Intimate Attachment of Escherichia coli O157:H7 to Urinary Bladder Epithelium in the Gnotobiotic Piglet Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Gnotobiotic Piglet Studies
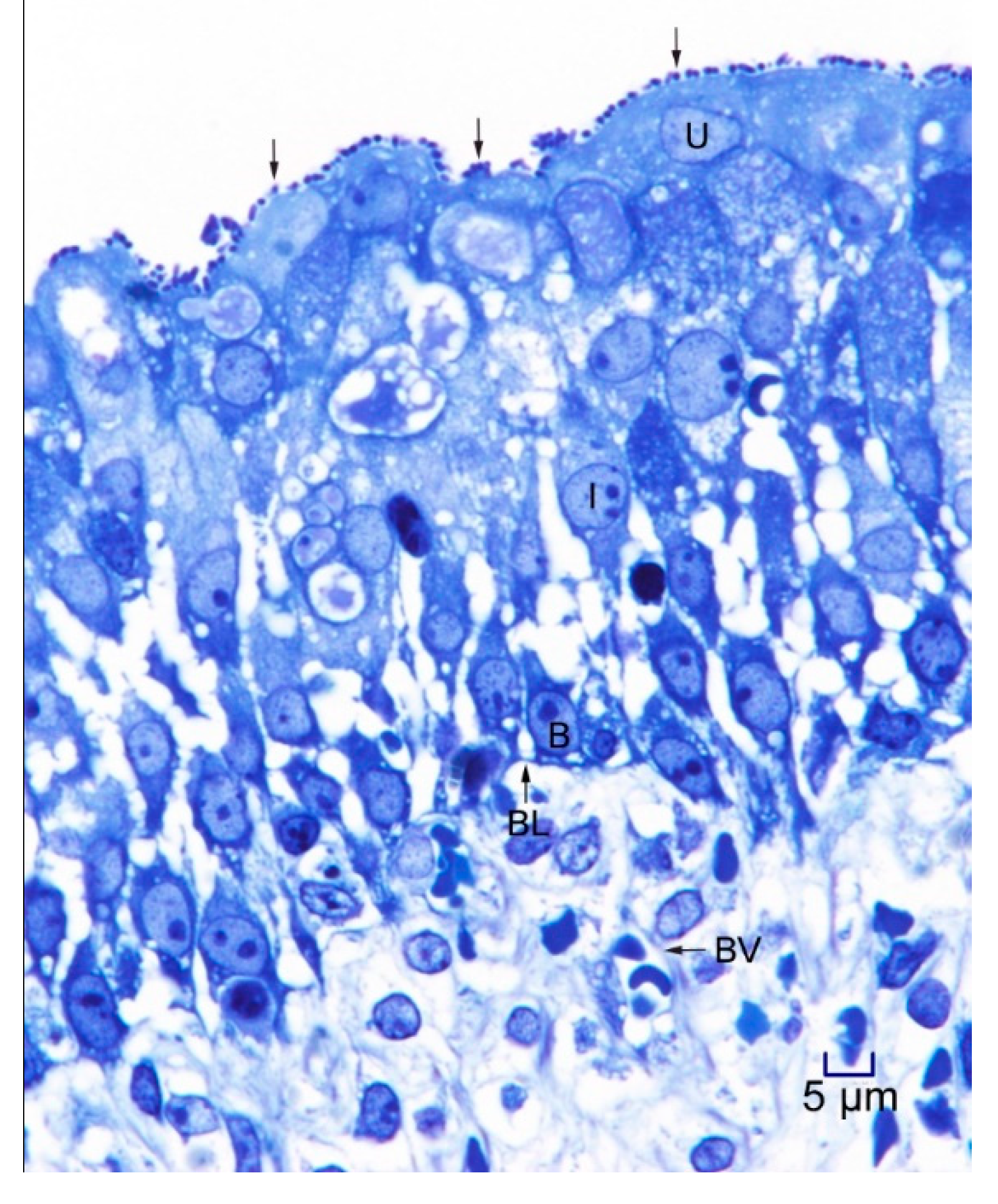
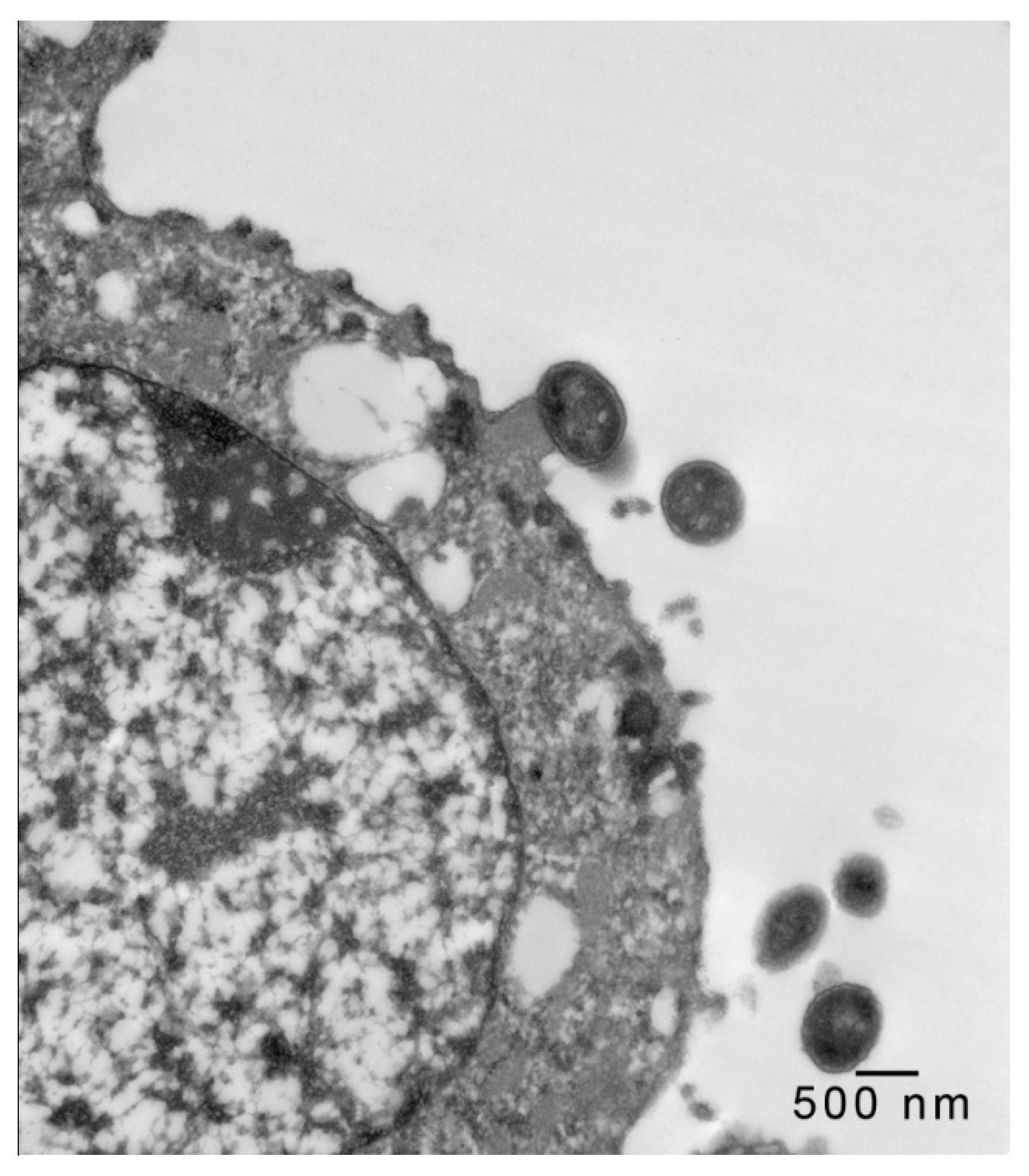
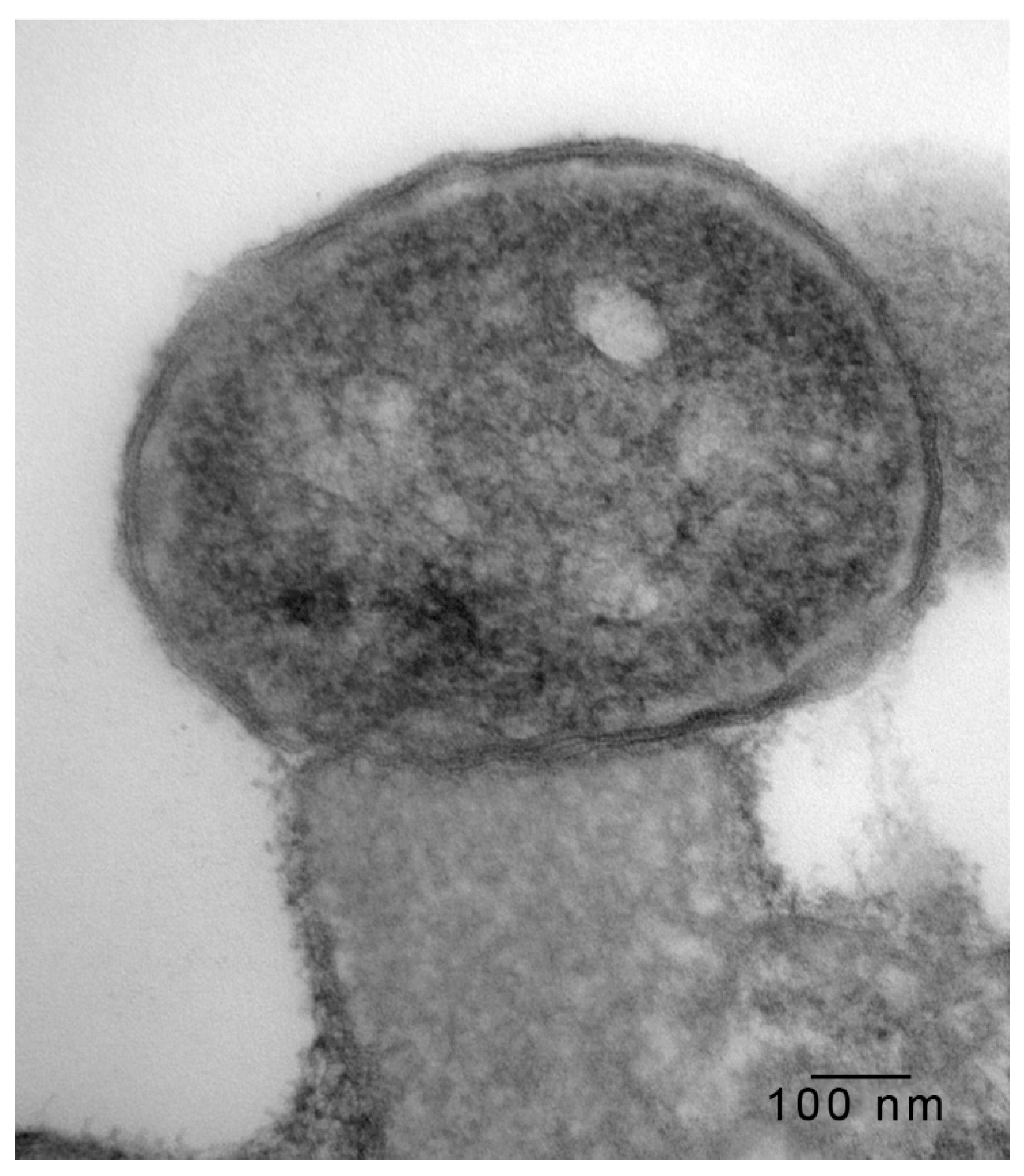
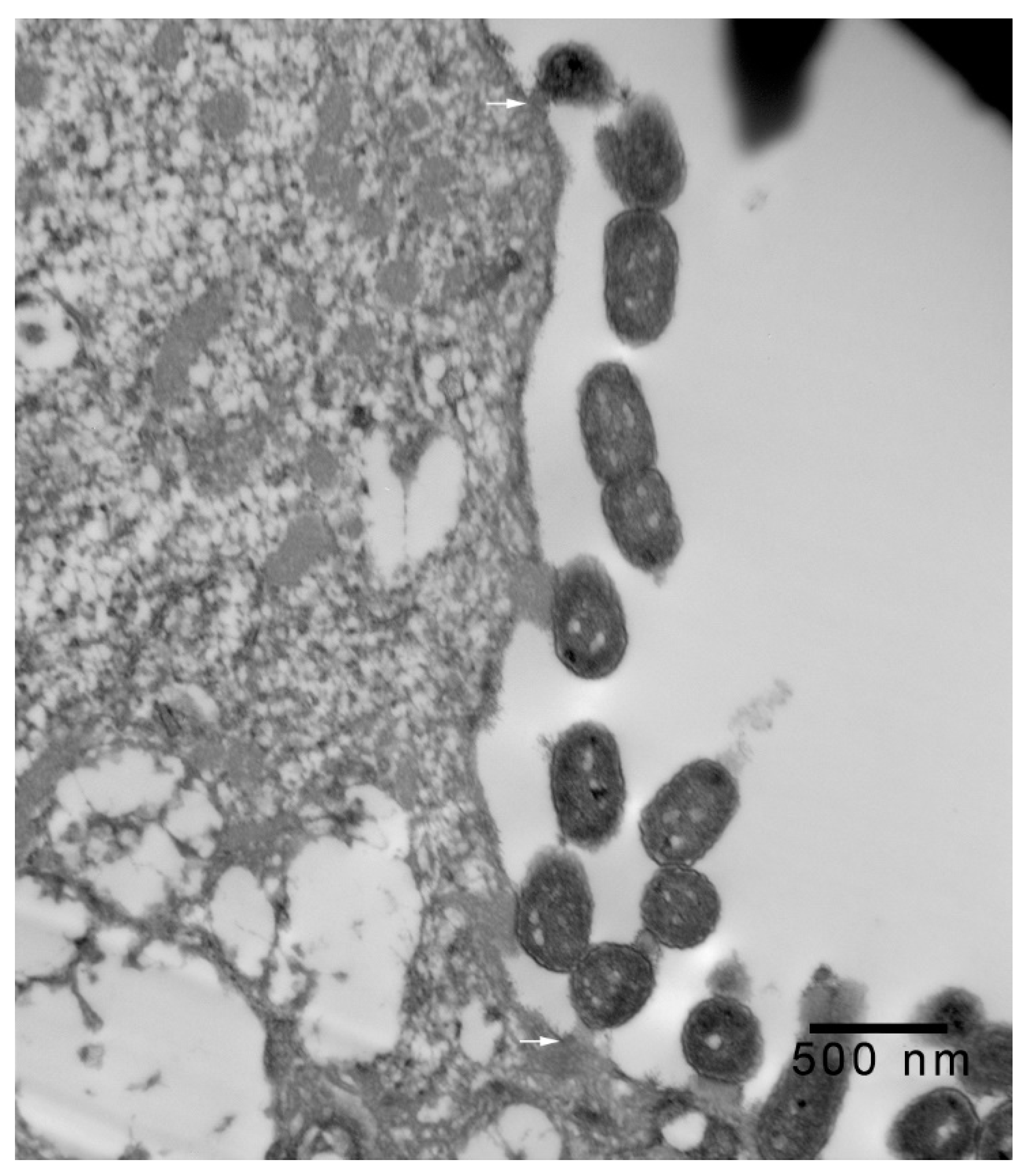
2.2. Transmission Electron Microscopy
2.3. Statistical Analyses
3. Results and Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Majowicz, S.E.; Scallan, E.; Jones-Bitton, A.; Sargeant, J.M.; Stapleton, J.; Angulo, F.J.; Yeung, D.H.; Kirk, M.D. Global incidence of human Shiga toxin-producing Escherichia coli infections and deaths: A systematic review and knowledge synthesis. Foodborne Pathog. Dis. 2014, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Tarr, P.I.; Gordon, C.A.; Chandler, W.L. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005, 365, 1073–1086. [Google Scholar] [CrossRef]
- Kavanagh, D.; Raman, S.; Sheerin, N.S. Management of hemolytic uremic syndrome. F1000Prime Rep. 2014, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control. Isolation of E. coli O157:H7 from sporadic cases of hemorrhagic colitis—United States. Morb. Mortal. Wkly. Rep. 1982, 31, 580–585. [Google Scholar]
- Riley, L.W.; Remis, R.S.; Helgerson, S.D.; McGee, H.B.; Wells, J.G.; Davis, B.R.; Hebert, R.J.; Olcott, E.S.; Johnson, L.M.; Hargrett, N.T.; et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 1983, 308, 681–685. [Google Scholar] [CrossRef]
