Influence of Low Dietary Inclusion of the Microalga Nannochloropsis gaditana (Lubián 1982) on Performance, Fish Morphology, and Muscle Growth in Juvenile Gilthead Seabream (Sparus aurata)
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Management
2.2. Experimental Diets
2.3. Sampling
2.4. Analysis of the Morphological Parameters
2.5. Quantitative Analysis of Muscle Growth
2.6. Statistical Analysis
3. Results
3.1. Body Growth Parameters and Survival
3.2. Fish Morphology Parameters
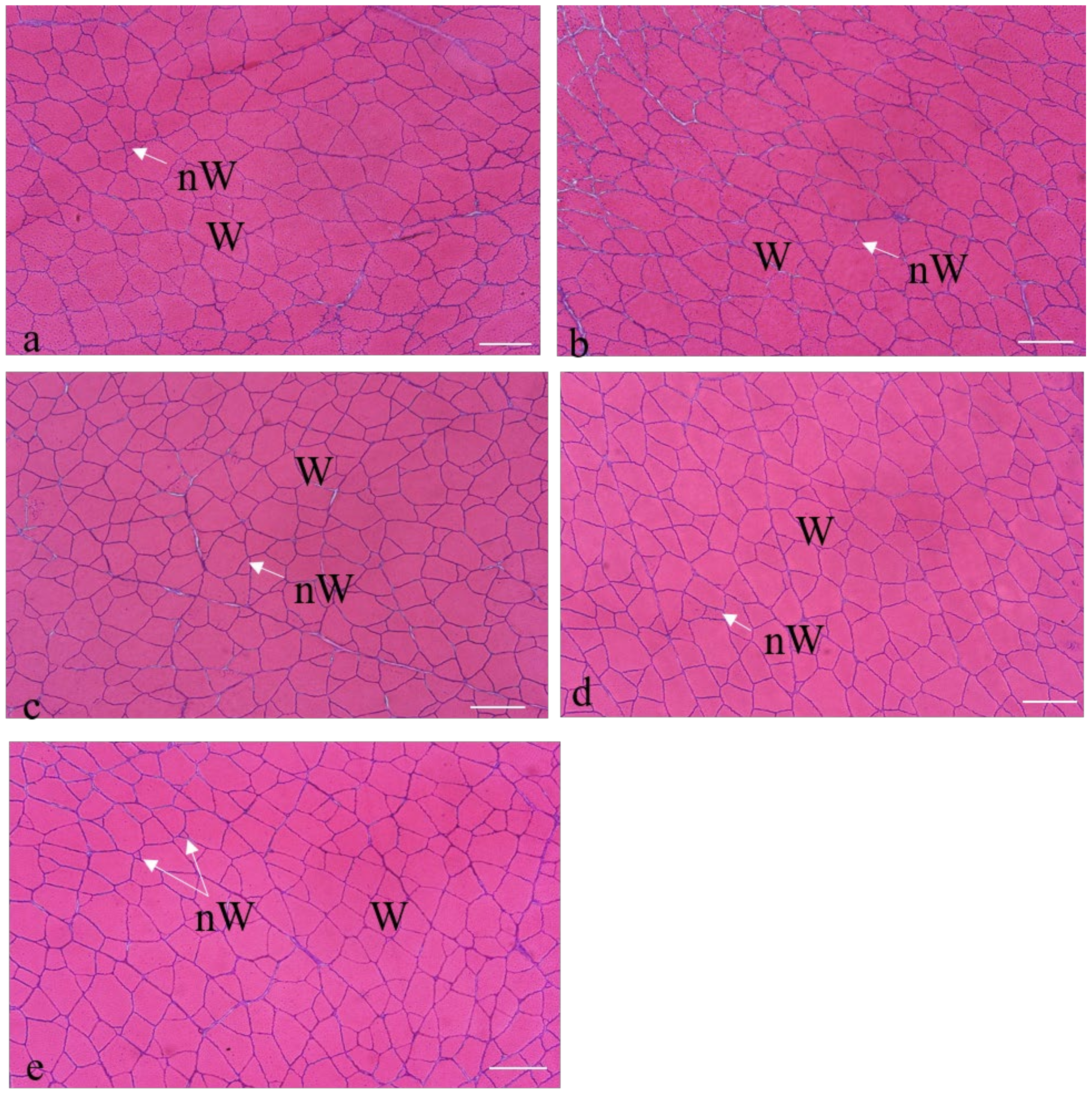
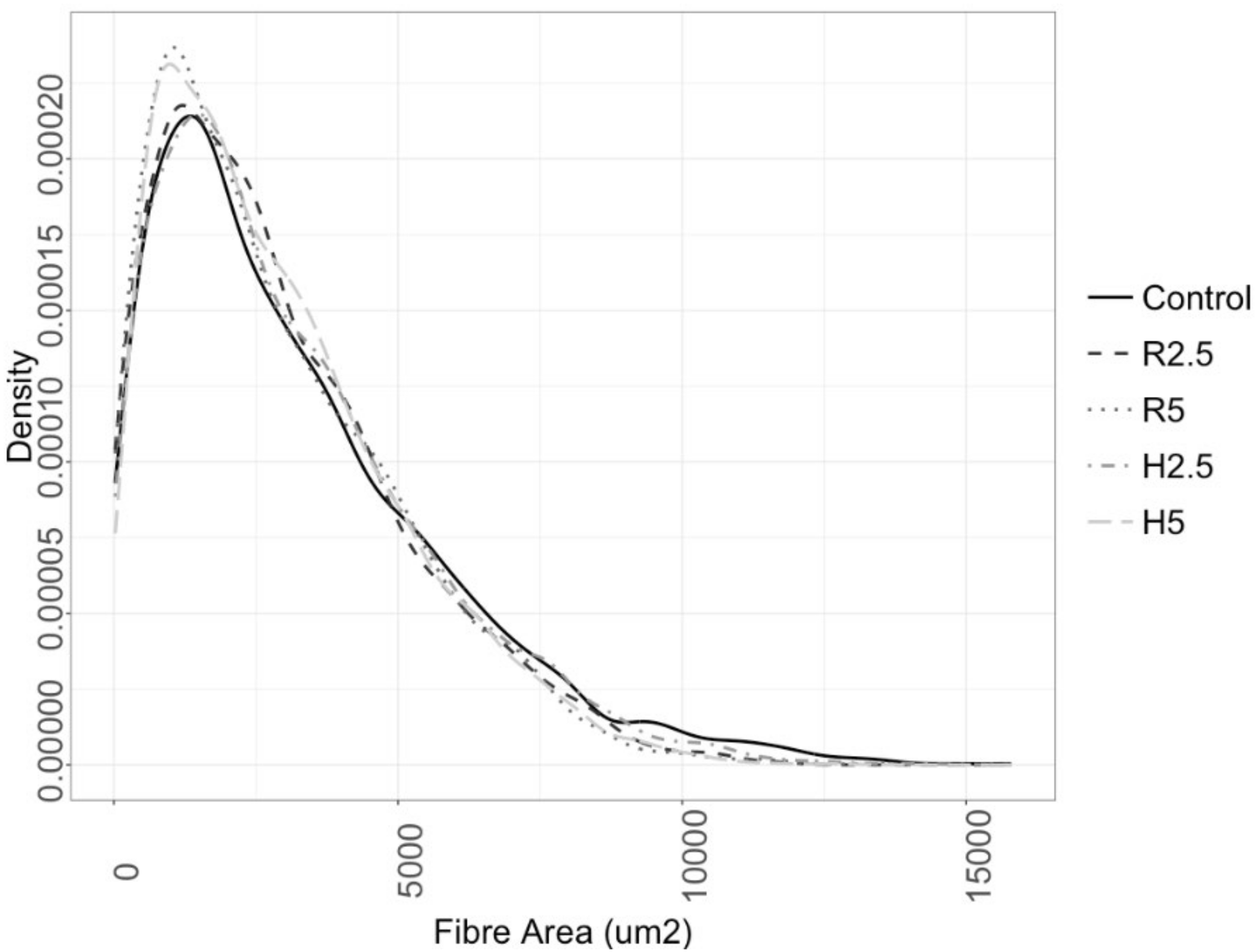
3.3. Muscle Growth
4. Discussion
4.1. Influence of the Diet on the Body Growth Parameters, Fish Morphology, and Survival
4.2. Influence of the Diet on the Muscle Growth
5. Conclusions
- 1.
- The highest body values were reached in the R2.5 group, which seems to indicate that the lowest level of inclusion of raw N. gaditana (2.5%) is sufficient to obtain adequate growth of juvenile gilthead seabream.
- 2.
- The inclusion of N. gaditana in juvenile gilthead seabream diets accelerated the acquisition of a discoid body shape in an inclusion level-dependent trend, but without affecting the total growth, since the conversion rates, growth rates and daily intake were similar in all groups.
- 3.
- The inclusion of hydrolyzed microalgae in gilthead seabream diets at 5% level affected the geometric morphology of fish compared to raw microalgae at the same inclusion level.
- 4.
- The muscle plasticity of gilthead seabream gave rise to differences in the muscular constitution among the groups, with the highest hyperplasia values in C group, and the highest hypertrophy values in R2.5 group. The groups with higher levels of microalgae inclusion (R5 and H5) showed a higher percentage of small and medium-sized fibers, which may indicate a greater potential for growth in later stages of cultivation. Long-term studies are now necessary to check the effects on subsequent stages of growth.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Knutsen, H.R.; Johnsen, I.H.; Keizer, S.; Sorensen, M.; Roques, J.A.C.; Heden, I.; Sundell, K. Fish welfare, fast muscle cellularity, fatty acid and body-composition of juvenile spotted wolffish (Anarhichas minor) fed a combination of plant proteins and microalgae (Nannochloropsis oceanica). Aquaculture 2019, 506, 212–223. [Google Scholar] [CrossRef]
- Gatlin, D.M.; Barrows, F.T.; Brown, P.; Dabrowski, K.; Gaylord, T.G.; Hardy, R.W.; Herman, E.; Hu, G.; Krogdahl, Å.; Nelson, R.; et al. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquac. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Hardy, R.W. Utilization of plant proteins in fish diets: Effects of global demand and supplies of fishmeal. Aquac. Res. 2010, 41, 770–776. [Google Scholar] [CrossRef]
- Bakke, A.M.; Chikwati, E.; Venold, F.F.; Sahlmann, C.; Holm, H.; Penn, M.H.; Oropeza-Moe, M.; Krogdahl, Å. Bile enhances glucose uptake, reduces permeability, and modulates effects of lectins, trypsin inhibitors and saponins on intestinal tissue. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 168, 96–109. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Penn, M.; Thorsen, J.; Refstie, S.; Bakke, A.M. Important antinutrients in plant feedstuffs for aquaculture: An update on recent findings regarding responses in salmonids. Aquac. Res. 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Marjara, I.S.; Chikwati, E.M.; Valen, E.C.; Krogdahl, Å.; Bakke, A.M. Transcriptional regulation of IL-17A and other inflammatory markers during the development of soybean meal-induced enteropathy in the distal intestine of Atlantic salmon (Salmo salar L.). Cytokine 2012, 60, 186–196. [Google Scholar] [CrossRef]
