An Investigation into the Critical Factors Influencing the Spread of Campylobacter during Chicken Handling in Commercial Kitchens in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection and Experimental Design
2.1.1. Sample Procedure
2.1.2. Sample Collection
2.1.3. Campylobacter Examination Method and Total Bacterial Count Analysis
2.1.4. Multilocus Sequence Typing (MLST)
2.1.5. Biofilm Formation
2.1.6. Statistical Analysis
3. Results
3.1. Occurrence of Campylobacter spp. in Commercial Kitchens
3.2. Occurrence of Campylobacter spp. and the Sanitary Conditions in Commercial Kitchens
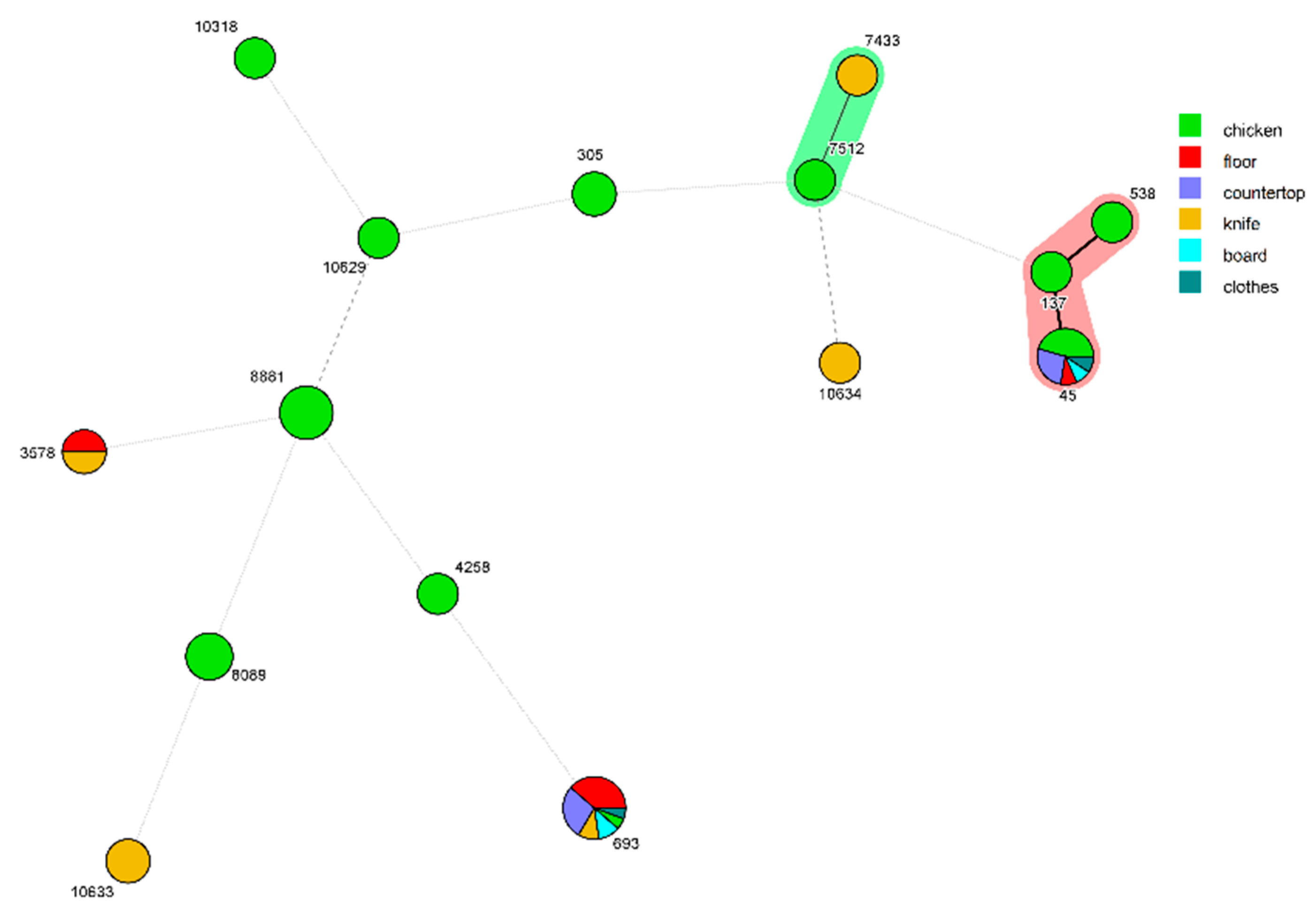
3.3. MLST Analysis of C. jejuni Isolates and the Analysis of Cross Contamination
3.4. Biofilm Assessment of Identified C. jejuni Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Tack, D.M.; Ray, L.; Griffin, P.M.; Cieslak, P.R.; Dunn, J.; Rissman, T.; Jervis, R.; Lathrop, S.; Muse, A.; Duwell, M.; et al. Preliminary incidence and trends of infections with pathogens transmitted commonly through food—Foodborne diseases active surveillance network, 10 U.S. Sites, 2016–2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, Y.C.; Li, N. Prevalence and risk assessment of Campylobacter jejuni in chicken in China. Biomed. Environ. Sci. 2013, 26, 243–248. [Google Scholar]
- Scharff, R.L. Economic burden from health losses due to foodborne illness in the United States. J. Food Prot. 2012, 75, 123–131. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on Campylobacter in broiler meat production: Control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011, 9, 2105. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA J. 2010, 8, 1437–1526. [Google Scholar] [CrossRef]
- National Institute for Public Health and the Environment; Iulietto, M.F.; Evers, E.G. Modelling and magnitude estimation of cross-contamination in the kitchen for quantitative microbiological risk assessment (QMRA). EFSA J. 2020, 18, e181106. [Google Scholar]
- Hu, Y.; Cheng, H.; Tao, S. Environmental and human health challenges of industrial livestock and poultry farming in China and their mitigation. Environ. Int. 2017, 107, 111–130. [Google Scholar] [CrossRef]
- Carrasco, E.; Morales-Rueda, A.; García-Gimeno, R.M. Cross-contamination and recontamination by Salmonella in foods: A review. Food Res. Int. 2012, 45, 545–556. [Google Scholar] [CrossRef]