- Remis, R.S.; MacDonald, K.L.; Riley, L.W.; Puhr, N.D.; Wells, J.G.; Davis, B.R.; Blake, P.A.; Cohen, M.L. Sporadic cases of hemorrhagic colitis associated with Escherichia coli O157:H7. Ann. Intern. Med. 1984, 101, 624–626. [Google Scholar] [CrossRef]
- Brooks, J.T.; Sowers, E.G.; Wells, J.G.; Greene, K.D.; Griffin, P.M.; Hoekstra, R.M.; Strockbine, N.A. Non-O157 Shiga toxin-producing Escherichia coli infections in the United States, 1983–2002. J. Infect. Dis. 2005, 192, 1422–1429. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Gould, L.H.; Mody, R.K.; Ong, K.L.; Clogher, P.; Cronquist, A.B.; Garman, K.N.; Lathrop, S.; Medus, C.; Spina, N.L.; Webb, T.H.; et al. Increased recognition of non-O157 Shiga toxin-producing Escherichia coli infections in the United States during 2000–2010: Epidemiologic features and comparison with E. coli O157 infections. Foodborne Pathog. Dis. 2013, 10, 453–460. [Google Scholar] [CrossRef]
- Luna-Gierke, R.E.; Griffin, P.M.; Gould, L.H.; Herman, K.; Bopp, C.A.; Strockbine, N.; Mody, R.K. Outbreaks of non-O157 Shiga toxin-producing Escherichia coli infection: USA. Epidemiol. Infect. 2014, 142, 2270–2280. [Google Scholar] [CrossRef]
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef] [PubMed]
- Lavrek, D.; Lava, S.A.G.; Milani, G.P.; Simonetti, G.D.; Bianchetti, M.G.; Giannini, O. Hemolytic-uremic syndrome after Escherichia coli urinary tract infection in humans: systematic review of the literature. J. Nephrol. 