- De Visser, C.; Schreuder, R.; Stoddard, F. The EU’s dependences on soya bean import for the animal feed industry and potential for EU produced alternatives. Oilseeds Fats Crops Lipids 2014, 21, 8. [Google Scholar]
- Sen Roy, S.; Pal, R. Microalgae in aquaculture: A review with special references to nutritional value and fish dietetics. Proc. Zool. Soc. 2015, 68, 1–8. [Google Scholar]
- Brown, M.R.; Jeffrey, S.W.; Volkman, J.K.; Dunstan, J.A. Nutritional properties of microalgae for mariculture. Aquaculture 1997, 151, 315–331. [Google Scholar] [CrossRef]
- Olvera-Novoa, M.A.; Domínguez-Cen, L.J.; Olivera-Castillo, L.; Martínez-Palacios, C.A. Effect of the use of the microalga Spirulina maxima as fish meal replacement in diets for tilapia, Oreochromis mossambicus (Peters), fry. Aquac. Res. 1998, 29, 709–715. [Google Scholar] [CrossRef]
- Atalah, E.; Cruz, C.M.H.; Izquierdo, M.S.; Rosenlund, G.; Caballero, M.J.; Valencia, A.; Robaina, L. Two microalgae Crypthecodinium cohnii and Phaeodactylum tricornutum as alternative source of essential fatty acids in starter feeds for seabream (Sparus aurata). Aquaculture 2007, 270, 178–185. [Google Scholar] [CrossRef]
- Tulli, F.; Chini Zittelli, G.; Giorgi, G.; Poli, B.M.; Tibaldi, E.; Tredici, M.R. Effect of the inclusion of dried Tetraselmis suecica on growth, feed utilization and fillet composition of European sea bass juveniles fed organic diets. J. Aquat. Food Prod. Technol. 2012, 21, 188–197. [Google Scholar] [CrossRef]
- Tibaldi, E.; ChiniZittelli, G.; Parisi, G.; Bruno, M.; Giorgi, G.; Tulli, F.; Venturini, S.; Tredici, M.R.; Poli, B.M. Growth performance and quality traits of European sea bass (D. labrax) fed diets including increasing levels of freez-dried Isochrysis sp. (T-ISO) biomass as a source protein and n-3 long chain PUFA in partial substitution of fish derivatives. Aquaculture 2015, 440, 60–66. [Google Scholar] [CrossRef]
- Vizcaíno, A.; López, G.; Sáez, M.; Jiménez, J.; Barros, A.; Hidalgo, L.; Camacho-Rodríguez, J.; Martínez, T.; Cerón-García, M.; Alarcón, F. Effects of the microalga Scenedesmus almerienses as fishmeal alternative in diets for gilthead sea bream, Sparus aurata, juveniles. Aquaculture 2014, 431, 34–43. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Saéz, M.I.; López, G.; Arizcun, M.; Abellán, E.; Martínez, T.F.; Cerón-García, M.C.; Alarcón, F.J. Tetraselmis suecia and Tisochrysis lutea meal as dietary ingredients for gilthead sea bream (Sparus aurata L.) fry. J. Appl. Phycol. 2016, 28, 2843–2855. [Google Scholar] [CrossRef]
- Otero, A.; García, D.; Fabregas, J. Factors controlling eico-sapentaenoic acid production in semicontinuous cultures of marine microalgae. J. Appl. Phycol. 1997, 9, 465–469. [Google Scholar] [CrossRef]
- Brown, M.; Mular, M.; Miller, I.; Farmer, C.; Trenerry, C. The vitamin content of microalgae used in aquaculture. J. Appl. Phycol. 1999, 11, 247–255. [Google Scholar] [CrossRef]
- Fawley, K.P. Observations on the Diversity and Ecology of Freshwater Nannochloropsis (Eustigmatophyceae), with Descriptions of New Taxa. Protist 2007, 158, 325–336. [Google Scholar] [CrossRef]
