Dynamics of Different Buffer Systems in Slurries Based on Time and Temperature of Storage and Their Visualization by a New Mathematical Tool
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Slurry Sampling
2.2. Storage
2.3. Analyses of the Ingredients
2.4. Titration
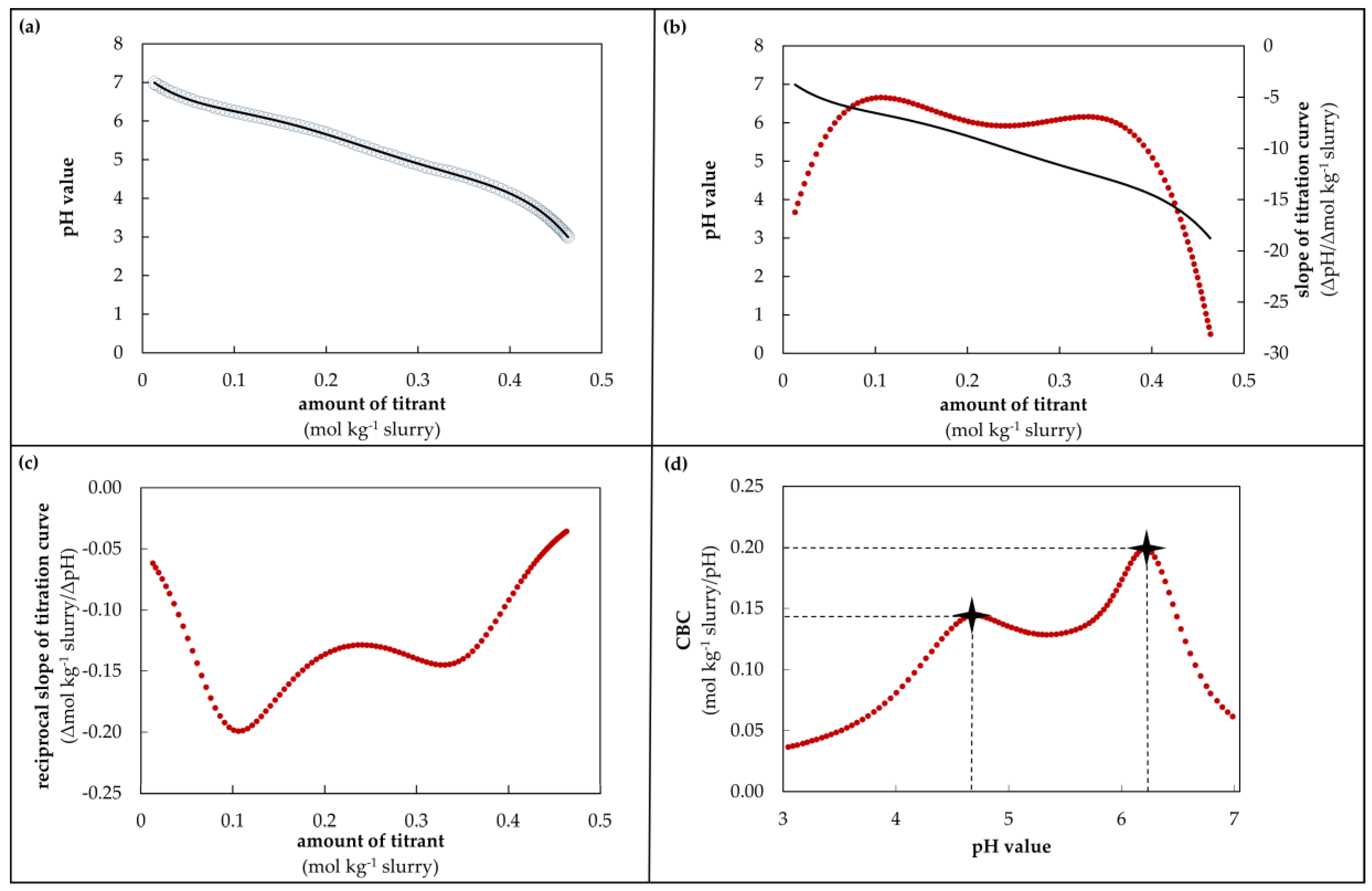
2.5. Calculations of the Titrations and New Determination Model for Buffer Capacity
2.6. Statistical Analysis
3. Results
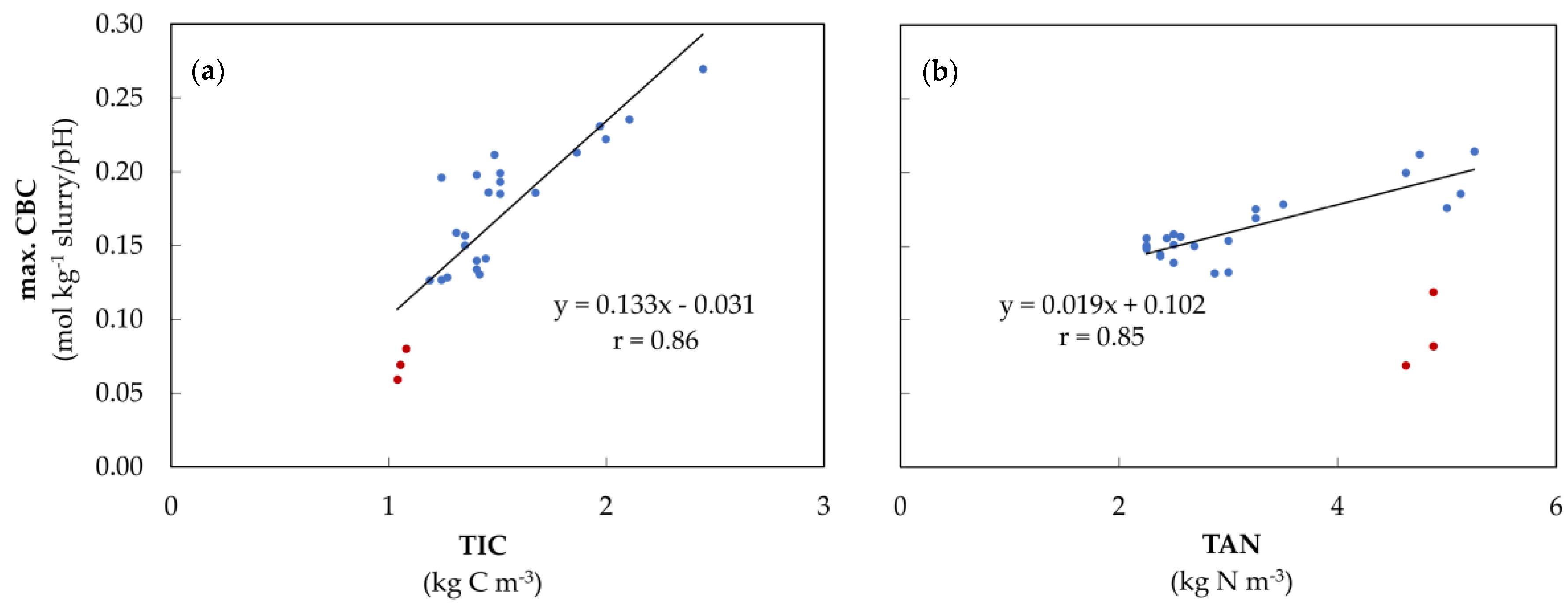
3.1. Slurry Characterization
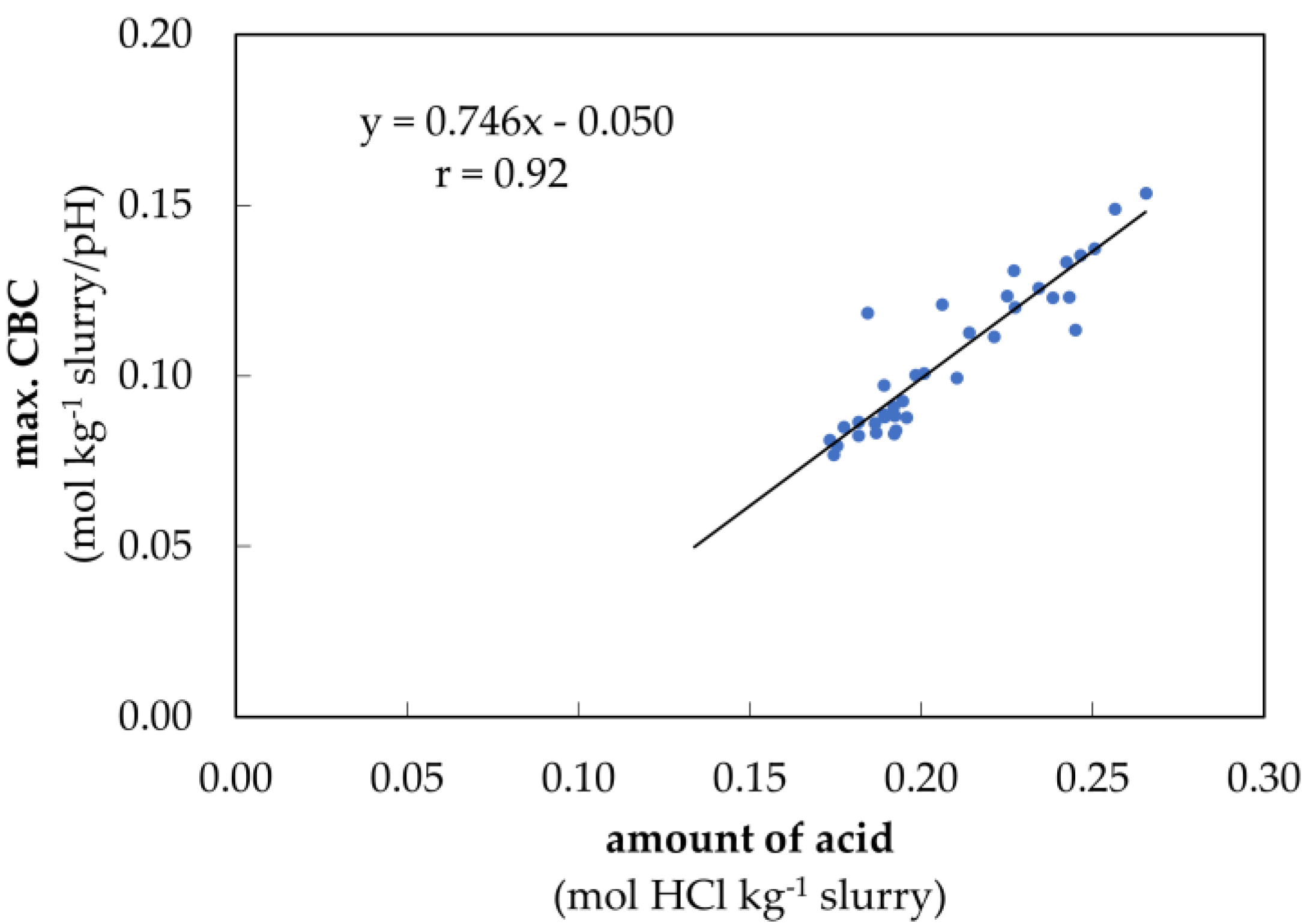
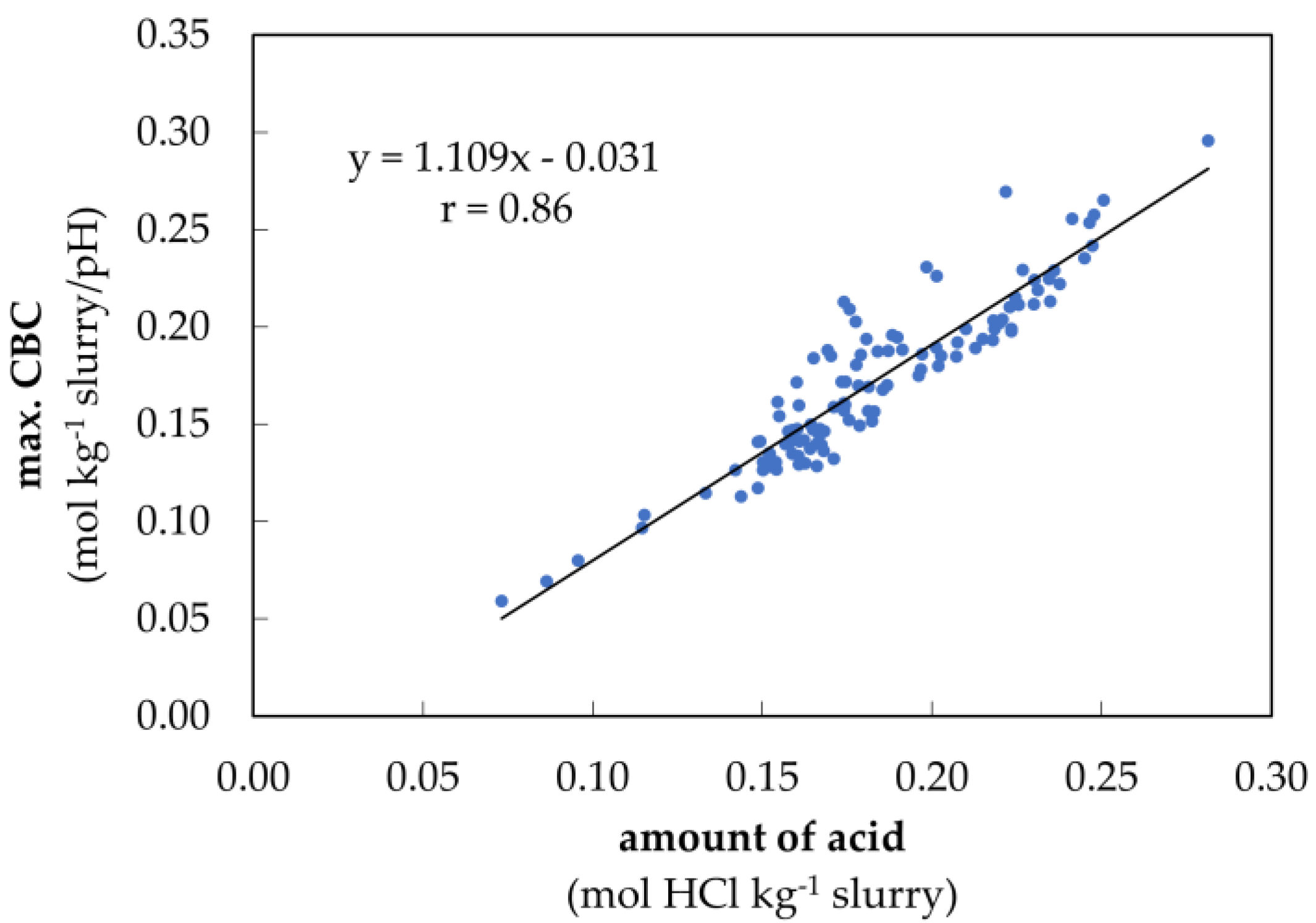
3.2. Verification of the Determination Model for Buffer Capacity
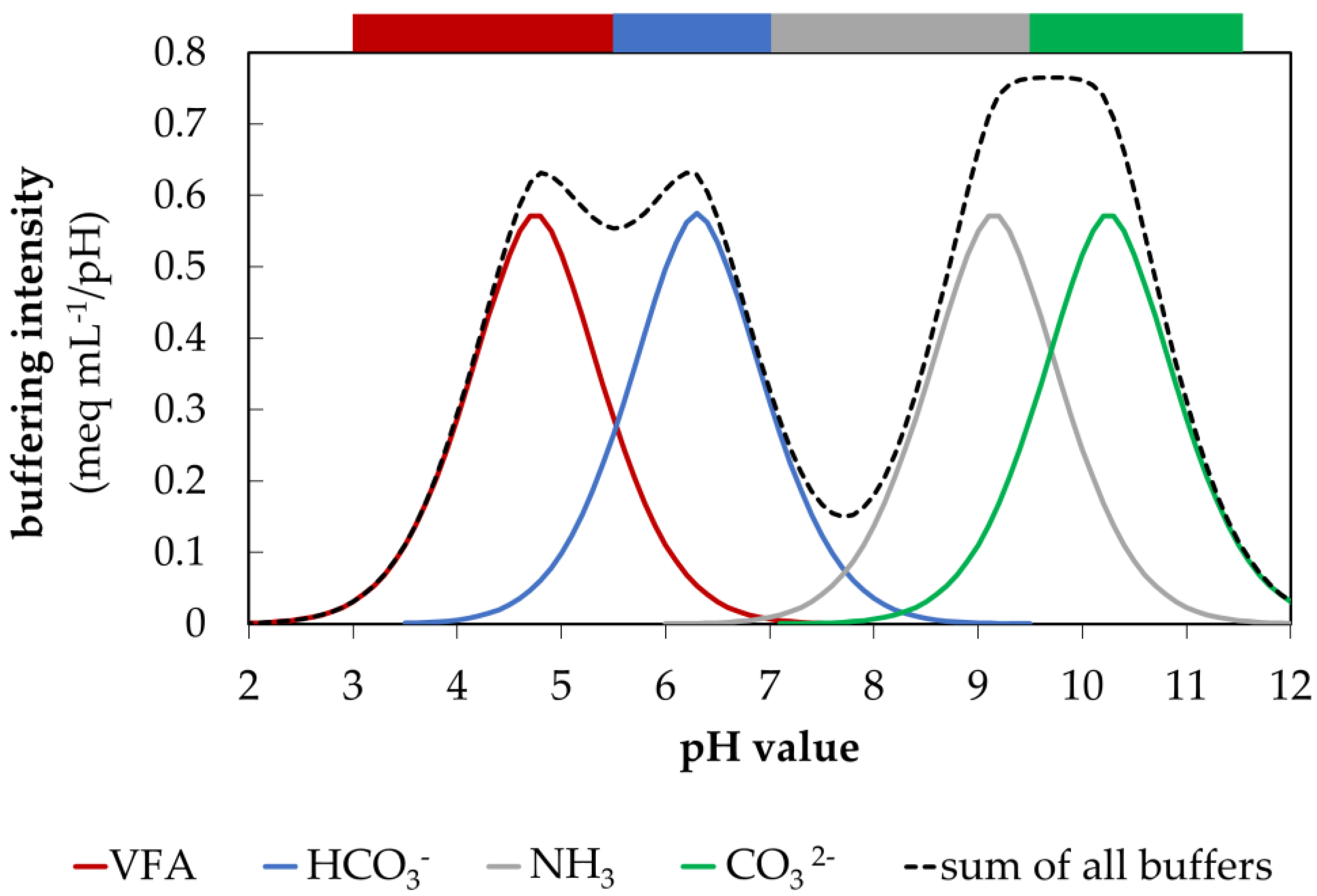
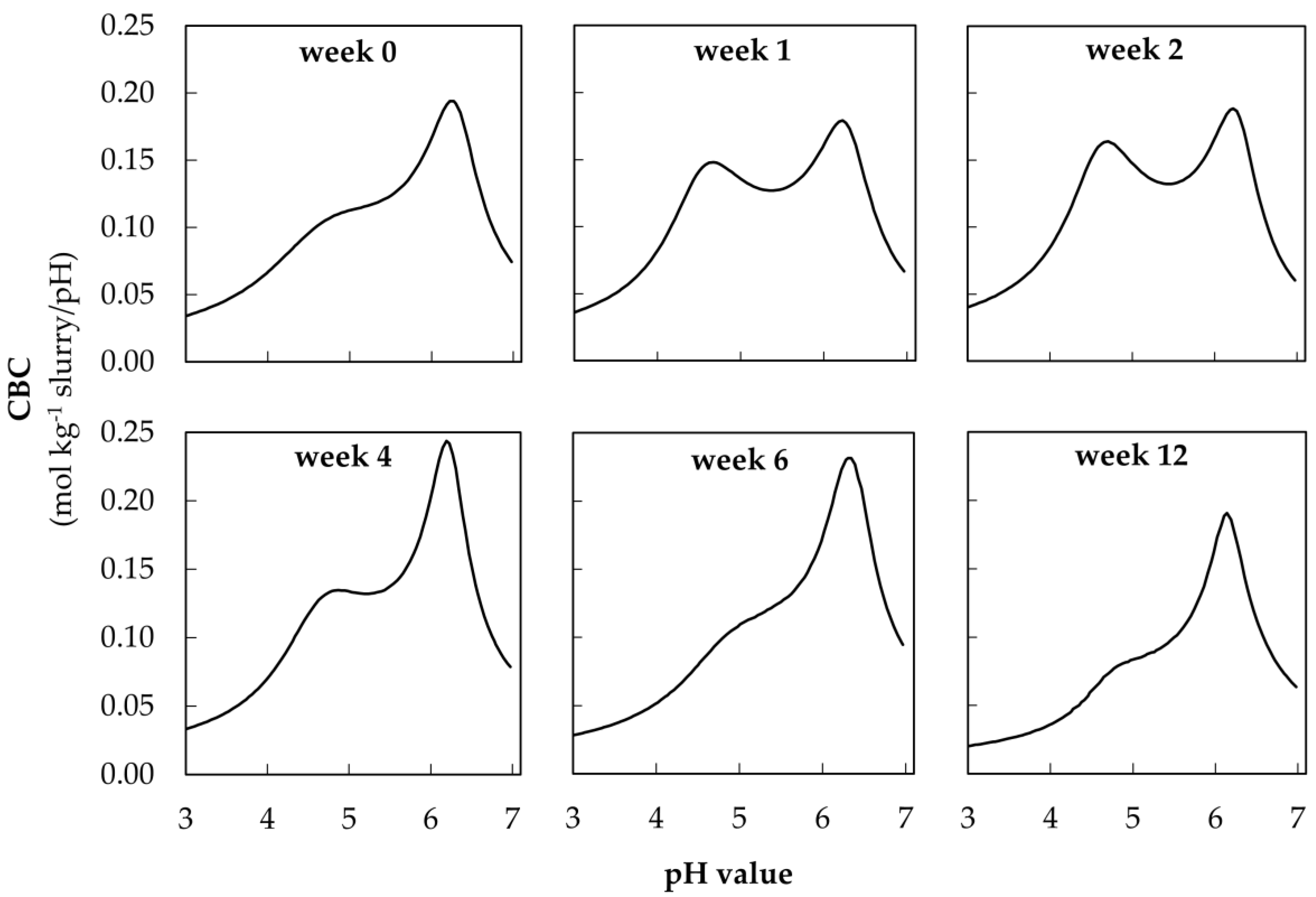
3.3. Visualization of CBC during Acidification
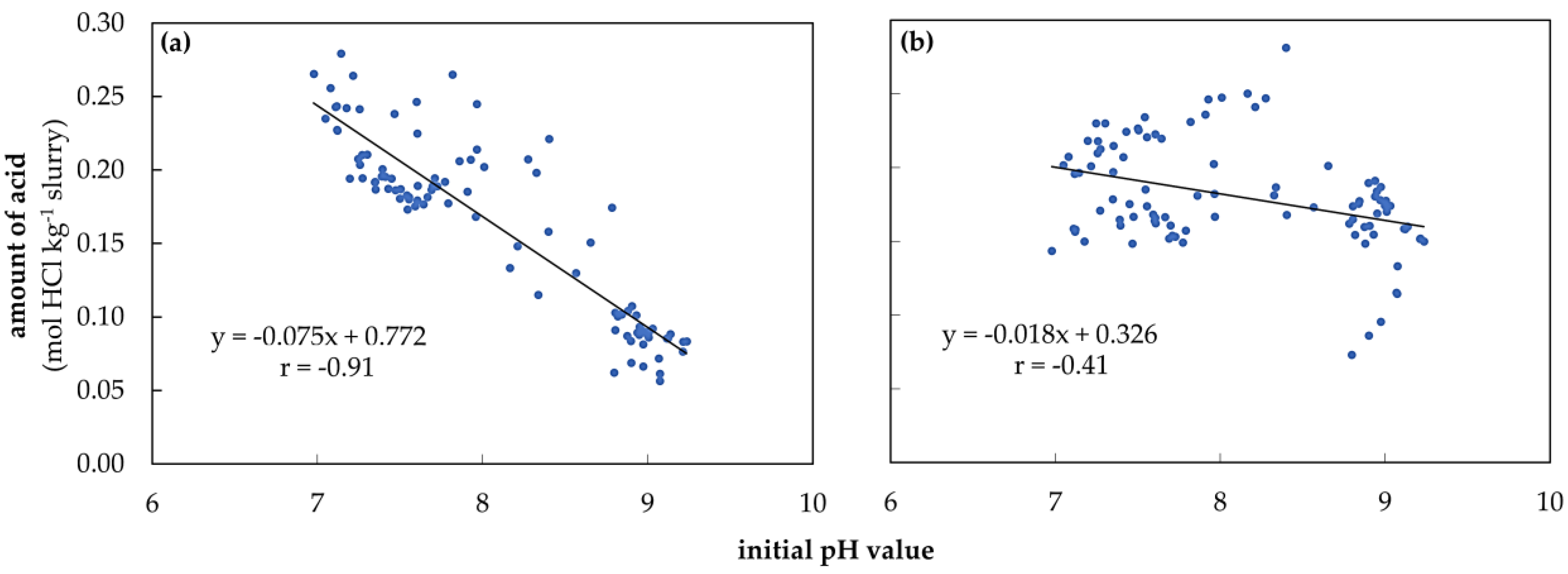
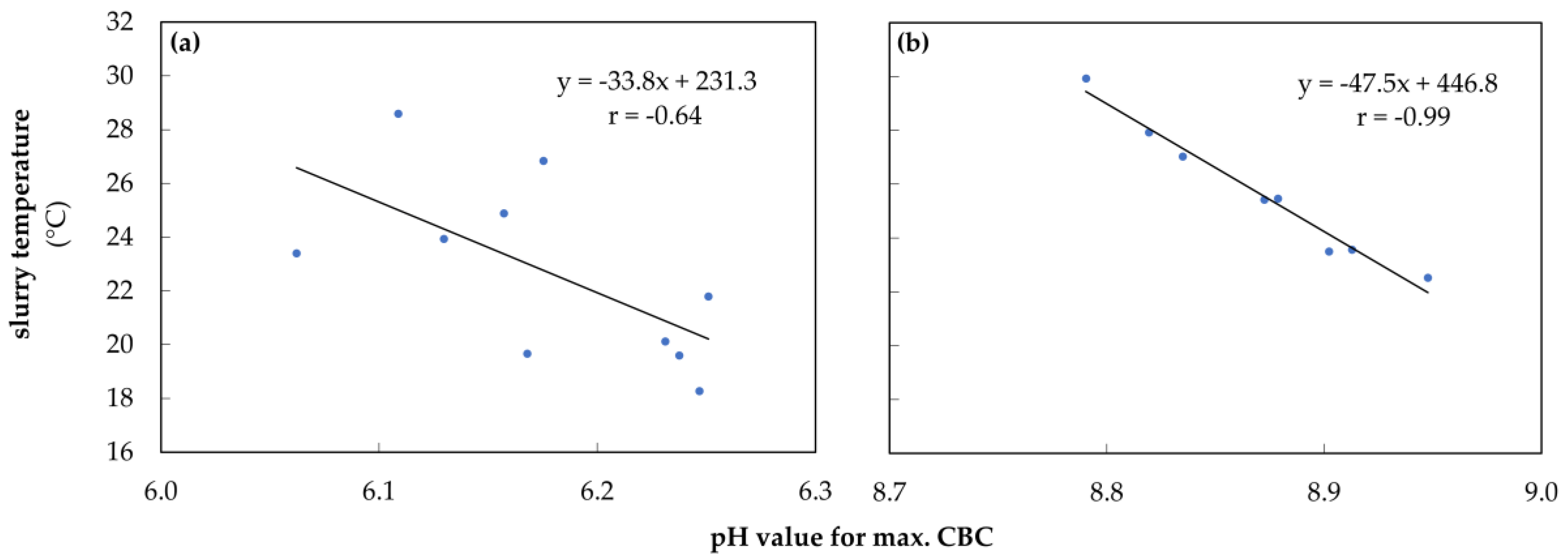
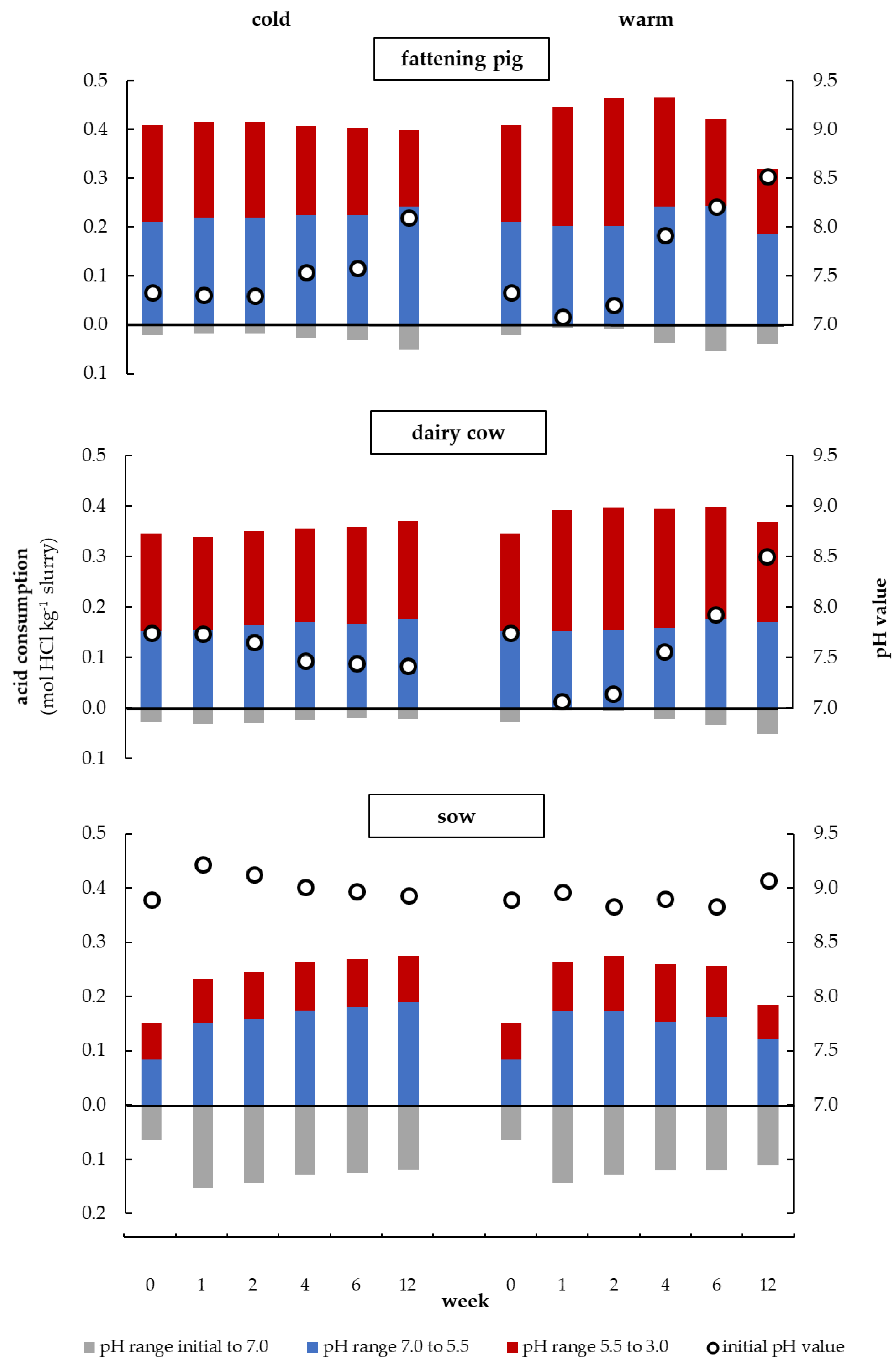
3.4. Initial pH Value and Amount of Acid for Titration during Storage at Different Temperatures
4. Discussion
4.1. Verification of the Determination Model for Buffer Capacity
4.2. Slurry Characterization, Initial pH Value and Amount of Acid during Storage at Different Temperatures
4.2.1. Slurry Characterization
4.2.2. Initial pH Value
4.2.3. Amount of Acid in the VFA Buffer Range
4.2.4. Dynamics of the Carbonate Buffer and its Impacts on the Alkaline Milieu
4.3. Degradation of Urea in Slurry
