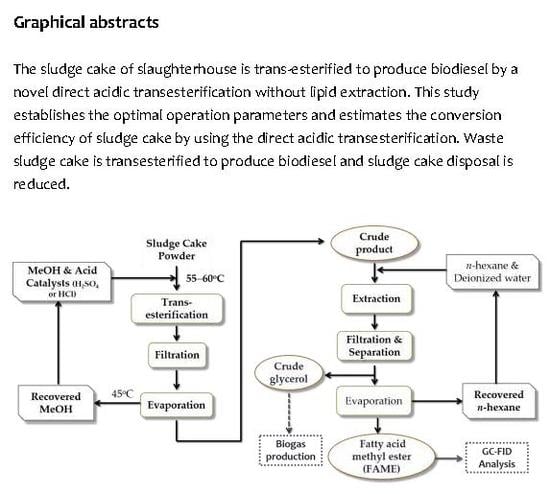
Biodiesel Production by Acid Methanolysis of Slaughterhouse Sludge Cake
Simple Summary
Abstract
1. Introduction
2. Material and Methods
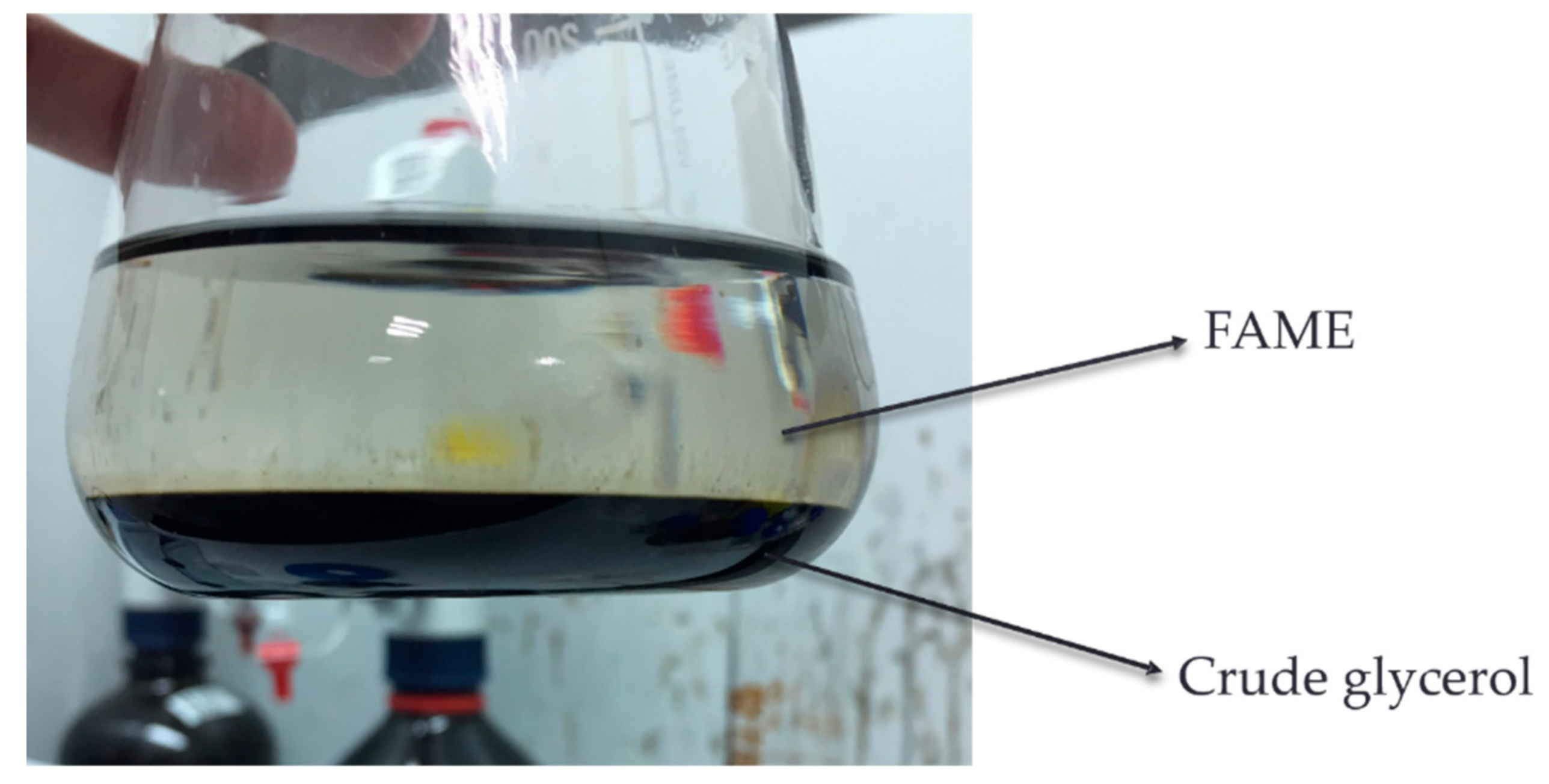
2.1. Feedstock for Acid Methanolysis
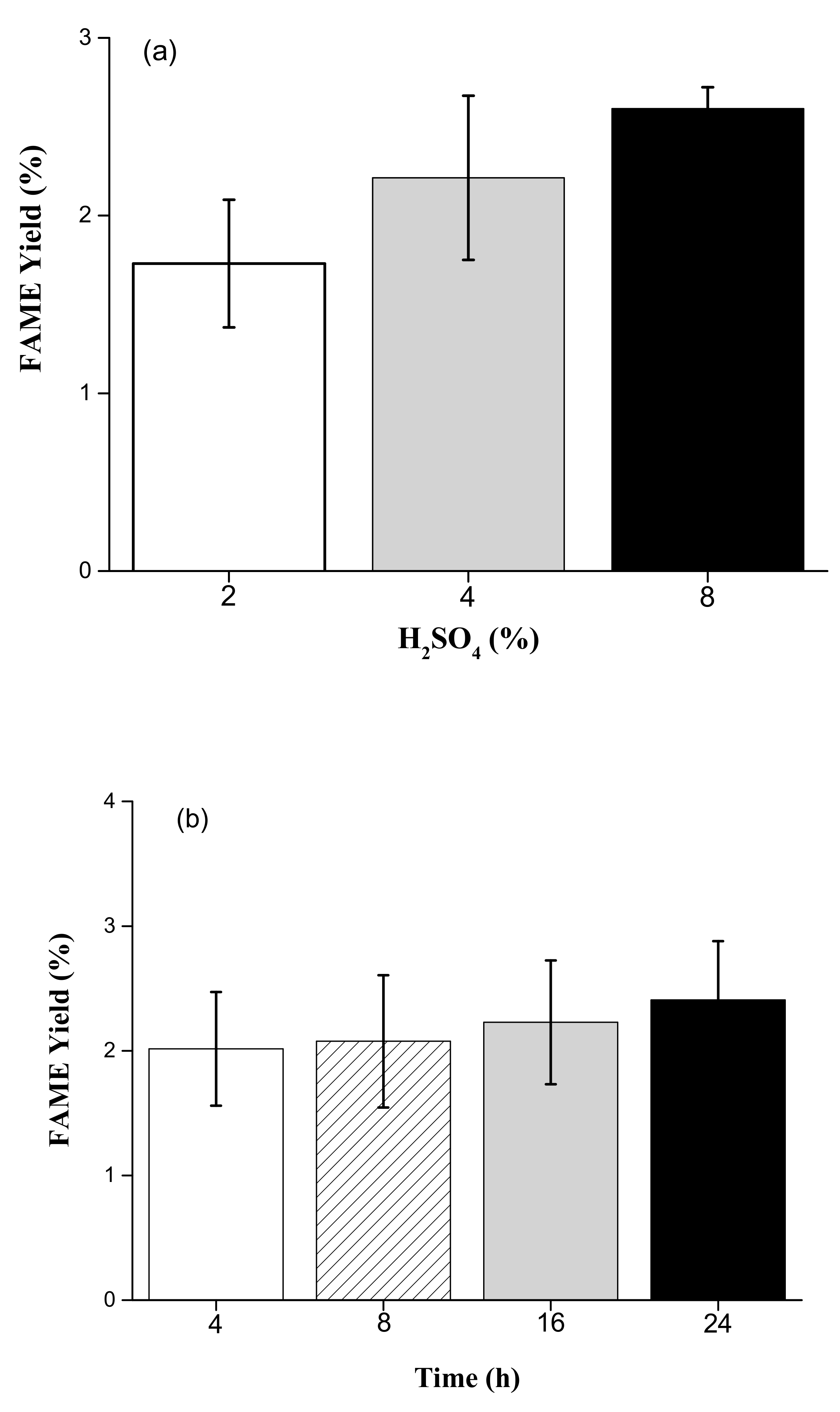
2.2. Preliminary Test for Optimal Concentrations of Acid and Reaction Time Based on Accumulated FAME Yield
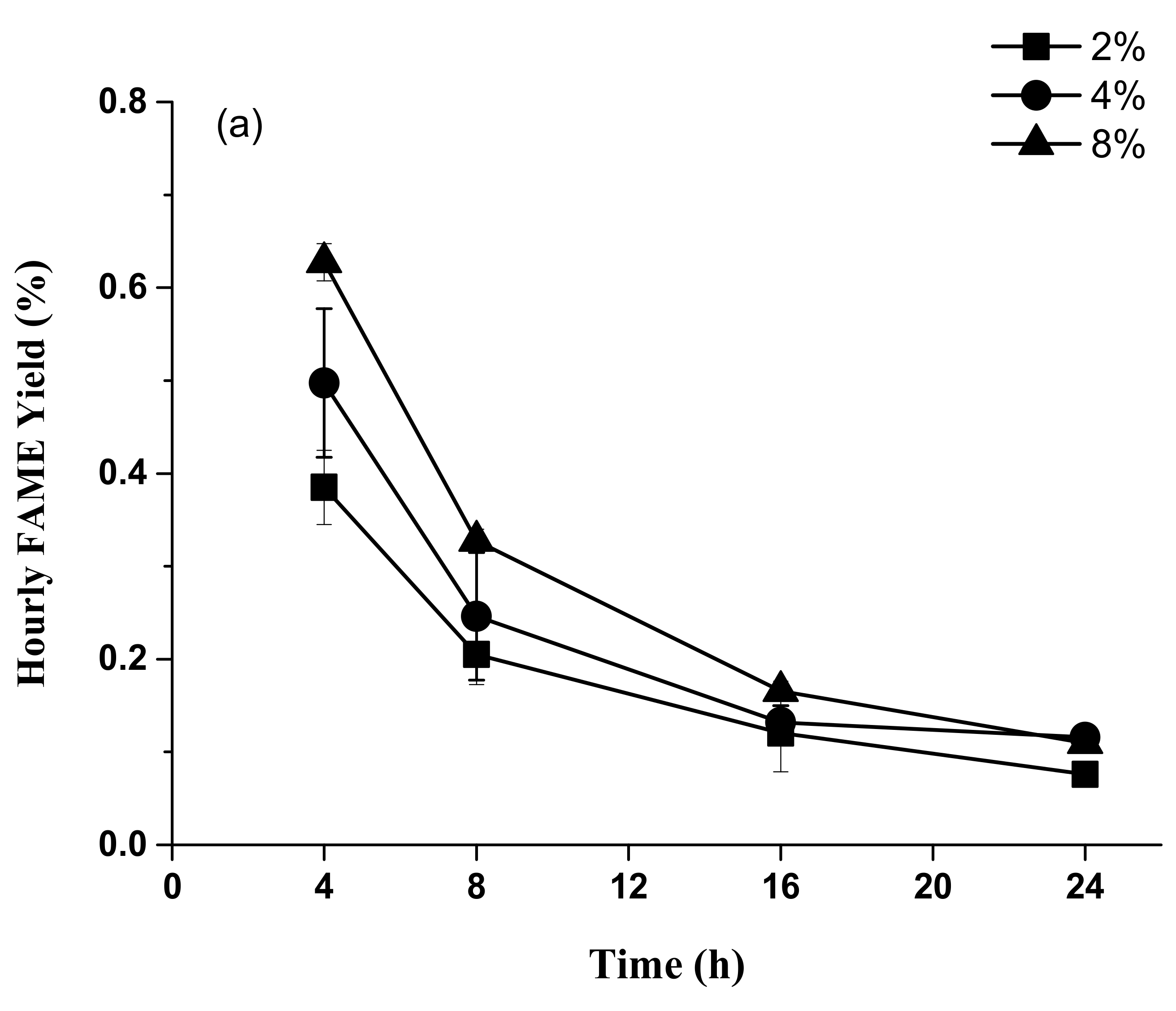
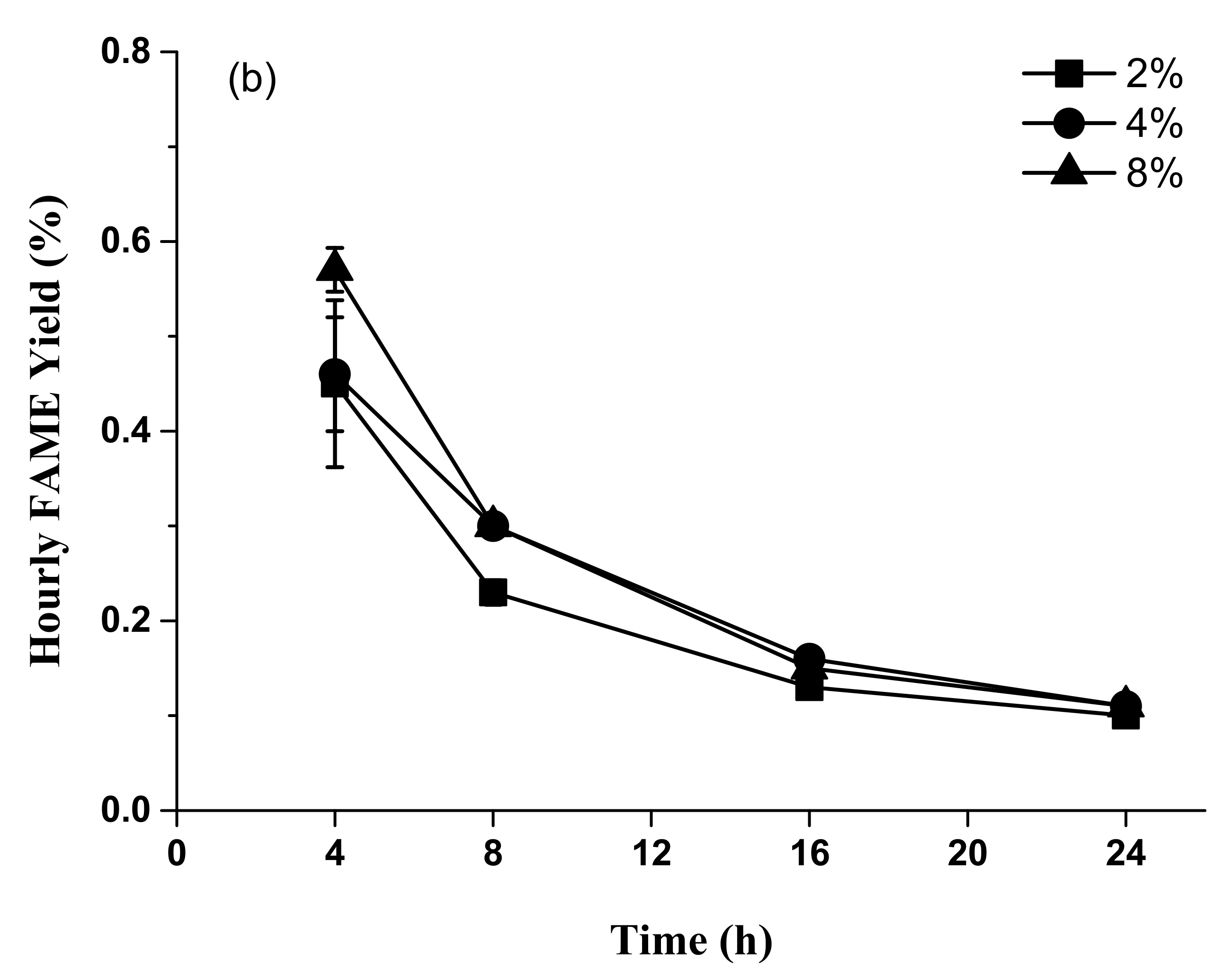
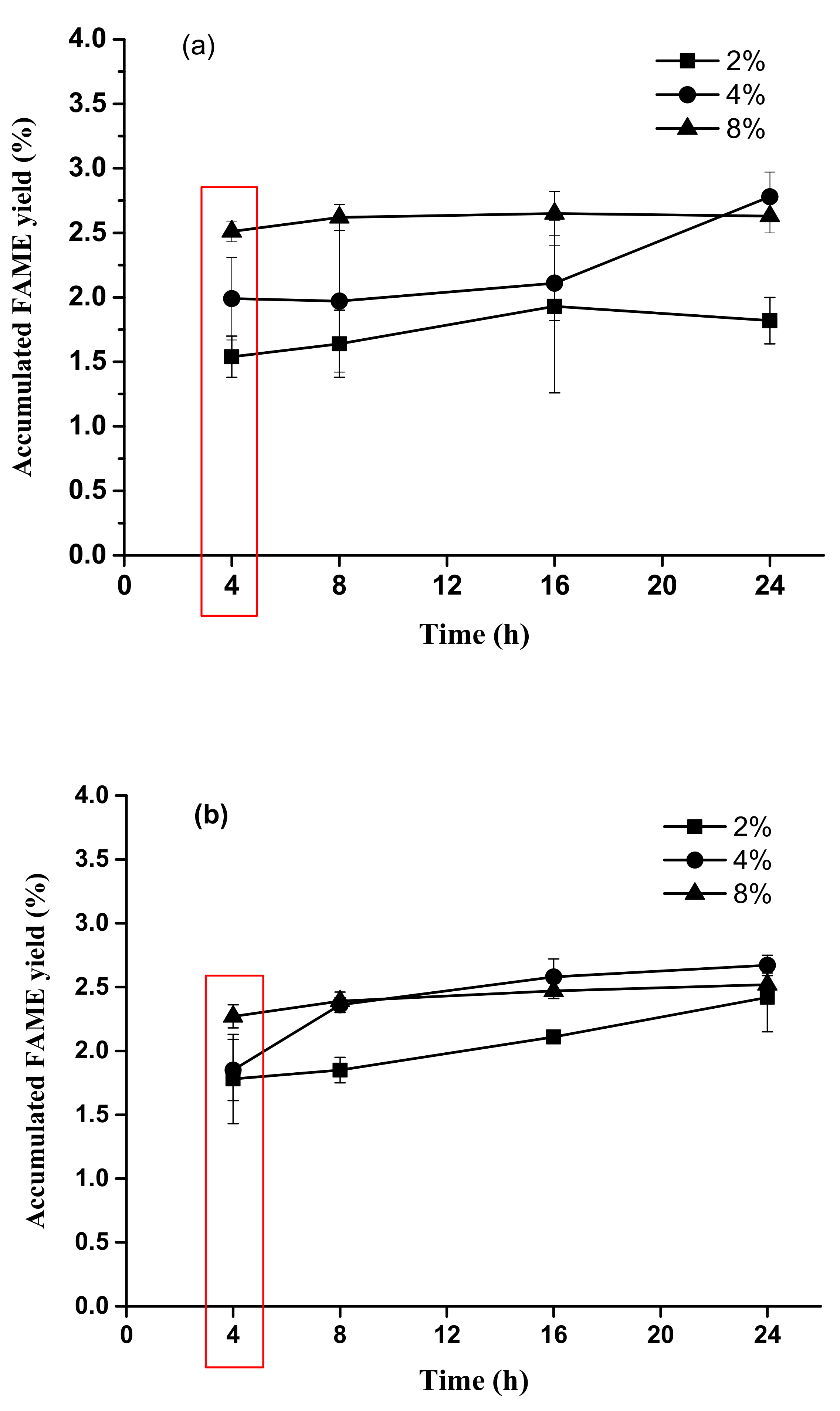
2.3. Time Course Experiments for Accumulated FAME Yield from Acid Methanolysis of Sludge Cake Powder
2.4. Qualitative and Quantitative Analysis of Fatty Acid Methyl Ester (FAME)
- F: Total amount of the FAME from the acid methanolysis samples (µg/mL)
- H: Amount of n-hexane used for each batch (75 mL)