- Sørensen, M.; Gong, Y.; Bjarnason, F.; Vasanth, G.K.; Dahle, D.; Huntley, M.; Kiron, V. Nannochloropsis oceanica-derived defatted meal as an alternative to fishmeal in Atlantic salmon feeds. PLoS ONE 2017, 12, e0179907. [Google Scholar] [CrossRef]
- Gong, Y.; Guterres, H.A.D.S.; Huntley, M.; Sørensen, M.; Kiron, V. Digestibility of the defatted microalgae Nannochloropsis sp. and Desmodesmus sp. when fed to Atlantic salmon. Salmo Salar. Aquac. Nutr. 2018, 24, 56–64. [Google Scholar] [CrossRef]
- Gbadamosi, O.K.; Lupatsch, I. Effects of dietary Nannochloropsis salina on the nutritional performance and fatty acid profile of Nile tilapia, Oreochromis niloticus. Algal Res. 2018, 33, 48–54. [Google Scholar] [CrossRef]
- Weatherley, A.H.; Gill, H.S.; Rogers, S.C. The relationship between mosaic muscle fibres and size in rainbow trout (Salmo gairdneri). J. Fish Biol. 1980, 17, 603–610. [Google Scholar] [CrossRef]
- Weatherley, A.H.; Gill, H.S.; Lobo, A.F. Recruitment and maximal diameter of axial muscle fibres in teleosts and their relationship to somatic growth and ultimate size. J. Fish Biol. 1988, 33, 851–859. [Google Scholar] [CrossRef]
- Fauconneau, B.; Alami-Durante, H.; Laroche, M.; Marcel, J.; Vallot, D. Growth and meat quality relations in carp. Aquaculture 1995, 129, 265–297. [Google Scholar] [CrossRef]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric assay using o- phthaldialdehyde for determination of proteolysis in milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Sáez, M.I.; Acién, F.G.; Alarcón, F.J. Differential hydrolysis of proteins of four microalgae by the digestive enzymes of gilthead sea bream and Senegalese sole. Algal Res. 2019, 37, 145–153. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Sanchez-Jerez, P.; Bayle-Sempere, J.T.; Sfakianakis, D.G.; Somarakis, S. Morphological differences between wild and farmed Mediterranean fish. Hydrobiologia 2012, 679, 217–231. [Google Scholar] [CrossRef]
- Ayala, M.D.; Abellán, E.; Arizcun, M.; García-Alcázar, A.; Navarro, F.; Blanco, A.; López-Albors, A. Muscle development and body growth in larvae and early postlarvae of shi drum, Umbrina cirrosa L., reared under different larval photoperiod. Muscle structural and ultrastructural study. Fish Physiol. Biochem. 2013, 39, 807–827. [Google Scholar] [CrossRef]
- Ayala, M.D.; Arizcun, M.; García-Alcázar, A.; Santaella, M.; Abellán, E. Long-term effects of the larval photoperiod on the subsequent growth of shi drum Umbrina cirrosa L. specimens and the fillet texture at commercial size. Turk. J. Fish Aquat. 2015, 15, 93–101. [Google Scholar] [CrossRef]
- Teissier, G. Relative growth. In The Physiology of Crustacea; Waterman, T.H., Ed.; Academic Press: New York, NY, USA, 1960; pp. 537–560. [Google Scholar]
- Reyment, R. Reification of classical multivariate analyses in morphometry. In Proceedings of the Michigan Morphometrics Workshop, Ann Arbor, MI, USA, 16–28 May 1988; Rohlf, F.J., Bookstein, F.L., Eds.; Special Publication; University of Michigan, Museum of Zoology: Ann Arbor, MI, USA, 1990; pp. 123–144. [Google Scholar]