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Fattening Pig | Dairy Cow | Sow | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week and Storage Conditions | 0 | 8 Cold | 8 Warm | 0 | 8 Cold | 8 Warm | 0 | 8 Cold | 8 Warm | |
| Ingredients 1 | ||||||||||
| MgO | kg m−3 | 2.01 | 2.22 | 2.51 | 0.81 | 1.03 | 1.02 | 0.62 | 0.61 | 0.64 |
| CaO | kg m−3 | 3.63 | 3.72 | 4.30 | 3.13 | 4.03 | 3.96 | 0.89 | 0.77 | 0.86 |
| S | kg m−3 | 0.55 | 0.65 | 0.69 | 0.53 | 0.67 | 0.60 | 0.35 | 0.36 | 0.35 |
| Cu | g m−3 | 15.00 | 16.10 | 18.47 | 3.50 | 4.48 | 4.47 | 2.87 | 2.75 | 2.93 |
| Zn | g m−3 | 80.40 | 90.87 | 102.40 | 17.40 | 22.10 | 21.97 | 16.20 | 15.47 | 16.47 |
| Butyric acid | g kg−1 | 0.82 | 0.77 | <0.05 | 0.51 | 0.50 | 0.37 | 0.09 | 0.09 | 0.11 |
| Iso-butyric acid | g kg−1 | 0.35 | 0.35 | 0.38 | 0.12 | 0.14 | 0.24 | <0.05 | 0.06 | 0.12 |
| Valeric acid | g kg−1 | 0.06 | 0.05 | 0.05 | 0.05 | <0.05 | 0.10 | <0.05 | <0.05 | <0.05 |
| Iso-valeric acid | g kg−1 | 0.38 | 0.38 | 0.37 | 0.08 | 0.09 | 0.16 | 0.05 | 0.06 | 0.10 |
| n-caproic acid | g kg−1 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Slurry Characteristics | Week | |||||||
|---|---|---|---|---|---|---|---|---|
| Fattening Pig | 0 | 1 | 2 | 4 | 6 | 12 | ||
| initial pH value | cold | 7.34 (0.05) ab | 7.31 (0.02) a | 7.29 (0.07) a | 7.54 (0.03) ab | 7.58 (0.03) b | 8.09 (0.09) c | |
| warm | 7.34 (0.05) a | 7.08 (0.02) a | 7.20 (0.03) a | 7.92 (0.06) b | 8.21 (0.13) bc | 8.52 (0.09) c | ||
| initial to 7.0 | mol HCl kg−1 slurry | cold | 0.021 (0.004) ab | 0.017 (0.001) a | 0.018 (0.002) a | 0.027 (0.001) ab | 0.032 (0.001) b | 0.051 (0.005) c |