- Gorman, R.; Bloomfield, S.; Adley, C.C. A study of cross-contamination of food-borne pathogens in the domestic kitchen in the Republic of Ireland. Int. J. Food Microbiol. 2002, 76, 143–150. [Google Scholar] [CrossRef]
- Kusumaningrum, H.D.; van Asselt, E.D.; Beumer, R.R.; Zwietering, M.H. A quantitative analysis of cross-contamination of Salmonella and Campylobacter spp. via domestic kitchen surfaces. J. Food Prot. 2004, 67, 1892–1903. [Google Scholar] [CrossRef] [PubMed]
- Mylius, S.D.; Nauta, M.J.; Havelaar, A.H. Cross-contamination during food preparation: A mechanistic model applied to chicken-borne Campylobacter. Risk Anal. 2007, 27, 803–813. [Google Scholar] [CrossRef]
- Van Asselt, E.D.; de Jong, A.E.; de Jonge, R.; Nauta, M.J. Cross-contamination in the kitchen: Estimation of transfer rates for cutting boards, hands and knives. J. Appl. Microbiol. 2008, 105, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Luber, P.; Brynestad, S.; Topsch, D.; Scherer, K.; Bartelt, E. Quantification of Campylobacter species cross-contamination during handling of contaminated fresh chicken parts in kitchens. Appl. Environ. Microbiol. 2006, 72, 66–70. [Google Scholar] [CrossRef]
- Bronowski, C.; James, C.E.; Winstanley, C. Role of environmental survival in transmission of Campylobacter jejuni. FEMS Microbiol. Lett. 2014, 356, 8–19. [Google Scholar] [CrossRef]
- Crofts, A.A.; Poly, F.M.; Ewing, C.P.; Kuroiwa, J.M.; Rimmer, J.E.; Harro, C.; Sack, D.; Talaat, K.R.; Porter, C.K.; Gutierrez, R.L.; et al. Campylobacter jejuni transcriptional and genetic adaptation during human infection. Nat. Microbiol. 2018, 3, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Borrusso, P.A.; Quinlan, J.J. Prevalence of pathogens and indicator organisms in home kitchens and correlation with unsafe food handling practices and conditions. J. Food Prot. 2017, 80, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Gunther, N.W.; Chen, C.Y. The biofilm forming potential of bacterial species in the genus Campylobacter. Food Microbiol. 2009, 26, 44–51. [Google Scholar] [CrossRef] [PubMed]
- USDA/FSIS. Isolation, Identification, and Enumeration of Campylobacter jejuni/lari from Pultry Rinse, Sponge, and Raw Product Samples. 2013. Available online: http://www.fsis.usda.gov/wps/portal/fsis/topics/science/laboratoriesand-procedures/guidebooks-and-methods/microbiology-laboratoryguidebook/microbiology-laboratory-guidebook (accessed on 15 June 2020).