- Jolicoeur, P.J. The multivariate generalization of the allometric equation. Biometrics 1963, 19, 497–499. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Multivariate allometry. In Advances in Morphometrics; Marcus, L.F., Corti, M., Loy, A., Naylor, G.J.P., Slice, D.E., Eds.; NATO ASI Series A, Life Science; Springer: Dordrecht, The Netherlands, 1996; Volume 284, pp. 23–49. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA. 2015. Available online: http://www.rstudio.com/ (accessed on 1 September 2019).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Verhaegen, Y.; Adriaens, D.; De Wolf, T.; Dhert, P.; Sorgeloos, P. Deformities in larval gilthead sea bream (Sparus aurata): A qualitative and quantitative analysis using geometric morphometrics. Aquaculture 2007, 268, 156–168. [Google Scholar] [CrossRef]
- Russo, T.; Costa, C.; Cataudella, S. Correspondence between shape and feeding habit changes throughout ontogeny of gilthead sea bream Sparus aurata L., 1758. J. Fish Biol. 2007, 71, 629–656. [Google Scholar] [CrossRef]
- Luczkovich, J.; Motta, P.; Norton, S.; Liem, K. (Eds.) Ecomorphology of Fishes; Springer: Dordrecht, The Netherlands, 2013; ISBN 978-90-481-4620-8. [Google Scholar]
- Osse, J.W.M.; van den Boogaart, J.G.M. Fish larvae, development, allometric growth, and the aquatic environment. ICES Mar. Sci. Symp. 1995, 201, 21–34. [Google Scholar]
- Hjelm, J.; Van De Weerd, G.H.; Sibbing, F.A. Functional link between foraging performance, functional morphology, and diet shift in roach (Rutilus rutilus). Can. J. Fish. Aquat. Sci. 2003, 60, 700–709. [Google Scholar] [CrossRef]
- Hjelm, J.; Svanbäck, R.; Byström, P.; Persson, L.; Wahlström, E. Diet-dependent body morphology and ontogenetic reaction norms in eurasian perch. Oikos 2001, 95, 311–323. [Google Scholar] [CrossRef]
- Rincón, D.; Velásquez, H.; Dávila, M.; Semprun, A.; Morales, E.; Hernández, J. Substitution levels of fish meal by Arthrospira (=Spirulina) maxima meal in experimental diets for red tilapia fingerlings (Oreochromis sp.). Rev. Colomb. Cienc. Pecu. 2012, 25, 430–437. [Google Scholar]
- Hussein, E.; Dabrowski, K.; El-Saidy, D.; Lee, B.J. Enhancing the growth of Nile tilapia larvae/juveniles by replacing plant (gluten) protein with algae protein. Aquac. Res. 2012, 44, 937–949. [Google Scholar] [CrossRef]
- Kim, S.; Rahimnejad, S.; Kim, K.; Lee, K. Partial replacement of fish meal with Spirulina pacifica in diets for Parrot fish (Oplegnathus fasciatus). Turk. J. Fish. Aquat. Sci. 2013, 13, 197–204. [Google Scholar] [CrossRef]
- Teimouri, M.; Amirkolaie, A.; Yeganeh, S. The effects of dietary supplement of Spirulina platensis on blood carotenoid concentration and fillet color stability in rainbow trout (Oncorhynchus mykiss). Aquaculture 2013, 414, 224–228. [Google Scholar] [CrossRef]
- Pérez-Velázquez, M.; Gatlin, D., III; González-Félix, M.; García-Ortega, A. Partial replacement of fishmeal and fish oil by algal meals in diets of red drum Sciaenops ocellatus. Aquaculture 2018, 487, 41–50. [Google Scholar] [CrossRef]
- Walker, A.; Berlinsky, D. Effects of partial replacement of fish meal protein by microalgae on growth, feed intake, and body composition of Atlantic cod. N. Am. J. Aquac. 2011, 73, 76–83. [Google Scholar] [CrossRef]
- Pereira, H.; Sardinha, M.; Santos, T.; Gouvela, L.; Barreira, L.; Días, J.; Varela, J. Incorporation of defatted microalgal biomass (Tetraselmis sp. CTP4) at the expense of soybean meal as a feed ingredient for juvenile gilthead seabream (Sparus aurata). Algal Res. 2020, 47, 101869. [Google Scholar] [CrossRef]
- Ortega, A. Cultivo de dorada (Sparus aurata). In Cuadernos de Acuicultura; Fundación Observatorio Español de Oceanografía: Madrid, Spain, 2008; 44p. [Google Scholar]