| warm | 0.021 (0.004) ab | 0.005 (0.001) a | 0.009 (0.002) a | 0.037 (0.003) bc | 0.053 (0.007) c | 0.038 (0.003) bc | ||
| 7.0 to 5.5 | cold | 0.211 (0.010) a | 0.219 (0.005) ab | 0.220 (0.002) ab | 0.225 (0.005) ab | 0.225 (0.005) ab | 0.243 (0.004) b | |
| warm | 0.211 (0.010) ab | 0.202 (0.003) ab | 0.203 (0.004) ab | 0.242 (0.005) b | 0.244 (0.023) b | 0.182 (0.008) a | ||
| 5.5 to 3.0 | cold | 0.198 (0.005) | 0.197 (0.007) | 0.195 (0.005) | 0.182 (0.002) | 0.180 (0.002) | 0.156 (0.015) | |
| warm | 0.198 (0.005) bc | 0.245 (0.006) cd | 0.262 (0.011) d | 0.225 (0.020) bcd | 0.178 (0.015) ab | 0.132 (0.010) a | ||
| Dairy cow | ||||||||
| initial pH value | cold | 7.74 (0.03) b | 7.74 (0.03) b | 7.66 (0.03) ab | 7.47 (0.07) ab | 7.45 (0.05) a | 7.42 (0.08) a | |
| warm | 7.74 (0.03) bc | 7.07 (0.05) a | 7.14 (0.03) a | 7.56 (0.05) b | 7.93 (0.03) c | 8.50 (0.14) bc | ||
| initial to 7.0 | mol HCl kg−1 slurry | cold | 0.029 (0.001) | 0.031 (0.001) | 0.030 (0.003) | 0.020 (0.002) | 0.021 (0.004) | 0.021 (0.004) |
| warm | 0.029 (0.001) c | 0.004 (0.002) a | 0.007 (0.001) ab | 0.022 (0.002) bc | 0.032 (0.001) bc | 0.052 (0.006) bc | ||
| 7.0 to 5.5 | cold | 0.152 (0.002) a | 0.154 (0.002) a | 0.163 (0.002) ab | 0.171 (0.004) b | 0.167 (0.004) ab | 0.177 (0.004) b | |
| warm | 0.152 (0.002) ab | 0.153 (0.005) a | 0.154 (0.004) ab | 0.160 (0.005) ab | 0.177 (0.005) b | 0.170 (0.006) ab | ||