- S: Amount of dried sludge cake powder for each sampling (5000 mg)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Slaughterhouse Sludge Cake
3.2. Preliminary Test for Optimal Concentrations of H2SO4 and Reaction Time Based on Accumulated FAME Yield
3.3. Acid Methanolysis of Sludge Cake with Different Concentrations of Sulfuric Acid and Hydrochloric Acid
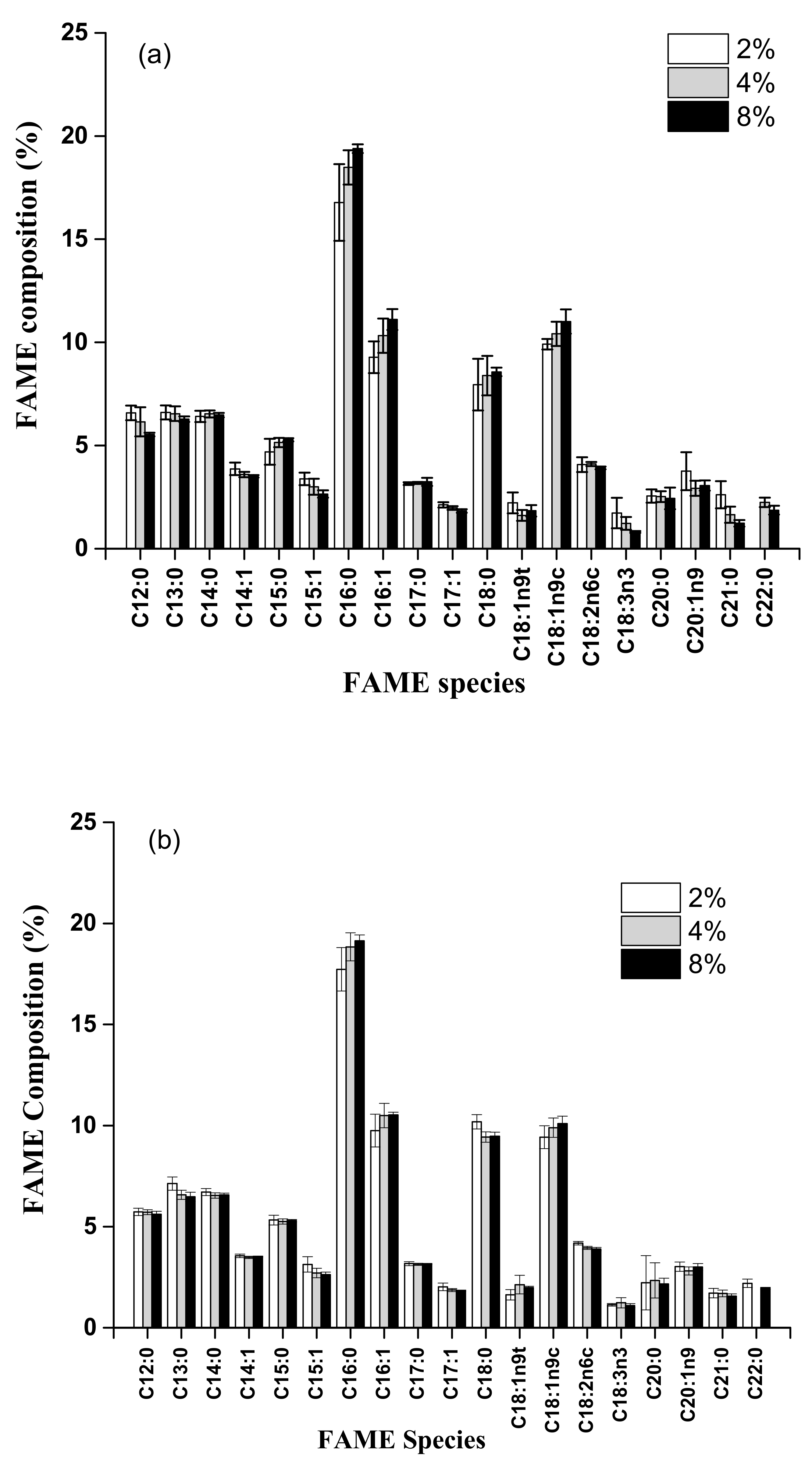
3.4. Comparison of FAME Components from Acid Methanolysis of Sludge Cake with Different Concentrations of H2SO4 and HCl
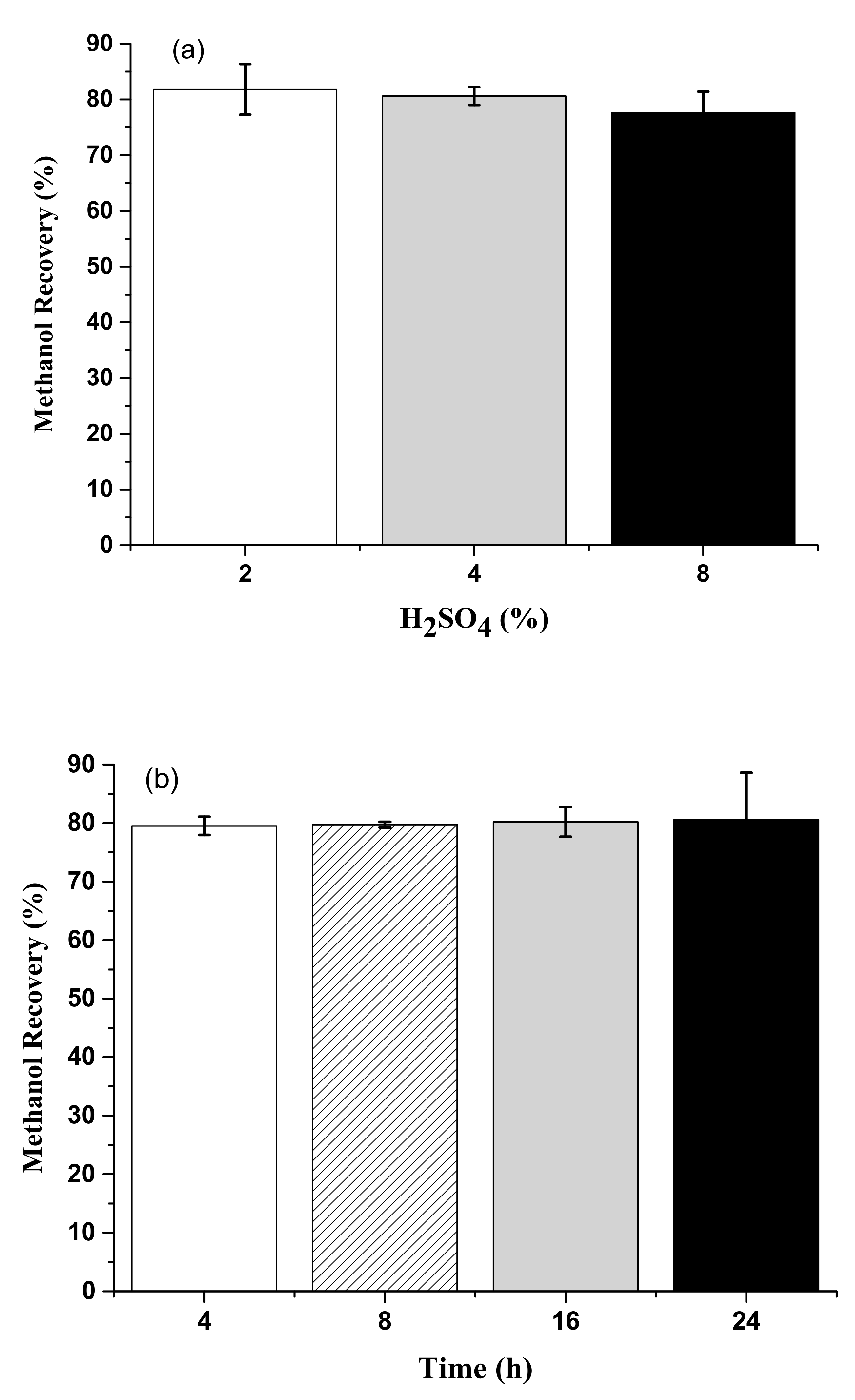
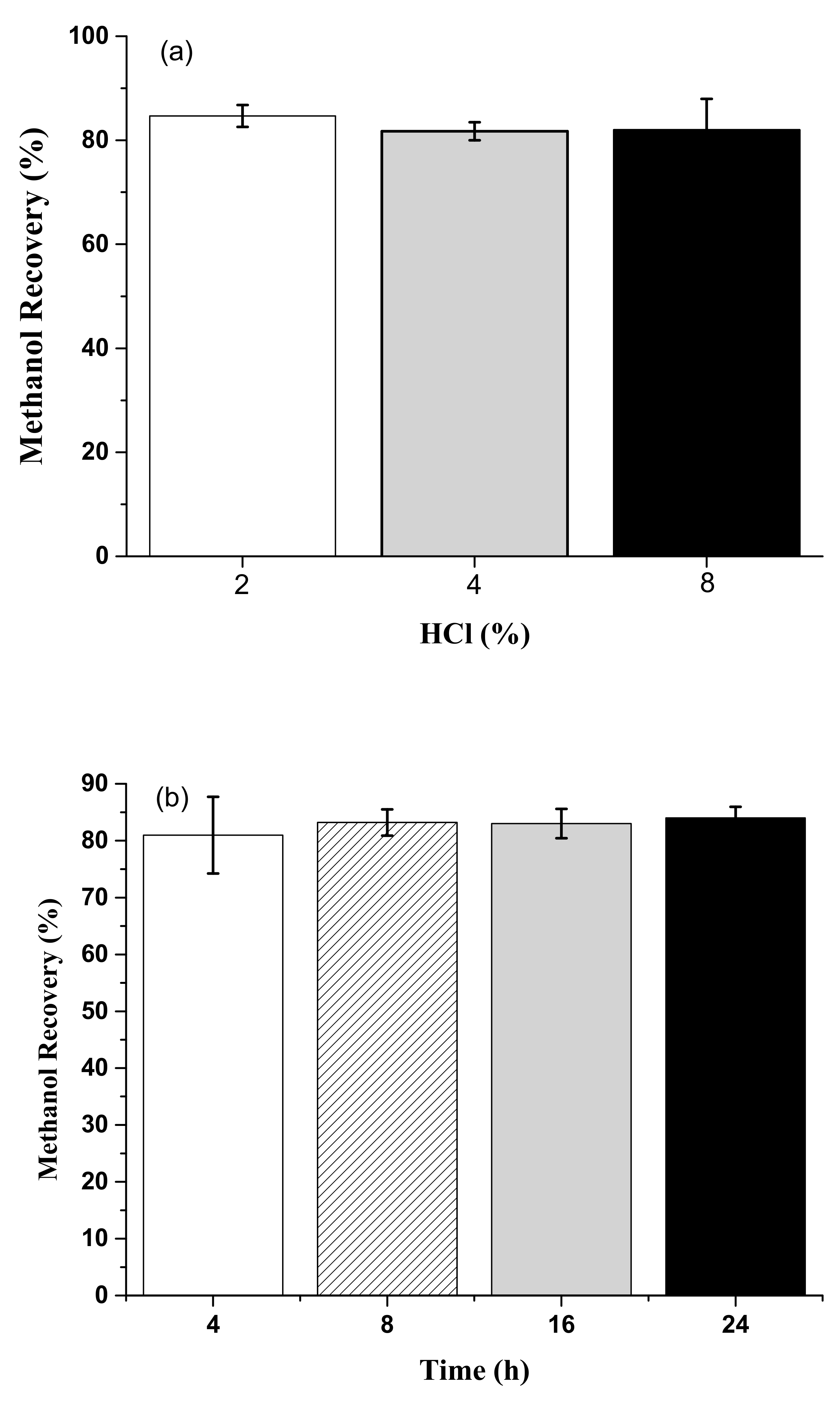
3.5. Methanol Recovery from Acid Methanolysis of Sludge Cake with Different Concentrations of Sulfuric Acid and Hydrochloric Acid