- Johnston, I.A.; Manthri, S.; Smart, A.; Campbell, P.; Nickell, D.; Alderson, R. Plasticity of muscle fibre number in seawater stages of Atlantic salmon in response to photoperiod manipulation. J. Exp. Biol. 2003, 206, 3425–3435. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.A. Environment and plasticity of myogenesis in teleost fish. J. Exp. Biol. 2006, 209, 2249–2264. [Google Scholar] [CrossRef] [PubMed]
- Ayala, M.D.; Erades, D.; Villafranca, S.; Arizcun, M. Influence of the partial replacement of fish meal and fish oil by vegetable products in growth and muscle cellularity of juvenile shi drum, Umbrina cirrosa L. Cienc. Mar. 2020, (in press). [CrossRef]
- Johnston, I.A.; Manthri, S.; Bickerdike, R.; Dingwall, A.; Luijkx, R.; Campbell, P.; Nickel, D.; Alderson, R. Growth performance, muscle structure and flesh quality in out-of-season Atlantic salmon (Salmo salar) smolts reared under two different photoperiod regimes. Aquaculture 2004, 237, 281–300. [Google Scholar] [CrossRef]
- Hatae, K.; Yoshimatsu, F.; Matsumoto, J.J. Discriminative characterization of different texture profiles of various cooked fish muscles. J. Food Sci. 1984, 49, 721–726. [Google Scholar] [CrossRef]
- Hatae, K.; Yoshimatsu, F.; Matsumoto, J.J. Role of muscle fibres in contributing firmness of cooked fish. J. Food Sci. 1990, 55, 693–696. [Google Scholar] [CrossRef]
- Periago, M.J.; Ayala, M.D.; López-Albors, O.; Abdel, I.; Martínez, C.; García-Alcázar, A.; Ros, G.; Gil, F. Muscle cellularity and flesh quality of wild and farmed sea bass, Dicentrarchus labrax L. Aquaculture 2005, 249, 175–188. [Google Scholar] [CrossRef]
- Knutsen, H.R.; Ottesen, O.H.; Palihawadana, A.M.; Sandaa, W.; Sørensen, M.; Hagen, Ø. Muscle growth and changes in chemical composition of spotted wolffish juveniles (Anarhichas minor) fed diets with and without microalgae (Scenedesmus obliquus). Aquac. Rep. 2019, 13, 100175. [Google Scholar] [CrossRef]
- McGlory, C.; Calder, P.C.; Nunes, E.A. The influence of omega-3 fatty acids on skeletal muscle protein turnover in health, disuse, and disease. Front. Nutr. 2019, 6, 144. [Google Scholar] [CrossRef] [PubMed]


| Ingredient Composition (% Dry Matter) | Diets | ||||
|---|---|---|---|---|---|
| C | R2.5 | R5 | H2.5 | H5 | |
| Fish meal LT94 1 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Raw N. gaditana | 2.5 | 5.0 | |||
| Hydrolyzed N. gaditana | 2.5 | 5.0 | |||
| Squid meal 2 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| CPSP90 3 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Krill meal 4 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Gluten meal 5 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Soybean protein concentrate 6 | 40.0 | 38.8 | 37.3 | 38.8 | 37.3 |
| Fish oil 7 | 11.4 | 11.0 | 10.5 | 11.0 | 10.5 |
| Soybean lecithin 8 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Wheat meal 9 | 5.4 | 4.5 | 4.0 | 4.5 | 4.0 |
| Choline chloride 10 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Betain 11 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Lysine 12 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Methionine 13 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Vitamin and mineral premix 14 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Vitamin C 15 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Guar gum 16 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Proximal analysis (% dry matter) | |||||