2018, 31, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Kalita, A.; Hu, J.; Torres, A.G. Recent advances in adherence and invasion of pathogenic Escherichia coli. Curr. Opin. Infect. Dis. 2014, 27, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Nielubowicz, G.R.; Mobley, H.L.T. Host-pathogen interactions in urinary tract infection. Nat. Rev. Urol. 2010, 7, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Robino, L.; Scavone, P.; Araujo, L.; Algorta, G.; Zunino, P.; Pírez, M.C. Intracellular bacteria in the pathogenesis of Escherichia coli urinary tract infection in children. Clin. Infect. Dis. 2014, 59, e158–e164. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, M.A.; Schilling, J.D.; Martinez, J.J.; Hultgren, S.J. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc. Natl. Acad. Sci. USA 2000, 97, 8829–8835. [Google Scholar] [CrossRef]
- Mulvey, M.A. Adhesion and entry of uropathogenic Escherichia coli. Cell. Microbiol. 2002, 4, 257–271. [Google Scholar] [CrossRef]
- Rosen, D.A.; Hooton, T.M.; Stamm, W.E.; Humphrey, P.A.; Hultgren, S.J. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007, 4, e329. [Google Scholar] [CrossRef]
- Wiles, T.J.; Kulesus, R.R.; Mulvey, M.A. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp. Mol. Pathol. 2008, 85, 11–19. [Google Scholar] [CrossRef]
- Mobley, H.L.; Donnenberg, M.S.; Hagan, E.C. Uropathogenic Escherichia coli. EcoSal Plus 2013. [Google Scholar] [CrossRef]
- Tamadonfar, K.O.; Omattage, N.S.; Spaulding, C.N.; Hulgren, S.J. Reaching the end of the line: urinary tract infections. Microbiol. Spectrum 2019, 7, BAI-0014-2019. [Google Scholar] [CrossRef]