- Scherer, K.; Bartelt, E.; Sommerfeld, C.; Hildebrandt, G. Comparison of different sampling techniques and enumeration methods for the isolation and quantification of Campylobacter spp. in raw retail chicken legs. Int. J. Food Microbiol. 2006, 108, 115–119. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yin, T.T.; Du, X.Q.; Yang, W.B.; Huang, J.L.; Jiao, X.A. Occurrence and genotypes of Campylobacter species in broilers during the rearing period. Avian Pathol. 2017, 46, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Cui, S.; Xu, X.; Li, F. Enumeration and characterization of Campylobacter species from retail chicken carcasses in Beijing, China. Foodborne Pathog. Dis. 2014, 11, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Y.; Jiang, Q.D.; Tang, H.Y.; Wang, Z.Y.; Yin, Y.; Ren, F.Z.; Kong, L.H.; Jiao, X.A.; Huang, J.L. Characterization and Prevalence of Campylobacter spp. From Broiler Chicken Rearing Period to the Slaughtering Process in Eastern China. Front. Vet. Sci. 2020, 7, 227. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; Xu, H.Y.; Bao, G.Y.; Zhou, X.H.; Ji, D.J.; Zhang, G.; Liu, P.H.; Jiang, F.; Pan, Z.M.; Liu, X.F.; et al. Epidemiological surveillance of Campylobacter jejuni in chicken, dairy cattle, and diarrhoea patients. Epidemiol. Infect. 2009, 137, 1111–1120. [Google Scholar] [CrossRef]
- Huang, J.L.; Zong, Q.; Zhao, F.; Zhu, J.Q.; Jiao, X.A. Quantitative surveys of Salmonella and Campylobacter on retail raw chicken in Yangzhou, China. Food Control 2016, 59, 68–73. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, G.; Wei, R.; Zhang, Y.; Li, S.; Chen, Y. Quality characteristics of fresh wet noodles treated with nonthermal plasma sterilization. Food Chem. 2019, 297, 124900. [Google Scholar] [CrossRef]
- Dingle, K.E.; Colles, F.M.; Wareing, D.R.; Ure, R.; Fox, A.J.; Bolton, F.E.; Bootsma, H.J.; Willems, R.J.; Urwin, R.; Maiden, M.C. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 2001, 39, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.L.; Reuter, M.; Salt, L.J.; Cross, K.L.; Betts, R.P.; van Vliet, A.H. Chicken juice enhances surface attachment and biofilm formation of Campylobacter jejuni. Appl. Environ. Microbiol. 2014, 80, 7053–7060. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Rasmussen, A.W.; Gudlavalleti, S.K.; Stephens, D.S.; Stojiljkovic, I. Biofilm formation by Neisseria meningitidis. Infect. Immun. 2004, 72, 6132–6138. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Ferreira, V.; Truninger, M.; Maia, R.; Teixeira, P. Cross-contamination events of Campylobacter spp. in domestic kitchens associated with consumer handling practices of raw poultry. Int. J. Food Microbiol. 2021, 338, 108984. [Google Scholar] [CrossRef]
- Cogan, T.A.; Bloomfield, S.F.; Humphrey, T.J. The effectiveness of hygiene procedures for prevention of cross-contamination from chicken carcases in the domestic kitchen. Lett. Appl. Microbiol. 1999, 29, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, H.C.; Bolton, F.J.; Hutchinson, D.N. A study of the spread of Campylobacter jejuni in four large kitchens. Epidemiol. Infect. 1984, 92, 357–364. [Google Scholar] [CrossRef]