| Crude protein | 45.7 | 47.6 | 48.1 | 46.0 | 47.6 |
| Crude lipid | 15.0 | 15.7 | 15.3 | 15.9 | 15.9 |
| Ash | 7.4 | 7.6 | 8.0 | 7.7 | 7.4 |
| Fiber | 3.7 | 3.4 | 3.3 | 3.4 | 3.3 |
| Nitrogen-free extract | 28.2 | 25.7 | 25.3 | 27.0 | 25.8 |
| Parameters | Experimental Groups | ||||
|---|---|---|---|---|---|
| C | R2.5 | R5 | H2.5 | H5 | |
| BL (cm) | 11.76 ± 0.06 a | 11.90 ± 0.05 ab | 12.02 ± 0.06 b | 11.80 ± 0.04 a | 11.80 ± 0.02 a |
| BW (g) | 23.65 ± 0.35 ab | 23.60 ± 0.31 ab | 24.55 ± 0.31 b | 23.13 ± 0.27 a | 24.10 ± 0.23 ab |
| CF (g cm−3) | 1.46 ± 0.01 a | 1.43 ± 0.00 ab | 1.40 ± 0.01 b | 1.46 ± 0.00 a | 1.47 ± 0.01 a |
| FCR | 1.16 ± 0.02 a | 1.12 ± 0.08 a | 1.09 ± 0.02 a | 1.23 ± 0.10 a | 1.19 ± 0.03 a |
| SGR (% d−1) | 1.90 ± 0.02 a | 1.96 ± 0.10 a | 2.02 ± 0.02 a | 1.93 ± 0.05 a | 1.96 ± 0.12 a |
| Experimental Groups | |||||
|---|---|---|---|---|---|
| Parameters | C | R2.5 | R5 | H2.5 | H5 |
| BL (cm) | 12.87± 0.04 a | 13.09 ± 0.03 b | 13.06 ± 0.03 b | 12.90 ± 0.04 a | 12.92 ± 0.03 a |
| BW (g) | 33.56 ± 0.26 a | 34.77 ± 0.25 b | 33.13 ± 0.33 a | 33.40 ± 0.28 a | 33.84 ± 0.24 ab |
| CF (g cm−3) | 1.56 ± 0.02 ab | 1.55 ± 0.00 ab | 1.47 ± 0.01 a | 1.55 ± 0.03 ab | 1.58 ± 0.01 b |
| FCR | 1.26 ± 0.00 a | 1.17 ± 0.08 a | 1.43 ± 0.00 a | 1.28 ± 0.00 a | 1.26 ± 0.10 a |
| SGR (% d−1) | 1.45 ± 0.02 a | 1.54 ± 0.14 a | 1.30 ± 0.00 a | 1.42 ± 0.00 a | 1.47 ± 0.12 a |
| DIR (% d−1) | 1.33 ± 0.00 a | 1.29 ± 0.01 a | 1.38 ± 0.00 a | 1.49 ± 0.22 a | 1.37 ± 0.07 a |
| Experimental Groups | |||||
|---|---|---|---|---|---|
| Parameters | C | R2.5 | R5 | H2.5 | H5 |
| BL (cm) | 14.40 ±0.10 | 14.63 ± 0.05 | 14.43 ± 0.10 | 14.45 ± 0.05 | 14.5 ± 0.05 |
| BW (g) | 49.10 ± 0.60 | 51.30 ± 0.50 | 49.50 ± 0.60 | 49.90 ± 0.60 | 50.2 ± 0.60 |
| CF (g cm−3) | 1.64 ± 0.01 | 1.64 ± 0.02 | 1.65 ± 0.01 | 1.65 ± 0.02 | 1.65 ± 0.03 |
| FCR | 1.07 ± 0.00 | 1.04 ± 0.01 | 1.01 ± 0.02 | 1.01 ± 0.01 | 1.01 ± 0.02 |
| SGR (% d−1) | 1.47 ± 0.01 | 1.49 ± 0.00 | 1.54 ± 0.00 | 1.54 ± 0.01 | 1.52 ± 0.01 |
| DIR (% d−1) | 1.31 ± 0.02 | 1.29 ± 0.03 | 1.28 ± 0.01 | 1.29 ± 0.01 | 1.28 ± 0.01 |
| Measurements | Day 0 | Day 40 | ||||
|---|---|---|---|---|---|---|
| C | C | R2.5 | R5 | H2.5 | H5 | |
| SL | 8.03 ± 0.08 | 9.70 ± 0.08 | 9.89 ± 0.08 | 9.88 ± 0.08 | 9.71 ± 0.06 | 9.84 ± 0.06 |
| TPM-MCH | 1.28 ± 0.02 | 1.43 ± 0.02 | 1.45 ± 0.02 | 1.49 ± 0.02 | 1.51 ± 0.02 | 1.49 ± 0.02 |
| MCH-ADF | 2.49 ± 0.04 | 1.99 ± 0.09 | 2.04 ± 0.04 | 2.01 ± 0.07 | 2.67 ± 0.05 | 2.76 ± 0.04 |
| ADF-PDF | 4.22 ± 0.04 | 6.02 ± 0.09 | 6.14 ± 0.06 | 6.16 ± 0.09 | 5.33 ± 0.05 | 5.41 ± 0.05 |
| PDF-PAF | 0.36 ± 0.12 | 1.00 ± 0.02 | 0.98 ± 0.01 | 0.97 ± 0.01 | 0.97 ± 0.01 | 1.00 ± 0.01 |
| PAF-AAF | 1.75 ± 0.03 | 2.29 ± 0.05 | 2.40 ± 0.03 | 2.43 ± 0.03 | 2.43 ± 0.04 | 2.51 ± 0.03 |
| AAF-PF | 2.45 ± 0.03 | 2.77 ± 0.05 | 2.78 ± 0.04 | 2.80 ± 0.03 | 2.61 ± 0.04 | 2.72 ± 0.03 |
| PF-OP | 1.56 ± 0.02 | 2.26 ± 0.0 | 2.25 ± 0.04 | 2.18 ± 0.04 | 2.12 ± 0.04 | 2.12 ± 0.03 |
| OP-TPM | 1.74 ± 0.04 | 1.95 ± 0.03 | 1.96 ± 0.04 | 1.98 ± 0.04 | 2.06 ± 0.03 | 2.04 ± 0.03 |
| OP-DPF | 1.63 ± 0.04 | 2.22 ± 0.05 | 2.24 ± 0.03 | 2.19 ± 0.03 | 2.14 ± 0.05 | 2.18 ± 0.03 |
| ED | ˂1 ± 0.00 | 1.02 ± 0.01 | 1.00 ± 0.01 | 1.01 ± 0.01 | 1.02 ± 0.03 | 1.00 ± 0.01 |
| MHC-OP | 1.99 ± 0.03 | 2.33 ± 0.03 | 2.35 ± 0.03 | 2.38 ± 0.03 | 2.41 ± 0.03 | 2.41 ± 0.02 |