| 5.5 to 3.0 | cold | 0.193 (0.001) | 0.184 (0.004) | 0.187 (0.003) | 0.185 (0.005) | 0.193 (0.006) | 0.193 (0.011) | |
| warm | 0.193 (0.001) | 0.240 (0.013) | 0.243 (0.000) | 0.237 (0.006) | 0.222 (0.012) | 0.198 (0.014) | ||
| Sow | ||||||||
| initial pH value | cold | 8.89 (0.05) a | 9.22 (0.01) d | 9.12 (0.01) cd | 9.01 (0.01) bc | 8.97 (0.02) ab | 8.94 (0.02) ab | |
| warm | 8.89 (0.05) ab | 8.97 (0.02) bc | 8.83 (0.01) a | 8.90 (0.01) ab | 8.83 (0.02) a | 9.07 (0.00) c | ||
| initial to 7.0 | mol HCl kg−1 slurry | cold | 0.064 (0.009) a | 0.152 (0.002) d | 0.143 (0.001) cd | 0.128 (0.001) bc | 0.125 (0.002) bc | 0.118 (0.002) b |
| warm | 0.064 (0.009) a | 0.144 (0.002) c | 0.127 (0.005) bc | 0.120 (0.006) bc | 0.120 (0.006) bc | 0.110 (0.006) b | ||
| 7.0 to 5.5 | cold | 0.085 (0.007) a | 0.151 (0.001) b | 0.159 (0.001) c | 0.174 (0.000) d | 0.180 (0.002) de | 0.189 (0.001) e | |
| warm | 0.085 (0.007) a | 0.173 (0.004) c | 0.173 (0.004) c | 0.155 (0.003) c | 0.163 (0.006) c | 0.121 (0.006) b | ||
| 5.5 to 3.0 | cold | 0.066 (0.002) a | 0.081 (0.002) b | 0.086 (0.001) bc | 0.090 (0.001) c | 0.088 (0.001) bc | 0.085 (0.002) bc | |
| warm | 0.066 (0.002) a | 0.091 (0.001) b | 0.102 (0.000) bc | 0.104 (0.002) c | 0.093 (0.004) bc | 0.063 (0.005) a | ||
References