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DGBAS. Green National Income. Directorate-General of Budget, Accounting and Statistics (DGBAS), Executive Yuan, Taiwan, ROC; 2017. Available online: https://ebook.dgbas.gov.tw/public/Data/9191653488VPAUQXD.pdf (accessed on 22 September 2019). (In Chinese)
- Kroiss, H. What is the potential for utilizing the resources in sludge? Water Sci. Technol. 2004, 49, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dufreche, S.; Hernandez, R.; French, T.; Sparks, D.; Zappi, M.; Alley, E. Extraction of lipids from municipal wastewater plant microorganisms for production of biodiesel. J. Am. Oil Chem. Soc. 2007, 84, 181–187. [Google Scholar] [CrossRef]
- You, Y.D.; Shie, J.L.; Chang, C.Y.; Huang, S.H.; Pai, C.Y.; Yu, Y.H.; Chang, C. Economic cost analysis of biodiesel production: Case in soybean oil. Energ. Fuels 2008, 22, 182–189. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Mondala, A.; Liang, K.; Toghiani, H.; Hernandez, R.; French, T. Biodiesel production by in situ transesterification of municipal primary and secondary sludges. Bioresour. Technol. 2009, 100, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Revellame, E.; Hernandez, R.; French, W.; Holmes, W.; Alley, E. Biodiesel from activated sludge through in situ transesterification. J. Chem. Technol. Biotechnol. 2010, 85, 614–620. [Google Scholar] [CrossRef]
- Wang, F.H.; Wang, H.J.; Hu, H.; Zeng, R.J. Applying rheological analysis to understand the mechanism of polyacrylamide (PAM) conditioning for sewage sludge dewatering. RSC Adv. 2017, 7, 30274–30282. [Google Scholar] [CrossRef]
- Pastore, C.; Lopez, A.; Lotito, V.; Mascolo, G. Biodiesel from dewatered wastewater sludge: A two-step process for a more advantageous production. Chemosphere 