- Medeiros, D.T.; Sattar, S.A.; Farber, J.M.; Carrillo, C.D. Occurrence of Campylobacter spp. in raw and ready-to-eat foods and in a Canadian food service operation. J. Food Prot. 2008, 71, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Mead, G.C.; Scott, M.J. Spread of an enteric ‘marker’ organism during evisceration of New York dressed poultry in a simulated kitchen environment. Br. Poult. Sci. 1997, 38, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Ngan, W.Y.; Rao, S.; Chan, L.C.; Sekoai, P.T.; Pu, Y.; Yao, Y.; Fung, A.H.Y.; Habimana, O. Impacts of wet market modernization levels and hygiene practices on the microbiome and microbial safety of wooden cutting boards in Hong Kong. Microorganisms 2020, 8, 1941. [Google Scholar] [CrossRef]
- Kusumaningrum, H.D.; van Asselt, E.D.; Beumer, R.R.; Zwietering, M.H. Food safety knowledge of consumers and the microbiological and temperature status of their refrigerators. J. Food Prot. 2005, 68, 1421–1430. [Google Scholar]
- Dharod, J.M.; Paciello, S.; Bermúdez-Millán, A.; Venkitanarayanan, K.; Damio, G.; Pérez-Escamilla, R. Bacterial contamination of hands increases risk of cross-contamination among low-income Puerto Rican meal preparers. J. Nutr. Educ. Behav. 2009, 41, 389–397. [Google Scholar] [CrossRef]
- Sarjit, A.; Dykes, G.A. Transfer of Campylobacter and Salmonella from poultry meat onto poultry preparation surfaces. J. Food Prot. 2017, 80, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, T.J.; Martin, K.W.; Slader, J.; Durham, K. Campylobacter spp. in the kitchen: Spread and persistence. J. Appl. Microbiol. 2001, 90, 115–120. [Google Scholar] [CrossRef]
- Allen, V.M.; Bull, S.A.; Corry, J.E.; Domingue, G.; Jørgensen, F.; Frost, J.A.; Whyte, R.; Gonzalez, A.; Elviss, N.; Humphrey, T.J. Campylobacter spp. contamination of chicken carcasses during processing in relation to flock colonisation. Int. J. Food Microbiol. 2007, 113, 54–61. [Google Scholar] [CrossRef]
- Parry, S.M.; Slader, J.; Humphrey, T.; Holmes, B.; Guildea, Z.; Palmer, S.R. A case-control study of domestic kitchen microbiology and sporadic Salmonella infection. Epidemiol. Infect. 2005, 133, 829–835. [Google Scholar] [CrossRef] [PubMed]
- APHA. Compendium of Methods for the Microbiological Examination of Foods, 4th ed.; American Public Health Association: Washington, DC, USA, 2001. [Google Scholar]
- Touimi, G.B.; Bennani, L.; Berrada, S.; Benboubker, M.; Bennani, B. Evaluation of hygienic conditions of food contact surfaces in a hospital kitchen in Morocco. Iran. J. Microbiol. 2019, 11, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.T.; Nou, X.; Bauchan, G.R.; Murphy, C.; Lefcourt, A.M.; Shelton, D.R.; Lo, Y.M. Effects of environmental parameters on the dual-species biofilms formed by Escherichia coli O157:H7 and Ralstonia insidiosa, a strong biofilm producer isolated from a fresh-cut produce processing plant. J. Food Prot. 2015, 78, 121–127. [Google Scholar] [CrossRef]
- Gomes, C.N.; Frazão, M.R.; Passaglia, J.; Duque, S.S.; Medeiros, M.I.C.; Falcão, J.P. Molecular Epidemiology and resistance profile of Campylobacter jejuni and Campylobacter coli strains isolated from different sources in Brazil. Microb. Drug Resist. 2020, 26, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Griekspoor, P.; Engvall, E.O.; Akerlind, B.; Olsen, B.; Waldenstrom, J. Genetic diversity and host associations in Campylobacter jejuni from human cases and broilers in 2000 and 2008. Vet. Microbiol. 2015, 178, 94–98. [Google Scholar] [CrossRef]