- Committee on Extreme Weather Events and Climate Change Attribution; Board on Atmospheric Sciences and Climate; Division on Earth and Life Studies; National Academies of Sciences, Engineering, and Medicine. Attribution of Extreme Weather Events in the Context of Climate Change; The National Academies Press: Washington, DC, USA, 2016; ISBN 978-0-309-38094-2. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. In A Policy Framework for Climate and Energy in the Period from 2020 to 2030; European Commission: Brussels, Belgium, 2014; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52012DC0673&from=EN (accessed on 19 April 2020).
- European Commission, DG Climate Action European Environment Agency. Annual European Union greenhouse gas inventory 1990–2017 and inventory report 2019. In Submission under the United Nations Framework Convention on Climate Change and the Kyoto Protocol; European Commission: Brussels, Belgium, 2019; Available online: https://www.eea.europa.eu/publications/european-union-greenhouse-gas-inventory-2019/at_download/file (accessed on 19 April 2020).
- Tista, M.; Gager, M.; Gaisbauer, S.; Ullrich, B. European Union Emission Inventory Report 1990-2017 under the UNECE Convention on Long-Range Transboundary Air Pollution (LRTAP); Publications Office of the European Union: Luxembourg, 2019; ISBN 978-92-9480-078-7. [Google Scholar]
- Rösemann, C.; Haenel, H.-D.; Dämmgen, U.; Döring, U.; Wulf, S.; Eurich-Menden, B.; Freibauer, A.; Döhler, H.; Schreiner, C.; Osterburg, B.; et al. Calculations of Gaseous and Particulate Emissions from German Agriculture 1990–2017: Report on Methods and Data (RMD) Submission 2019; Thünen Rep 67; Johann Heinrich von Thünen-Institut: Braunschweig, Germany, 2019. [Google Scholar] [CrossRef]