- Eberly, A.R.; Beebout, C.J.; Tong, C.M.C.; Van Horn, G.T.; Green, H.D.; Fitzgerald, M.J.; De, S.; Apple, E.K.; Schrimpe-Rutledge, A.C.; Codreanu, S.G.; et al. Defining a molecular signature for uropathogenic versus urocolonizing Escherichia coli: The status of the field and new clinical opportunities. J. Mol. Biol. 2019. [Google Scholar] [CrossRef]
- Moon, H.W.; Whipp, S.C.; Argenzio, R.A.; Levine, M.M.; Giannella, R.A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect. Immun. 1983, 41, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Rosenshine, I.; Leong, J.M.; Frankel, G. Intimate host attachment: Enteropathogenic and enterohaemorrhagic Escherichia coli. Cell. Microbiol. 2013, 15, 1796–1808. [Google Scholar] [PubMed]
- Baker, D.R.; Moxley, R.A.; Steele, M.B.; LeJeune, J.T.; Christopher-Hennings, J.; Chen, D.-G.; Hardwidge, P.R.; Francis, D.H. Differences in virulence among Escherichia coli O157:H7 strains isolated from humans during disease outbreaks and from healthy cattle. Appl. Environ. Microbiol. 2007, 73, 7338–7346. [Google Scholar] [CrossRef] [PubMed]
- Scheidegger, G. Der aufbau des übergangsepithels der harnblase bei schwein, schaf, ratte und spitzmaus (Structure of the transitional epithelium in the urinary bladder of the pig, sheep, rat and shrew). Acta Anat. 1980, 107, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Liebhold, M.; Wendt, M.; Kaup, F.-J.; Drommer, W. Licht- und elektronenmikroskopische studien zur struktur des normalen blasenepithels beim weiblichen schwein (Light- and electron-microscope study of the structure of the normal bladder epithelium in female pigs). Anat. Histol. Embryol. 1995, 24, 47–52. [Google Scholar] [CrossRef]
