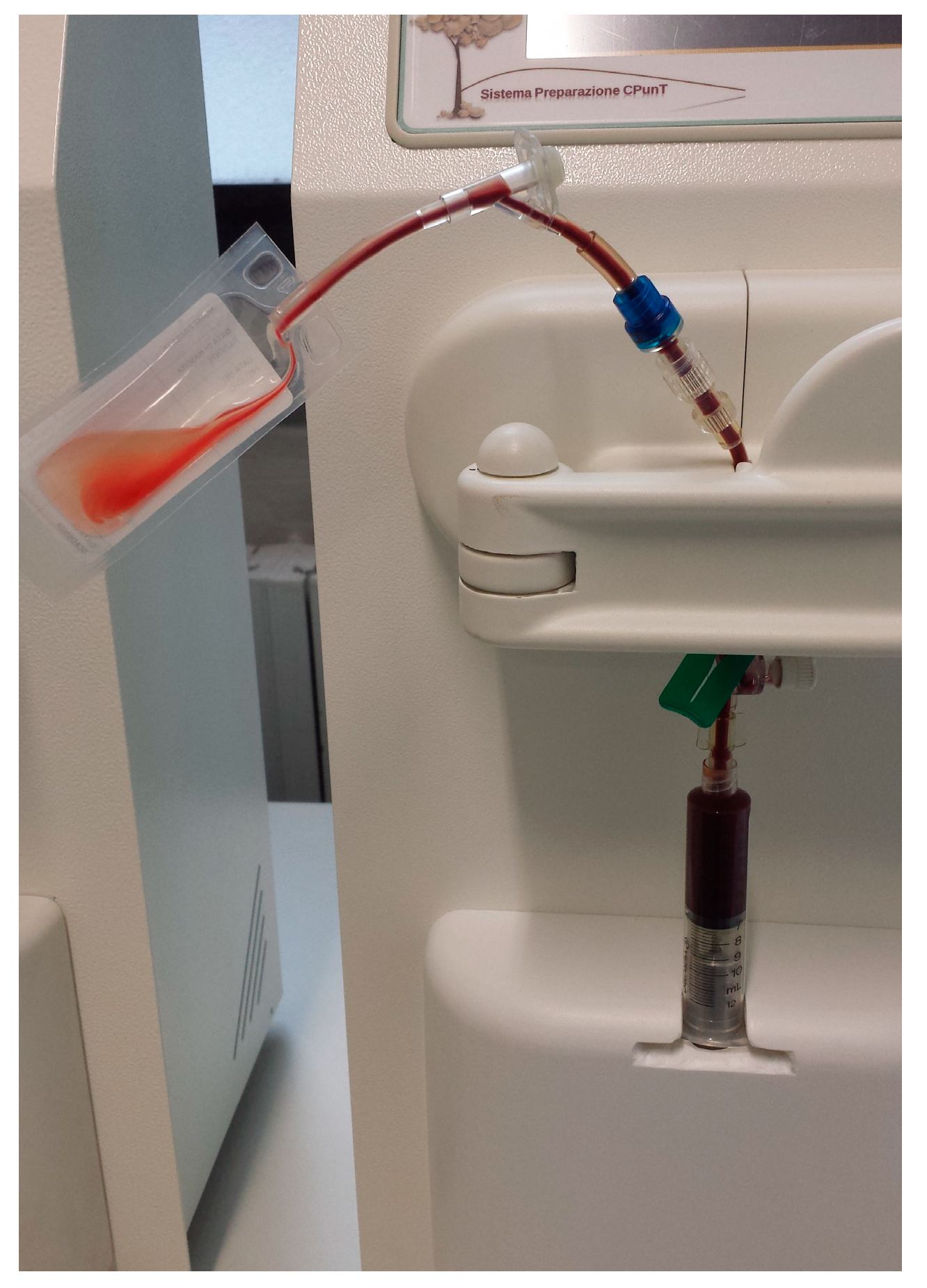
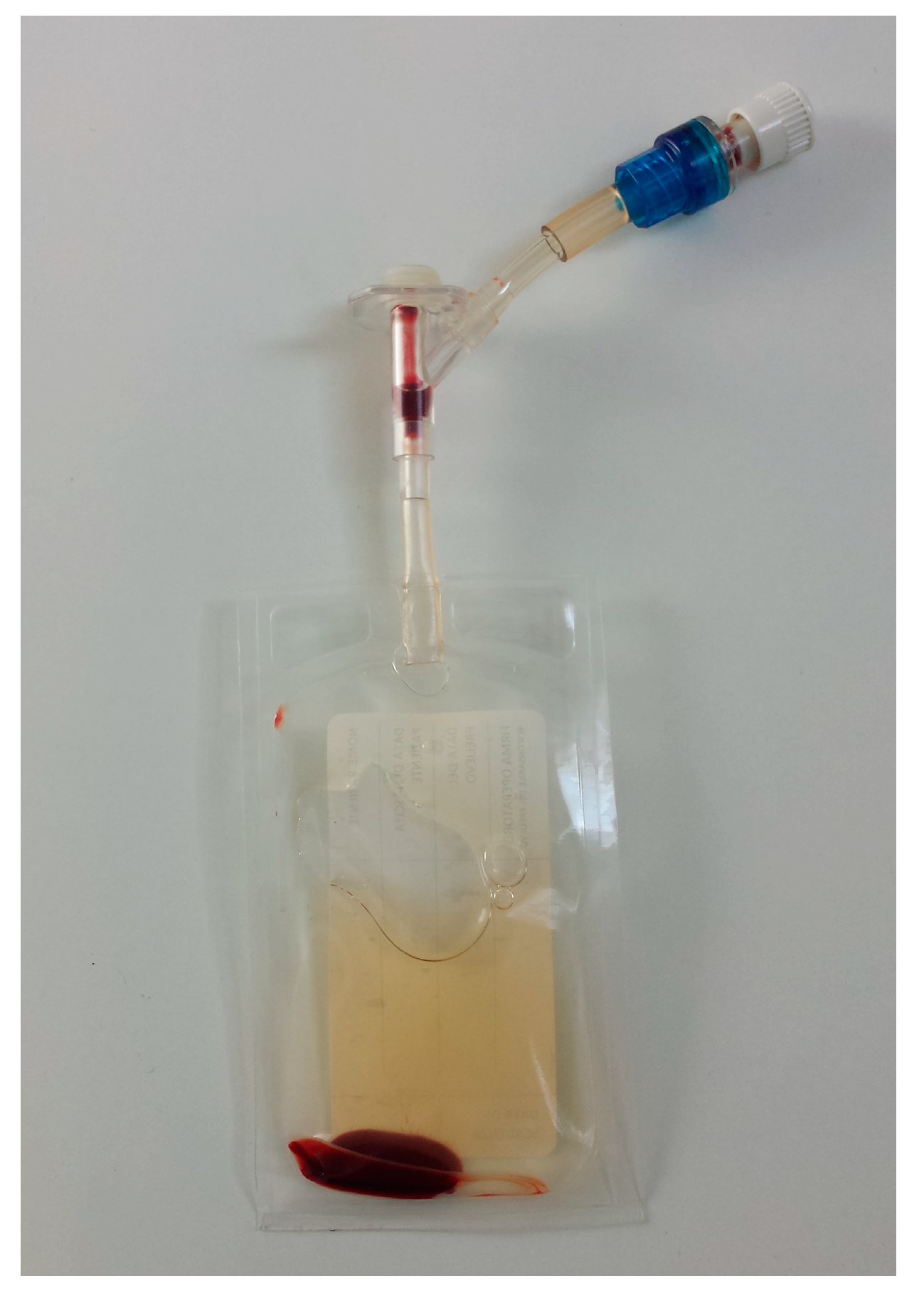
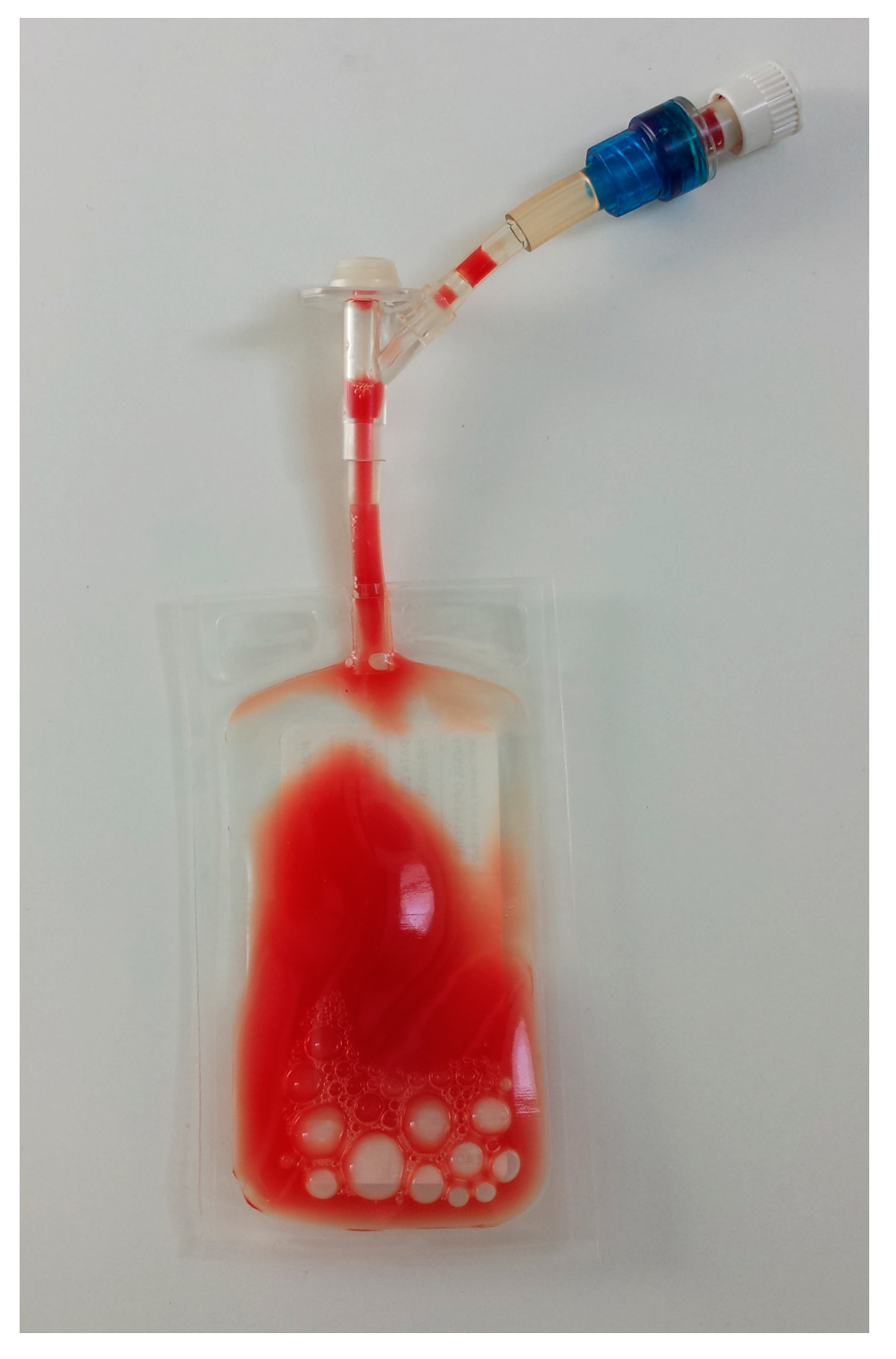
| Cellular Type (Number of Subject) | WB (%) | L-PRP (%) | p (° Wilcoxon; * t-Test) | Spearman’s Coefficient |
|---|---|---|---|---|
| Platelet (μL) (30/30) | 185,300 ± 39,576 | 767,633 ± 291,001 | <0.0001 ° | rho: 0.259 p: 0.1673 |
| Leucocytes (μL) (30/30) | 8167 ± 3425 | 8422 ± 6346 | 0.9918 ° | rho: 0.387 p: 0.034 |
| Neutrophils (μL) (19/30) | 6303 ± 3459 (63.7) | 1250 ± 1960 (14.8) | <0.0001 ° | rho: 0.428 p: 0.0762 |
| Lymphocytes (μL) (19/30) | 2500 ± 1329 (29.3) | 5231 ± 2620 (71.7) | 0.0001 * | rho: 0.558 p: 0.016 |