- Zbrun, M.V.; Rossler, E.; Soto, L.P.; Rosmini, M.R.; Sequeira, G.J.; Frizzo, L.S.; Signorini, M.L. Molecular epidemiology of Campylobacter jejuni isolates from the broiler production chain: First report of MLST profiles in Argentina. Rev. Argent. Microbiol. 2021, 53, 59–63. [Google Scholar] [PubMed]
- Zhang, G.; Zhang, X.Y.; Hu, Y.Q.; Jiao, X.A.; Huang, J.L. Multilocus Sequence Types of Campylobacter jejuni Isolates from Different Sources in Eastern China. Curr. Microbiol. 2015, 71, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.Y.; Zhang, M.J.; Ma, Y.P.; Lu, J.R.; Yu, M.H.; Chen, H.; Liu, C.Y.; Gu, Y.X.; Fu, Y.Y.; Duan, Y.X. Genetic and antibiotic resistance characteristics of Campylobacter jejuni isolated from diarrheal patients, poultry and cattle in Shenzhen. Biomed. Environ. Sci. 2018, 31, 579–585. [Google Scholar]
- Kärenlampi, R.; Rautelin, H.; Schönberg-Norio, D.; Paulin, L.; Hänninen, M.L. Longitudinal study of Finnish Campylobacter jejuni and C. coli isolates from humans, using multilocus sequence typing, including comparison with epidemiological data and isolates from poultry and cattle. Appl. Environ. Microbiol. 2007, 73, 148–155. [Google Scholar] [CrossRef]
- García-Sánchez, L.; Melero, B.; Jaime, I.; Hänninen, M.L.; Rossi, M.; Rovira, J. Campylobacter jejuni survival in a poultry processing plant environment. Food Microbiol. 2017, 65, 185–192. [Google Scholar] [CrossRef]
- Lamas, A.; Regal, P.; Vázquez, B.; Miranda, J.M.; Cepeda, A.; Franco, C.M. Salmonella and Campylobacter biofilm formation: A comparative assessment from farm to fork. J. Sci. Food Agric. 2018, 98, 4014–4032. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Lee, J.; Kim, J.-S.; Koo, O. Isolation of indigenous bacteria from a cafeteria kitchen and their biofilm formation and disinfectant susceptibility. LWT Food Sci. Technol. 2017, 77, 376–382. [Google Scholar] [CrossRef]
- Marouani-Gadri, N.; Augier, G.; Carpentier, B. Characterization of bacterial strains isolated from a beef-processing plant following cleaning and disinfection—Influence of isolated strains on biofilm formation by Sakai and EDL 933 E. coli O157:H7. Int. J. Food Microbiol. 2009, 133, 62–67. [Google Scholar] [CrossRef] [PubMed]

| Kitchen | Source of Sample | No. of Samples | Positive Samples | Prevalence (%) |
|---|---|---|---|---|
| A | Chicken | 153 | 120 | 78.43 |
| Sample sites | 174 | 78 | 44.82 | |
| B | Chicken | 96 | 79 | 82.29 |
| Sample sites | 121 | 36 | 29.75 | |
| C | Chicken | 66 | 52 | 78.79 |
| Sample sites | 136 | 53 | 38.97 | |
| D | Chicken | 10 | 5 | 50 |
| Sample sites | 17 | 6 | 35.29 | |
| E | Chicken | 38 | 25 | 65.79 |
| Sample sites | 31 | 6 | 19.35 | |
| Total | Chicken | 363 | 281 | 77.41 |
| Sample sites | 479 | 179 | 37.37 |
| Sample Source | Procedure | Detection of Campylobacter in Commercial Kitchen | ||
|---|---|---|---|---|
| No. of Positive Samples | Total | Prevalence % | ||
| Chicken | Defeathering | 41 | 70 | 58.57 a |
| Evisceration | 65 | 74 | 87.84 b | |
| Washing | 175 | 219 | 79.91 b | |
| Total | 281 | 363 | 77.41 | |
| Countertop (NFCS*) | Before | 2 | 29 | 6.90 a |
| Knife (FCS**) | Before | 1 | 26 | 3.85 a |
| Board (FCS) | Before | 5 | 28 | 17.86 a |
| Hand (FCS) | Before | 7 | 26 | 26.92 a |
| Container (FCS) | Before | 6 | 26 | 23.08 a |
| Sink (NFCS) | Before | 4 | 25 | 16.00 a |
| Floor (NFCS) | Before | 2 | 26 | 7.69 a |