- Khandelwal, P.; Abraham, S.N.; Apodaca, G. Cell biology and physiology of the uroepithelium. Am. J. Physiol. Renal Physiol. 2009, 297, F1477–F1501. [Google Scholar] [CrossRef]
- Hicks, R.M. The mammalian urinary bladder: An accommodating organ. Biol. Rev. Camb. Phil. Soc. 1975, 50, 215–246. [Google Scholar] [CrossRef]
- Staley, T.E.; Jones, E.W.; Corley, L.D. Attachment and penetration of Escherichia coli into intestinal epithelium of the ileum in newborn pigs. Am. J. Pathol. 1969, 56, 371–392. [Google Scholar]
- Takeuchi, A.; Inman, L.R.; O’Hanley, P.D.; Cantey, J.R.; Lushbaugh, W.B. Scanning and transmission electron microscopic study of Escherichia coli O15 (RDEC-1) enteric infection in rabbits. Infect. Immun. 1978, 19, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Ulshen, M.H.; Rollo, J.L. Pathogenesis of Escherichia coli gastroenteritis in man—Another mechanism. N. Engl. J. Med. 1980, 302, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Rothbaum, R.; McAdams, A.J.; Giannella, R.; Partin, J.C. A clinicopathologic study of enterocyte-adherent Escherichia coli: A cause of protracted diarrhea in infants. Gastroenterology 1982, 83, 441–454. [Google Scholar] [CrossRef]