2013, 92, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Olkiewicz, O.; Fortuny, A.; Stüber, F.; Fabregat, A.; Font, J.; Bengoa, C. Evaluation of different sludges from WWTP as a potential source for biodiesel production. Procedia Eng. 2012, 42, 634–643. [Google Scholar] [CrossRef]
- di Bitonto, L.; Lopez, A.; Mascolo, G.; Mininni, G.; Pastore, C. Efficient solvent-less separation of lipids from municipal wet sewage scum and their sustainable conversion into biodiesel. Renew. Energ. 2016, 90, 55–61. [Google Scholar] [CrossRef]
- Pastore, C.; Lopez, A.; Mascolo, G. Efficient conversion of brown grease produced by municipal wastewater treatment plant into biofuel using aluminum chlorine hexahydrate under very mild conditions. Bioresour. Technol. 2014, 155, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-Y.; Yoon, Y.-M. Energy recovery efficiency of poultry slaughterhouse sludge cake by hydrothermal carbonization. Energies 2017, 10, 1876. [Google Scholar] [CrossRef]
- Choi, O.K.; Song, J.S.; Cha, D.K.; Lee, J.W. Biodiesel production from wet municipal sludge: Evaluation of in situ transesterification using xylene as a cosolvent. Bioresour. Technol. 2014, 166, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy. 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Chou, Y.C.; Su, J.J. Biogas production by anaerobic co-digestion of dairy wastewater with the crude glycerol from slaughterhouse sludge cake transesterification. Animals 2019, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Revellame, E.; Hernandez, R.; French, W.; Holmes, W.; Alley, E.; Robert, C. Production of biodiesel from wet activated sludge. J. Chem. Technol. Biotechnol. 2011, 86, 61–68. [Google Scholar] [CrossRef]