- IPCC. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty. 2018. Available online: https://www.ipcc.ch/site/assets/uploads/sites/2/2019/06/SR15_Full_Report_High_Res.pdf (accessed on 19 April 2020).
- Denmead, O.T.; Chen, D.; Griffith, D.W.T.; Loh, Z.M.; Bai, M.; Naylor, T. Emissions of the indirect greenhouse gases NH3 and NOx from Australian beef cattle feedlots. Aust. J. Exp. Agric. 2008, 48, 213. [Google Scholar] [CrossRef]
- Sigurdarson, J.J.; Svane, S.; Karring, H. The molecular processes of urea hydrolysis in relation to ammonia emissions from agriculture. Rev. Environ. Sci. Biotechnol. 2018, 17, 241–258. [Google Scholar] [CrossRef] [Green Version]
- Scarlat, N.; Fahl, F.; Dallemand, J.-F.; Monforti, F.; Motola, V. A spatial analysis of biogas potential from manure in Europe. Renew. Sust. Energ. Rev. 2018, 94, 915–930. [Google Scholar] [CrossRef]
- Clarisse, L.; Clerbaux, C.; Dentener, F.; Hurtmans, D.; Coheur, P.-F. Global ammonia distribution derived from infrared satellite observations. Nat. Geosci. 2009, 2, 479–483. [Google Scholar] [CrossRef]
- Oenema, O.; Oudendag, D.; Velthof, G.L. Nutrient losses from manure management in the European Union. Livest.Sci. 2007, 112, 261–272. [Google Scholar] [CrossRef]
- Council Directive 91/676/EEC of 12 December 1991 concerning the protection of waters against pollution caused by nitrates from agricultural sources. Off. J. Eur. Commun. 1991, 375.