- Knutton, S.; Baldwin, T.; Williams, P.H.; McNeish, A.S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: Basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 1989, 57, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, S.E. Clinical presentations and epidemiology of urinary tract infections. Microbiol. Spectrum 2016, 4, UTI-0002-2012. [Google Scholar] [CrossRef] [PubMed]
- Oelschlaeger, T.A.; Barrett, T.J.; Kopecko, D.J. Some structures and processes of human epithelial cells involved in uptake of enterohemorrhagic Escherichia coli O157:H7 strains. Infect. Immun. 1994, 62, 5142–5150. [Google Scholar] [CrossRef]
- Turner, A.M.; Subramaniam, R.; Thomas, D.F.M.; Southgate, J. Generation of a functional, differentiated porcine urothelial tissue in vitro. Eur. Urol. 2008, 54, 1423–1432. [Google Scholar] [CrossRef]
| Group a | Strain b | Number of Piglets Inoculated | Number of Piglets with Urinary Bladder Examined | Number of Piglets with Cystitis | Number of Days Post-Inoculation Tissues Examined | Identification Numbers of Litters with Piglets Having Cystitis | Identification Numbers of Piglets with Cystitis |
|---|---|---|---|---|---|---|---|
| Control | EDL933 | 13 | 13 | 0 | NA c | NA | NA |
| Human | 3234-86 | 5 | 2 | 0 | NA | NA | NA |
| B8763 | 5 | 4 | 1 | 5 | 2 | 2818 d | |
| A7785 | 5 | 5 | 0 | NA | NA | NA | |
| C9490 | 8 | 8 | 1 | 8 | 8 | 15637 d | |
| B1189 | 5 | 3 | 0 | NA | NA | NA | |
| C509 | 5 | 2 | 0 | NA | NA | NA | |
| C6183 | 6 | 5 | 1 | 8 | 5 | 9875 | |
| C8779 | 8 | 7 | 0 | NA | NA | NA | |
| C7927 | 5 | 3 | 0 | NA | NA | NA | |
| C4193 | 5 | 4 | 1 | 8 | 9 | 16506 | |
| Bovine | 2890 | 5 | 4 | 0 | NA | NA | NA |