| Sources of Sludge | Catalysts & Concentrations | Addition of MeOH (mL/g) | Reaction Temp. (°C) | Reaction Time (h) | Accumulated FAME Yield (%, w/w) | References |
|---|---|---|---|---|---|---|
| Municipal wastewater treatment plant (secondary sludge) | H2SO4 (1%) | 5 | 50 | 24 | 6.23 | Dufreche et al. [3] |
| Municipal wastewater treatment plant (primary sludge) | H2SO4 (5%) | 12 | 75 | 8 | 14.5 | Mondala et al. [6] |
| Municipal wastewater treatment plant (secondary sludge) | H2SO4 (5%) | 12 | 75 | 8 | 2.5 | |
| Municipal wastewater treatment plant (secondary sludge) | H2SO4 (4%) | 30 | 55 | 24 | 4.79 | Revellame et al. [7] |
| Municipal wastewater treatment plant (secondary sludge) | H2SO4 (10%) | 30 | 75 | 24 | 3.93 | Revellame et al. [17] |
| Slaughterhouse sludge cake | H2SO4 (8%) | 25 | 55 | 4 | 2.51 | This study (Su and Chou) |
| HCl (8%) | 25 | 55 | 4 | 2.27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.-J.; Chou, Y.-C. Biodiesel Production by Acid Methanolysis of Slaughterhouse Sludge Cake. Animals 2019, 9, 1029. https://doi.org/10.3390/ani9121029
Su J-J, Chou Y-C. Biodiesel Production by Acid Methanolysis of Slaughterhouse Sludge Cake. Animals. 2019; 9(12):1029. https://doi.org/10.3390/ani9121029
Chicago/Turabian StyleSu, Jung-Jeng, and Yu-Chun Chou. 2019. "Biodiesel Production by Acid Methanolysis of Slaughterhouse Sludge Cake" Animals 9, no. 12: 1029. https://doi.org/10.3390/ani9121029
APA StyleSu, J.-J., & Chou, Y.-C. (2019). Biodiesel Production by Acid Methanolysis of Slaughterhouse Sludge Cake. Animals, 9(12), 1029. https://doi.org/10.3390/ani9121029