- Conn, K.L.; Topp, E.; Lazarovits, G. Factors influencing the concentration of volatile fatty acids, ammonia, and other nutrients in stored liquid pig manure. J. Environ. Qual. 2007, 36, 440–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arogo, J.; Westerman, P.W.; Liang, Z.S. Comparing Ammonium ion Dissociation Constant in Swine Anaerobic Lagoon Liquid and Deionized Water. Trans. ASAE 2003, 46, 1415–1419. [Google Scholar] [CrossRef]
- Misselbrook, T.; Hunt, J.; Perazzolo, F.; Provolo, G. Greenhouse Gas and Ammonia Emissions from Slurry Storage: Impacts of Temperature and Potential Mitigation through Covering (Pig Slurry) or Acidification (Cattle Slurry). J. Environ. Qual. 2016, 45, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, A.R.; Carswell, A.M.; Shaw, R.; Hunt, J.; Saunders, K.; Cotton, J.; Chadwick, D.R.; Jones, D.L.; Misselbrook, T.H. Advanced Processing of Food Waste Based Digestate for Mitigating Nitrogen Losses in a Winter Wheat Crop. Front. Sustain. Food Syst. 2018, 2, 136. [Google Scholar] [CrossRef]
- Jacobsen, B.H. Why is acidification a success only in Denmark?-Transfer of technology across boarders. In Proceedings of the 21st International Farm Management Association Congress, Edinburgh, UK, 2–7 July 2017; International Farm Management Association, Ed.; [Google Scholar]
- Kaupenjohann, M.; Schnug, E.; Haneklaus, S.; Döhler, H.G.; Nebelsieck, R.; Fock, M. Gutachten zur Anwendung von Minderungstechniken für Ammoniak durch “Ansäuerung von Gülle“ und deren Wirkungen auf Boden und Umwelt; Umweltbundesamt: Dessau-Roßlau, Germany, 2019. [Google Scholar]
- Cornel, P.; Schaum, C. Phosphorus recovery from wastewater: Needs, technologies and costs. Water Sci. Technol. 2009, 59, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Mohammed-Nour, A.; Al-Sewailem, M.; El-Naggar, A.H. The Influence of Alkalization and Temperature on Ammonia Recovery from Cow Manure and the Chemical Properties of the Effluents. Sustainability 2019, 11, 2441. [Google Scholar] [CrossRef] [Green Version]
- Laureni, M.; Palatsi, J.; Llovera, M.; Bonmatí, A. Influence of pig slurry characteristics on ammonia stripping efficiencies and quality of the recovered ammonium-sulfate solution. J. Chem. Technol. Biotechnol. 2013, 88, 1654–1662. [Google Scholar] [CrossRef]
- Georgacakis, D.; Sievers, D.M.; Iannotti, E.L. Buffer stability in manure digesters. Agric. Wastes 1982, 4, 427–441. [Google Scholar] [CrossRef]
- Patni, N.K.; Jui, P.Y. Volatile fatty acids in stored dairy-cattle slurry. Agric. Wastes 1985, 13, 159–178. [Google Scholar] [CrossRef]
- Paul, J.W.; Beauchamp, E.G. Relationship between volatile fatty acids, total ammonia, and pH in manure slurries. Biol. Wastes 1989, 29, 313–318. [Google Scholar] [CrossRef]
- Christensen, M.L.; Sommer, S.G. Manure Characterisation and Inorganic Chemistry. In Animal Manure: Recycling, Treatment, and Management; Jensen, L.S., Christensen, M.L., Sommer, S.G., Schmidt, T., Eds.; John Wiley & Sons Inc.: Chichester, West Sussex, UK, 2013; pp. 41–65. ISBN 9781118676677. [Google Scholar]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 9780471206934. [Google Scholar]
- Vandré, R.; Clemens, J. Studies on the relationship between slurry pH, volatilization processes and the influence of acidifying additives. Nutr. Cycl. Agroecosyst. 1996, 47, 157–165. [Google Scholar] [CrossRef]
- Klasse, H.-J.; Werner, W. Method for rapid determination of ammonia nitrogen in animal slurries and sewage sludge. In Agricultural Waste Management and Environmental Protection. 4th International CIEC Symposium; Welte, E., Szaboles, I., Eds.; FAL: Braunschweig-Völkenrode, Germany, 1987; pp. 119–123. [Google Scholar]
- Tunney, H.; Bertrand, M. Rapid field tests for estimating dry matter and fertiliser value of animal slurries. In Proceedings of the Agricultural Engineering: Proceedings of the Eleventh International Congress on Agricultural Engineering, Dublin, Ireland, 4–8 September 1989; Dodd, V.A., Ed.; Balkema: Rotterdam, The Netherlands, 1989; pp. 363–370, ISBN 9061919800. [Google Scholar]
- Klasse, H.-J. Entwicklung einer Schnellmethode zur Bestimmung des Ammoniumstickstoffs in Güllen und Klärschlamm sowie Lysimeterversuche zur Beurteilung von Strohdüngung und Zwischenfruchtanbau als Maßnahmen zur Konservierung von Stickstoff aus der Gülledüngung im Herbst. Ph.D. Dissertation, Rheinische Friedrich-Wilhelms-Universität Bonn, Bonn, Germnay, 1988. [Google Scholar]
- Clemens, J.; Seufert, H. Wissen, was im Reaktor abläuft. In Bayerisches Landwirtschaftliches Wochenblatt; Sonderdruck aus Heft 26 vom 20.07.2007; Deutscher Landwirtschaftsverlag GmbH: München, Germany, 2007. [Google Scholar]
- Hecht, M. Die Bedeutung des Carbonat-Puffersystems für die Stabilität des Gärprozesses landwirtschaftlicher Biogasanlagen. Ph.D. Dissertation, Rheinische Friedrich-Wilhelms-Universität Bonn, Bonn, Germnay, 2008. [Google Scholar]
- Millero, F.J.; Graham, T.B.; Huang, F.; Bustos-Serrano, H.; Pierrot, D. Dissociation constants of carbonic acid in seawater as a function of salinity and temperature. Mar. Chem. 2006, 100, 80–94. [Google Scholar] [CrossRef]
- Moosbrugger, R.E.; Wentzel, M.C.; Ekama, G.A.; Marais, G.v.R. Weak acid/bases and pH control in anaerobic systems–A review. Water SA 1993, 19, 1–10. [Google Scholar]
- Husted, S.; Jensen, L.S.; Jørgensen, S.S. Reducing ammonia loss from cattle slurry by the use of acidifying additives: The role of the buffer system. J. Sci. Food Agric. 1991, 57, 335–349. [Google Scholar] [CrossRef]
- Lahav, O.; Morgan, B.E. Titration methodologies for monitoring of anaerobic digestion in developing countries-a review. J. Chem. Technol. Biotechnol. 2004, 79, 1331–1341. [Google Scholar] [CrossRef] [Green Version]
- Christensen, M.L.; Hjorth, M.; Keiding, K. Characterization of pig slurry with reference to flocculation and separation. Water Res. 2009, 43, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Cassity-Duffey, K.; Cabrera, M.; Mowrer, J.; Kissel, D. Titration and Spectroscopic Measurements of Poultry Litter pH Buffering Capacity. J. Environ. Q. 2015, 44, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Costello, R.C.; Sullivan, D.M. Determining the pH Buffering Capacity of Compost Via Titration with Dilute Sulfuric Acid. Waste Biomass Valor 2014, 5, 505–513. [Google Scholar] [CrossRef]
- Cooper, P.; Cornforth, I.S. Volatile fatty acids in stored animal slurry. J. Sci. Food Agric. 1978, 29, 19–27. [Google Scholar] [CrossRef]