| 2893 | 5 | 5 | 1 | 8 | 1 | 27 d | |
| 2903 | 5 | 5 | 1 | 8 | 2 | 2825d | |
| 2909 | 8 | 5 | 1 | 8 | 10 | 4099d | |
| 3032 | 5 | 4 | 0 | NA | NA | NA | |
| 2922 | 5 | 5 | 3 | 8 | 4, 9 | 8581 d, 8582, 16507 e | |
| 2918 | 5 | 5 | 1 | 8 | 7 | 12580 | |
| 2939 | 5 | 5 | 1 | 6 | 5 | 9877 | |
| 2891 | 8 | 7 | 2 | 8 | 6, 8 | 11090 d, 15627 df | |
| 2977 | 5 | 4 | 0 | NA | NA | NA | |
| Total | 126 | 105 | 14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moxley, R.A.; Bargar, T.W.; Kachman, S.D.; Baker, D.R.; Francis, D.H. Intimate Attachment of Escherichia coli O157:H7 to Urinary Bladder Epithelium in the Gnotobiotic Piglet Model. Microorganisms 2020, 8, 263. https://doi.org/10.3390/microorganisms8020263
Moxley RA, Bargar TW, Kachman SD, Baker DR, Francis DH. Intimate Attachment of Escherichia coli O157:H7 to Urinary Bladder Epithelium in the Gnotobiotic Piglet Model. Microorganisms. 2020; 8(2):263. https://doi.org/10.3390/microorganisms8020263
Chicago/Turabian StyleMoxley, Rodney A., Tom W. Bargar, Stephen D. Kachman, Diane R. Baker, and David H. Francis. 2020. "Intimate Attachment of Escherichia coli O157:H7 to Urinary Bladder Epithelium in the Gnotobiotic Piglet Model" Microorganisms 8, no. 2: 263. https://doi.org/10.3390/microorganisms8020263
APA StyleMoxley, R. A., Bargar, T. W., Kachman, S. D., Baker, D. R., & Francis, D. H. (2020). Intimate Attachment of Escherichia coli O157:H7 to Urinary Bladder Epithelium in the Gnotobiotic Piglet Model. Microorganisms, 8(2), 263. https://doi.org/10.3390/microorganisms8020263