| Total | Before | 27 | 186 | 14.52 |
| Countertop (NFCS) | After | 10 | 31 | 32.26 a |
| Knife (FCS) | After | 20 | 35 | 57.14 ab |
| Board (FCS) | After | 27 | 35 | 77.14 b |
| Hand (FCS) | After | 24 | 35 | 68.57 b |
| Container (FCS) | After | 29 | 35 | 82.86 bc |
| Sink (NFCS) | After | 4 | 27 | 14.81 a |
| Floor (NFCS) | After | 10 | 30 | 33.33 a |
| Total | After | 124 | 228 | 54.39 |
| Knife (FCS) | Cleaning | 10 | 22 | 45.45 a |
| Board (FCS) | Cleaning | 12 | 23 | 52.17 a |
| Hand (FCS) | Cleaning | 6 | 20 | 30.00 a |
| Total | Cleaning | 28 | 65 | 43.08 |
| Sample Source | Procedure | Loads of Campylobacter spp. and Total Bacterial Count (Mean ± SEM log CFU/100 cm2) | |
|---|---|---|---|
| Campylobacter | T.B.C. | ||
| Chicken carcasses | Defeathering | 2.67 ± 0.90 a | 6.91 ± 1.20 a |
| Evisceration | 2.57 ± 0.89 a | 6.35 ± 1.39 b | |
| Washing | 2.16 ± 0.80 b | 5.89 ± 0.66 c | |
| Total | 2.47 ± 0.86 | 6.38 ± 1.08 | |
| Countertop (NFCS) | Before | 1.95 ± 0.35 a | 6.29 ± 0.87 a |
| Knife (FCS) | Before | 1.80 ± 0.20 a | 6.40 ± 0.87 b |
| Board (FCS) | Before | 2.14 ± 0.24 a | 7.03 ± 1.10 a |
| Hand (FCS) | Before | 2.17 ± 0.29 a | 6.20 ± 1.04 b |
| Container (FCS) | Before | 2.17 ± 0.31 a | 6.36 ± 1.15 b |
| Sink (NFCS) | Before | 2.02 ± 0.16 a | 6.37 ± 0.92 b |
| Floor (NFCS) | Before | 1.60 ± 0.00 a | 6.07 ± 1.13 b |
| Total | Before | 1.98 ± 0.22 | 6.29 ± 1.01 |
| Countertop (NFCS) | After | 2.01 ± 0.21 c | 6.67 ± 0.72 bc |
| Knife (FCS) | After | 2.54 ± 0.23 ab | 6.61 ± 0.87 bc |
| Board (FCS) | After | 2.86 ± 0.18 a | 7.53 ± 0.74 a |
| Hand (FCS) | After | 2.47 ± 0.19 ab | 6.71 ± 0.73 bc |
| Container (FCS) | After | 2.35 ± 0.14 b | 6.97 ± 0.86 b |
| Sink (NFCS) | After | 3.04 ± 0.56 ab | 6.66 ± 0.95 bc |
| Floor (NFCS) | After | 1.86 ± 0.21 c | 6.27 ± 0.94 c |
| Total | After | 2.45 ± 0.25 | 6.77 ± 0.83 |
| Knife (FCS) | Cleaning | 2.33 ± 0.17 a | 6.35 ± 0.72 b |
| Board (FCS) | Cleaning | 2.10 ± 0.17 a | 7.04 ± 0.83 a |
| Hand (FCS) | Cleaning | 2.44 ± 0.17 a | 6.02 ± 0.99 ab |
| Total | Cleaning | 2.29 ± 0.16 | 6.47 ± 0.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, H.; Tang, Y.; Ren, F.; Li, Z.; Li, F.; Cui, C.; Jiao, X.; Huang, J. An Investigation into the Critical Factors Influencing the Spread of Campylobacter during Chicken Handling in Commercial Kitchens in China. Microorganisms 2021, 9, 1164. https://doi.org/10.3390/microorganisms9061164
Lai H, Tang Y, Ren F, Li Z, Li F, Cui C, Jiao X, Huang J. An Investigation into the Critical Factors Influencing the Spread of Campylobacter during Chicken Handling in Commercial Kitchens in China. Microorganisms. 2021; 9(6):1164. https://doi.org/10.3390/microorganisms9061164
Chicago/Turabian StyleLai, Honggang, Yuanyue Tang, Fangzhe Ren, Zeng Li, Fengming Li, Chaoyue Cui, Xinan Jiao, and Jinlin Huang. 2021. "An Investigation into the Critical Factors Influencing the Spread of Campylobacter during Chicken Handling in Commercial Kitchens in China" Microorganisms 9, no. 6: 1164. https://doi.org/10.3390/microorganisms9061164
APA StyleLai, H., Tang, Y., Ren, F., Li, Z., Li, F., Cui, C., Jiao, X., & Huang, J. (2021). An Investigation into the Critical Factors Influencing the Spread of Campylobacter during Chicken Handling in Commercial Kitchens in China. Microorganisms, 9(6), 1164. https://doi.org/10.3390/microorganisms9061164