- Popovic, O.; Jensen, L.S. Storage temperature affects distribution of carbon, VFA, ammonia, phosphorus, copper and zinc in raw pig slurry and its separated liquid fraction. Water Res. 2012, 46, 3849–3858. [Google Scholar] [CrossRef]
- Conn, K.L.; Tenuta, M.; Lazarovits, G. Liquid Swine Manure Can Kill Verticillium dahliae Microsclerotia in Soil by Volatile Fatty Acid, Nitrous Acid, and Ammonia Toxicity. Phytopathology 2005, 95, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Sommer, S.G.; Sherlock, R.R. pH and buffer component dynamics in the surface layers of animal slurries. J. Agric. Sci. 1996, 127, 109–116. [Google Scholar] [CrossRef]
- Miller, D.N.; Varel, V.H. Swine manure composition affects the biochemical origins, composition, and accumulation of odorous compounds. J. Anim. Sci. 2003, 81, 2131–2138. [Google Scholar] [CrossRef]
- Miller, D.N.; Varel, V.H. In vitro study of the biochemical origin and production limits of odorous compounds in cattle feedlots. J. Anim. Sci. 2001, 79, 2949–2956. [Google Scholar] [CrossRef]
- Petersen, S.O.; Lind, A.-M.; Sommer, S.G. Nitrogen and organic matter losses during storage of cattle and pig manure. J. Agric. Sci. 1998, 130, 69–79. [Google Scholar] [CrossRef]
- Møller, H.B.; Sommer, S.G.; Ahring, B.K. Separation efficiency and particle size distribution in relation to manure type and storage conditions. Bioresour. Technol. 2002, 85, 189–196. [Google Scholar] [CrossRef]
- Sommer, S.G.; Clough, T.J.; Balaine, N.; Hafner, S.D.; Cameron, K.C. Transformation of Organic Matter and the Emissions of Methane and Ammonia during Storage of Liquid Manure as Affected by Acidification. J. Environ. Q. 2017, 46, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.G.; Husted, S. The chemical buffer system in raw and digested animal slurry. J. Agric. Sci. 1995, 124, 45–53. [Google Scholar] [CrossRef]
- Sommer, S.G.; Husted, S. A simple model of pH in slurry. J. Agric. Sci. 1995, 124, 447. [Google Scholar] [CrossRef]
- Stevens, R.J.; Cornforth, I.S. The effect of aeration on the gases produced by slurry during storage. J. Sci. Food Agric. 1974, 25, 1249–1261. [Google Scholar] [CrossRef]
- Eriksen, J.; Nørgaard, J.V.; Poulsen, H.D.; Poulsen, H.V.; Jensen, B.B.; Petersen, S.O. Effects of Acidifying Pig Diets on Emissions of Ammonia, Methane, and Sulfur from Slurry during Storage. J. Environ. Q. 2014, 43, 2086–2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommer, S.G.; Petersen, S.O.; Sørensen, P.; Poulsen, H.D.; Møller, H.B. Methane and carbon dioxide emissions and nitrogen turnover during liquid manure storage. Nutr. Cycl. Agroecosyst. 2007, 78, 27–36. [Google Scholar] [CrossRef]
- Aguerre, M.J.; Wattiaux, M.A.; Hunt, T.; Lobos, N.E. Effect of nitrogen content and additional straw on changes in chemical composition, volatile losses, and ammonia emissions from dairy manure during long-term storage. J. Dairy Sci. 2012, 95, 3454–3466. [Google Scholar] [CrossRef] [Green Version]
- Hobson, P.N.; Bousfield, S.; Summers, R.; Kirsch, E.J. Anaerobic digestion of organic matter. Crit. Rev. Environ. Sci. Technol. 1974, 4, 131–191. [Google Scholar] [CrossRef]
- Canh, T.T.; Sutton, A.L.; Aarnink, A.J.; Verstegen, M.W.; Schrama, J.W.; Bakker, G.C. Dietary carbohydrates alter the fecal composition and pH and the ammonia emission from slurry of growing pigs. J. Anim. Sci. 1998, 76, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- McGill, A.E.J.; Jackson, N. Changes in the short-chain carboxylic acid content and chemical oxygen demand of stored pig slurry. J. Sci. Food Agric. 1977, 28, 424–430. [Google Scholar] [CrossRef]
- Dinuccio, E.; Berg, W.; Balsari, P. Gaseous emissions from the storage of untreated slurries and the fractions obtained after mechanical separation. Atmos. Environ. 2008, 42, 2448–2459. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Karring, H. A determination and comparison of urease activity in feces and fresh manure from pig and cattle in relation to ammonia production and pH changes. PLoS ONE 2014, 9, e110402. [Google Scholar] [CrossRef] [PubMed]

| Fattening Pig | Dairy Cow | Sow | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week and Storage Conditions | 0 | 8 Cold | 8 Warm | 0 | 8 Cold | 8 Warm | 0 | 8 Cold | 8 Warm | |
| Ingredients 1 | ||||||||||
| Dry residue | % | 8.30 | 8.27 | 7.23 | 9.80 | 10.03 | 8.90 | 2.50 | 2.37 | 2.20 |
| N | kg m−3 | 4.81 | 4.92 | 5.02 | 4.20 | 4.22 | 4.11 | 5.37 | 5.41 | 5.03 |
| NH4-N | kg m−3 | 2.88 | 2.99 | 3.17 | 2.42 | 2.37 | 2.51 | 4.97 | 4.20 | 4.37 |
| P2O5 | kg m−3 | 2.56 | 2.94 | 3.36 | 1.16 | 1.53 | 1.58 | 0.92 | 0.87 | 0.93 |
| K2O | kg m−3 | 4.14 | 4.72 | 5.03 | 4.14 | 5.31 | 5.36 | 1.70 | 1.72 | 1.78 |
| Acetic acid | g kg−1 | 7.00 | 5.83 | 2.67 | 6.20 | 5.83 | 6.87 | 2.70 | 2.77 | 2.50 |
| Propionic acid | g kg−1 | 1.60 | 1.53 | 2.17 | 1.20 | 1.17 | 1.97 | 0.14 | 0.15 | 0.29 |
| Acetic acid equivalent 2 | g kg−1 | 9.30 | 8.10 | 4.90 | 7.70 | 7.27 | 9.03 | 2.90 | 3.00 | 2.93 |
| TAN | kg N m−3 | 2.50 | 2.35 | 3.33 | 2.48 | 2.31 | 2.96 | 4.80 | 4.88 | 4.71 |
| TIC | kg C m−3 | 1.48 | 1.48 | 1.99 | 1.43 | 1.31 | 1.33 | 1.06 | 1.45 | 1.89 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Overmeyer, V.; Holtkamp, F.; Clemens, J.; Büscher, W.; Trimborn, M. Dynamics of Different Buffer Systems in Slurries Based on Time and Temperature of Storage and Their Visualization by a New Mathematical Tool. Animals 2020, 10, 724. https://doi.org/10.3390/ani10040724
Overmeyer V, Holtkamp F, Clemens J, Büscher W, Trimborn M. Dynamics of Different Buffer Systems in Slurries Based on Time and Temperature of Storage and Their Visualization by a New Mathematical Tool. Animals. 2020; 10(4):724. https://doi.org/10.3390/ani10040724
Chicago/Turabian StyleOvermeyer, Veronika, Felix Holtkamp, Joachim Clemens, Wolfgang Büscher, and Manfred Trimborn. 2020. "Dynamics of Different Buffer Systems in Slurries Based on Time and Temperature of Storage and Their Visualization by a New Mathematical Tool" Animals 10, no. 4: 724. https://doi.org/10.3390/ani10040724
APA StyleOvermeyer, V., Holtkamp, F., Clemens, J., Büscher, W., & Trimborn, M. (2020). Dynamics of Different Buffer Systems in Slurries Based on Time and Temperature of Storage and Their Visualization by a New Mathematical Tool. Animals, 10(4), 724. https://doi.org/10.3390/ani10040724