| MHC-PF | 3.12 ± 0.05 | 4.00 ± 0.04 | 4.01 ± 0.03 | 3.96 ± 0.03 | 3.94 ± 0.03 | 3.98 ± 0.02 |
| MHC-AAF | 5.06 ± 0.05 | 6.22 ± 0.06 | 6.24 ± 0.05 | 6.20 ± 0.05 | 6.00 ± 0.05 | 6.10 ± 0.04 |
| ADF-PF | 3.08 ± 0.04 | 3.76 ± 0.04 | 3.75 ± 0.03 | 3.76 ± 0.03 | 3.71 ± 0.02 | 3.81 ± 0.03 |
| ADF-AAF | 3.71 ± 0.04 | 5.16 ± 0.08 | 5.14 ± 0.05 | 5.13 ± 0.06 | 4.60 ± 0.04 | 4.68 ± 0.04 |
| ADF-PAF | 4.57 ± 0.04 | 6.44 ± 0.08 | 6.52 ± 0.06 | 6.52 ± 0.08 | 5.78 ± 0.05 | 5.88 ± 0.05 |
| 64 Days | |||||
|---|---|---|---|---|---|
| Measurements | C | R2.5 | R5 | H2.5 | H5 |
| SL | 11.19 ± 0.06 | 11.36 ± 0.05 | 11.15 ± 0.07 | 11.03 ± 0.06 | 11.05 ± 0.06 |
| TPM-MCH | 1.90 ± 0.03 | 1.75 ± 0.02 | 1.63 ± 0.03 | 1.77 ± 0.02 | 1.56 ± 0.02 |
| MCH-ADF | 2.97 ± 0.06 | 3.01 ± 0.06 | 3.36 ± 0.06 | 3.07 ± 0.05 | 2.85 ± 0.05 |
| ADF-PDF | 6.03 ± 0.06 | 6.19 ± 0.06 | 5.86 ± 0.08 | 5.94 ± 0.05 | 6.39 ± 0.05 |
| PDF-PAF | 1.21 ± 0.02 | 1.24 ± 0.02 | 1.20 ± 0.02 | 1.19 ± 0.01 | 1.18 ± 0.01 |
| PAF-AAF | 2.78 ± 0.04 | 2.81 ± 0.03 | 2.66 ± 0.04 | 2.50 ± 0.04 | 2.42 ± 0.03 |
| AAF-PF | 3.41 ± 0.05 | 3.45 ± 0.04 | 3.51 ± 0.05 | 3.49 ± 0.04 | 3.45 ± 0.04 |
| PF-OP | 2.37 ± 0.03 | 2.33 ± 0.03 | 2.22 ± 0.03 | 2.32 ± 0.03 | 2.35 ± 0.03 |
| OP-TPM | 2.20 ± 0.03 | 2.27 ± 0.03 | 2.20 ± 0.03 | 2.27 ± 0.02 | 2.25 ± 0.02 |
| OP-DPF | 2.57 ± 0.03 | 2.60 ± 0.02 | 2.40 ± 0.03 | 2.47 ± 0.02 | 2.32 ± 0.02 |
| ED | 1.04 ± 0.01 | 1.05 ± 0.01 | 1.02 ± 0.01 | 1.06 ± 0.01 | 1.06 ± 0.01 |
| MHC-OP | 2.74 ± 0.02 | 2.74 ± 0.03 | 2.65 ± 0.02 | 2.75 ± 0.02 | 2.66 ± 0.02 |
| MHC-PF | 4.29 ± 0.03 | 4.37 ± 0.03 | 4.27 ± 0.03 | 4.37 ± 0.03 | 4.44 ± 0.03 |
| MHC-AAF | 6.90 ± 0.05 | 7.08 ± 0.04 | 7.10 ± 0.05 | 7.10 ± 0.05 | 7.26 ± 0.05 |
| ADF-PF | 4.37 ± 0.03 | 4.36 ± 0.03 | 4.28 ± 0.03 | 4.35 ± 0.03 | 4.27 ± 0.03 |
| ADF-AAF | 5.39 ± 0.05 | 5.52 ± 0.04 | 5.33 ± 0.05 | 5.47 ± 0.05 | 5.74 ± 0.04 |
| ADF-PAF | 6.65 ± 0.06 | 6.84 ± 0.06 | 6.50 ± 0.06 | 6.56 ± 0.06 | 6.89 ± 0.05 |
| Experimental Diets | |||||
|---|---|---|---|---|---|
| Measurements | C | R2.5 | R5 | H2.5 | H5 |
| SL | 12.01 ± 0.07 | 12.03 ± 0.07 | 11.99 ± 0.08 | 12.20 ± 0.07 | 12.12 ± 0.06 |
| TPM-MCH | 1.61 ± 0.02 | 1.56 ± 0.02 | 1.54 ± 0.02 | 1.64 ± 0.02 | 1.59 ± 0.02 |
| MCH-ADF | 3.64 ± 0.04 | 3.86 ± 0.04 | 3.75 ± 0.04 | 3.60 ± 0.04 | 3.56 ± 0.05 |
| ADF-PDF | 5.89 ± 0.05 | 5.73 ± 0.05 | 5.84 ± 0.06 | 6.11 ± 0.06 | 6.20 ± 0.06 |
| PDF-PAF | 1.69 ± 0.02 | 1.69 ± 0.02 | 1.66 ± 0.02 | 1.71 ± 0.02 | 1.62 ± 0.02 |
| PAF-AAF | 2.36 ± 0.03 | 2.33 ± 0.03 | 2.31 ± 0.03 | 2.41 ± 0.04 | 2.46 ± 0.03 |
| AAF-PF | 3.90 ± 0.04 | 3.99 ± 0.04 | 3.92 ± 0.05 | 4.04 ± 0.05 | 3.94 ± 0.05 |
| PF-OP | 2.25 ± 0.03 | 2.25 ± 0.03 | 2.23 ± 0.03 | 2.17 ± 0.04 | 2.25 ± 0.03 |
| OP-TPM | 2.25 ± 0.03 | 2.20 ± 0.03 | 2.21 ± 0.03 | 2.33 ± 0.02 | 2.20 ± 0.03 |
| OP-DPF | 2.28 ± 0.04 | 2.20 ± 0.03 | 2.30 ± 0.04 | 2.35 ± 0.03 | 2.34 ± 0.03 |
| ED | 0.98 ± 0.01 | 0.97 ± 0.01 | 0.99 ± 0.01 | 0.98 ± 0.01 | 0.98 ± 0.01 |
| MHC-OP | 2.85 ± 0.02 | 2.81 ± 0.03 | 2.79 ± 0.03 | 2.96 ± 0.03 | 2.83 ± 0.03 |
| MHC-PF | 4.43 ± 0.03 | 4.42 ± 0.03 | 4.42 ± 0.04 | 4.53 ± 0.04 | 4.45 ± 0.03 |
| MHC-AAF | 7.52 ± 0.05 | 7.53 ± 0.05 | 7.52 ± 0.06 | 7.75 ± 0.05 | 7.54 ± 0.05 |
| ADF-PF | 4.77 ± 0.04 | 4.82 ± 0.03 | 4.78 ± 0.04 | 4.88 ± 0.03 | 4.75 ± 0.04 |
| ADF-AAF | 5.71 ± 0.05 | 5.64 ± 0.04 | 5.66 ± 0.07 | 5.96 ± 0.05 | 5.82 ± 0.04 |
| ADF-PAF | 6.62 ± 0.05 | 6.46 ± 0.04 | 6.52 ± 0.07 | 6.87 ± 0.05 | 6.78 ± 0.05 |