| Monocytes (μL) (19/30) | 248 ± 174 (3) | 823 ± 792 (10.7) | 0.0008 ° | rho: −0.164 p: 0.52 |
| Erythrocytes (μL) (30/30) | 5,907,433 ± 704,099 | 528,600 ± 222,773 | <0.0001 * | rho: −0.108 p: 0.57 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perego, R.; Spada, E.; Baggiani, L.; Martino, P.A.; Proverbio, D. Efficacy of a Semi Automated Commercial Closed System for Autologous Leukocyte- and Platelet-Rich Plasma (l-prp) Production in Dogs: A Preliminary Study. Animals 2020, 10, 1342. https://doi.org/10.3390/ani10081342
Perego R, Spada E, Baggiani L, Martino PA, Proverbio D. Efficacy of a Semi Automated Commercial Closed System for Autologous Leukocyte- and Platelet-Rich Plasma (l-prp) Production in Dogs: A Preliminary Study. Animals. 2020; 10(8):1342. https://doi.org/10.3390/ani10081342
Chicago/Turabian StylePerego, Roberta, Eva Spada, Luciana Baggiani, Piera Anna Martino, and Daniela Proverbio. 2020. "Efficacy of a Semi Automated Commercial Closed System for Autologous Leukocyte- and Platelet-Rich Plasma (l-prp) Production in Dogs: A Preliminary Study" Animals 10, no. 8: 1342. https://doi.org/10.3390/ani10081342
APA StylePerego, R., Spada, E., Baggiani, L., Martino, P. A., & Proverbio, D. (2020). Efficacy of a Semi Automated Commercial Closed System for Autologous Leukocyte- and Platelet-Rich Plasma (l-prp) Production in Dogs: A Preliminary Study. Animals, 10(8), 1342. https://doi.org/10.3390/ani10081342