| Measurements | PC1 | PC2 | |
|---|---|---|---|
| SL | 0.721 | 0.690 | |
| TPM-MCH | 0.722 | 0.684 | |
| MCH-ADF | 0.742 | 0.664 | |
| ADF-PDF | 0.708 | 0.701 | |
| PDF-PAF | 0.720 | 0.688 | |
| PAF-AAF | 0.702 | 0.703 | |
| AAF-PF | 0.746 | 0.661 | |
| PF-OP | 0.661 | 0.747 | |
| OP-TPM | 0.743 | 0.664 | |
| OP-DPF | 0.665 | 0.741 | |
| ED | 0.712 | 0.694 | |
| MCH-OP | 0.740 | 0.668 | |
| MCH-PF | 0.713 | 0.698 | |
| MCH-AAF | 0.730 | 0.680 | |
| ADF-PF | 0.729 | 0.687 | |
| ADF-AAF | 0.723 | 0.687 | |
| ADF-PAF | 0.719 | 0.692 | |
| Variance % | 51.523 | 47.783 | |
| Cumulative % | 51.523 | 99.306 | |
| Eigen values | 16.864 | 0.018 | |
| ANOVA | Treatment | 0.0000 | 0.0000 |
| Time | 0.0000 | 0.0000 | |
| Treatment × Time | 0.0000 | 0.0000 |
| Section | Experimental Groups | |||||
|---|---|---|---|---|---|---|
| C | R2.5 | R5 | H2.5 | H5 | ||
| B (mm2) | Day 0 | 122.7 ± 4.1 | ||||
| Day 40 | 212.0 ± 8.3 a | 206.48 ± 8.1 ab | 210.5 ± 6.3 a | 177.2 ± 5.9 b | 206.0 ± 9.4 ab | |
| Day 90 | 391.2 ±13.6 a | 358.9 ± 14.7 ab | 341.2 ± 13.0 ab | 340.1 ± 12.9 ab | 332.8 ± 9.9 b | |
| Groups | Day 0 | Day 90 | ||||
|---|---|---|---|---|---|---|
| C | C | R2.5 | R5 | H2.5 | H5 | |
| A (μm2) | 1791.60 ± 1.94 | 2644.80 ± 524.30 | 3133.90 ± 167.60 | 2924.60 ± 78.03 | 2921.30 ± 112.90 | 2853.56 ± 67.41 |
| D (μm) | 35.93 ± 1.44 | 49.50 ± 1.60 | 48.80 ± 1.50 | 48.70 ± 0.80 | 47.56 ± 1.16 | 48.18 ± 0.84 |
| N (×103) | 70.49 ± 4.32 | 120.87 ± 8.08 | 118.15 ± 9.63 | 117.03 ± 4.17 | 117.51 ± 7.35 | 116.16 ± 4.80 |
| Dens | 569.12 ± 31.04 | 309.20 ± 13.50 | 329.20 ± 21.70 | 344.04 ±8.80 | 346.87 ± 14.90 | 350.71 ± 9.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala, M.D.; Galián, C.; Fernández, V.; Chaves-Pozo, E.; García de la Serrana, D.; Sáez, M.I.; Galafaz Díaz, A.; Alarcón, F.J.; Martínez, T.F.; Arizcun, M. Influence of Low Dietary Inclusion of the Microalga Nannochloropsis gaditana (Lubián 1982) on Performance, Fish Morphology, and Muscle Growth in Juvenile Gilthead Seabream (Sparus aurata). Animals 2020, 10, 2270. https://doi.org/10.3390/ani10122270
Ayala MD, Galián C, Fernández V, Chaves-Pozo E, García de la Serrana D, Sáez MI, Galafaz Díaz A, Alarcón FJ, Martínez TF, Arizcun M. Influence of Low Dietary Inclusion of the Microalga Nannochloropsis gaditana (Lubián 1982) on Performance, Fish Morphology, and Muscle Growth in Juvenile Gilthead Seabream (Sparus aurata). Animals. 2020; 10(12):2270. https://doi.org/10.3390/ani10122270
Chicago/Turabian StyleAyala, María Dolores, Carolina Galián, Victoria Fernández, Elena Chaves-Pozo, Daniel García de la Serrana, María Isabel Sáez, Alba Galafaz Díaz, Francisco Javier Alarcón, Tomás Francisco Martínez, and Marta Arizcun. 2020. "Influence of Low Dietary Inclusion of the Microalga Nannochloropsis gaditana (Lubián 1982) on Performance, Fish Morphology, and Muscle Growth in Juvenile Gilthead Seabream (Sparus aurata)" Animals 10, no. 12: 2270. https://doi.org/10.3390/ani10122270
APA StyleAyala, M. D., Galián, C., Fernández, V., Chaves-Pozo, E., García de la Serrana, D., Sáez, M. I., Galafaz Díaz, A., Alarcón, F. J., Martínez, T. F., & Arizcun, M. (2020). Influence of Low Dietary Inclusion of the Microalga Nannochloropsis gaditana (Lubián 1982) on Performance, Fish Morphology, and Muscle Growth in Juvenile Gilthead Seabream (Sparus aurata). Animals, 10(12), 2270. https://doi.org/10.3390/ani10122270







