Abstract
Since no recent data characterizing Shiga toxin-producing E. coli (STEC) from human infections in Brazil are available, the present study aimed to investigate serotypes, stx genotypes, and accessory virulence genes, and also to perform pulsed-field gel electrophoresis (PFGE) and multi-locus sequence typing (MLST) of 43 STEC strains recovered from 2007 to 2017. Twenty-one distinct serotypes were found, with serotype O111:H8 being the most common. However, serotypes less frequently reported in human diseases were also found and included a hybrid STEC/ETEC O100:H25 clone. The majority of the strains carried stx1a as the sole stx genotype and were positive for the eae gene. Regarding the occurrence of 28 additional virulence genes associated with plasmids and pathogenicity islands, a diversity of profiles was found especially among the eae-harboring strains, which had combinations of markers composed of up to 12 distinct genes. Although PFGE analysis demonstrated genetic diversity between serotypes such as O157:H7, O111:H8, O26:H11, O118:H16, and O123:H2, high genetic relatedness was found for strains of serotypes O24:H4 and O145:H34. MLST allowed the identification of 17 distinct sequence types (STs) with ST 16 and 21 being the most common ones. Thirty-five percent of the strains studied were not typeable by the currently used MLST approach, suggesting new STs. Although STEC O111:H8 remains the leading serotype in Brazil, a diversity of other serotypes, some carrying virulence genes and belonging to STs incriminated as causing severe disease, were found in this study. Further studies are needed to determine whether they have any epidemiological relevance.
1. Introduction
Shiga toxin-producing Escherichia coli (STEC), one of the six recognized pathotypes of diarrheagenic E. coli (DEC) [1], is a worldwide foodborne pathogen responsible for causing enteric infections in humans, which can vary from mild and self-limiting diarrhea to exacerbated clinical conditions such as bloody diarrhea (BD) and hemorrhagic colitis [2]. Furthermore, patients infected with STEC may develop hemolytic uremic syndrome (HUS), which is characterized by acute renal failure, hemolytic anemia, and thrombotic thrombocytopenic purpura [3]. HUS is a major cause of permanent renal injury and can be fatal in a variable proportion of cases but especially in children [4]. The production of Shiga toxins (Stx) is the main virulence property associated with STEC pathogens [2]. These toxins comprise two major antigenically distinct groups, Stx1 and Stx2, and each group has toxin subtypes. For Stx1, there are subtypes 1a, 1c, and 1d, while for Stx2 there are currently subtypes 2a, 2b, 2c, 2d, 2e, 2f, 2g, 2h, 2i and 2k [5,6,7].
In addition to Stx production, most of the STEC isolates causing severe disease in humans may harbor other virulence determinants. The presence of the adhesin called intimin, produced by the eae gene [8], in such isolates and the secretion of a plasmid hemolysin called EHEC enterohemolysin [9], are known to enhance their pathogenic potential. The concomitant occurrence of these two additional factors in association with specific stx subtypes such as stx2a or stx2c, is a predictor of greater probability of HUS developing [10,11]. In fact, the presence of many other putative virulence factors beyond Stx, such as adhesins, toxins and autotransporter proteins [12,13,14,15] have been detected in the STEC pathotype, but their individual role in the pathogenesis of human infections is still poorly understood.
STEC strains are zoonotic pathogens, having cattle and other ruminants as their natural reservoirs [8]. Transmission to humans occurs through direct or indirect contact with the animals or their feces, or through ingestion of a variety of contaminated food or water [16,17,18]. In addition, a low infectious dose and the ability to remain viable and to respond to environmental stress provide favorable conditions for bacterial spread and outbreak occurrence [2,19].
Although hundreds of O:H E. coli serotypes are associated with the STEC pathotype [20], less than 20 of them are more often implicated with severe human illness. Serotype O157:H7 receives greater attention because of its ability to cause infections that more often evolve to BD and HUS [2], but the occurrence of complicated infections due to non-O157 serogroups has been steadily growing in recent years [21], so that in many countries, some non-O157 STEC serogroups have become a priority for public health policies and diagnostic and regulatory processes. For instance, serogroups O26, O45, O103, O111, O121, and O145, which are currently designated the “big six,” represent the most common non-O157 serogroups associated with clinical severity and HUS in the USA and Europe [22,23], and since 2011, they have been considered adulterants in food.
Despite the fact that the most serious STEC infections in humans are linked to bacterial clones belonging to a restricted number of serotypes that show specific virulence factor combinations, there are reports on the occurrence of less frequently isolated and uncommon STEC serotypes causing BD and HUS [24,25], so virtually all the Stx-producing E. coli strains may represent a risk for the human host and must, therefore, have their virulence properties determined as fully as possible.
There have been few studies describing the occurrence and characterization of the virulence potential of STEC strains isolated from human infections including HUS in Brazil [26,27,28]. On the other hand, due to the body of knowledge that has accumulated more recently about the STEC pathotype, the available information on STEC of clinical origin in Brazil can be considered out of date, and there is a gap regarding the current patterns of virulence and circulation of STEC pathogens in Brazilian settings. The detection of changes in serotype circulation and virulence properties is essential for public health policies and infection management.
Accordingly, the present study can be considered timely being aimed at characterizing STEC strains isolated from cases of human disease in Brazil, in recent years, in relation to a comprehensive set of virulence markers. Furthermore, molecular typing was also performed to compare the pattern of circulating clones with that in other countries.
2. Materials and Methods
2.1. Bacterial Strains
A total of 43 STEC strains isolated in Brazil from cases of acute and BD and HUS were studied. These strains were recovered between 2007 and 2017 as a part of the activities performed in the surveillance programs established in Brazil focusing on foodborne and waterborne pathogens. Twenty-nine of the 43 strains had been previously investigated regarding their serotypes, stx gene types and subtypes, the presence of the eae gene and the ability to produce Shiga toxins in Vero cells cultivated in vitro [29]. Presently, the remaining 15 strains had the same characteristics determined by standard procedures [30,31,32]. Moreover, since some of the strains previously serotyped by tube agglutination tests were phenotypically non-motile, all the 43 strains were now further analyzed for flagellar (H) antigens by using a multiplex PCR strategy with specific primers directed to each of the fliC gene alleles [33]. Alleles of eae were also determined in all the strains harboring this gene by Sanger sequencing [34].
2.2. Determination of Virulence Genes
A panel of several genes considered virulence markers for STEC/EHEC pathogens and other DEC pathotypes were investigated. Genes estA (ETEC thermo stable toxin), ehxA (pO157 marker), toxB (pO157 marker), katP (pO157 marker), espP (pO157 marker), iha (OI-48) efa-1 (OI-122 marker), nleB (OI-122 marker), nleE (OI-122 marker), sen (OI-122 marker), pagC (OI-122 marker), etpD (pO157 marker), cdt-V (cytolethal distending toxin -V), astA (EAST-1 toxin) subAB (pO113 marker), saa (pO113 marker), sab (STEC pO113 marker) and hes (pO113 marker) were searched by conventional PCR, while genes Z2098 (OI-57 marker), Z2099 (OI-57 marker), Z2121 (OI-57 marker), espK (OI-50 marker), ureD (OI-43/48 marker), espM1 (OI-71 marker), espN (OI-50 marker), terE (chromosomal gene associated with tellurite resistance) and espV (OI-44 marker) were investigated by real-time PCR. Primers and probes employed in these assays were as previously described [29,35,36,37,38,39,40,41,42].
2.3. Pulsed-Field Gel Electrophoresis (PFGE)
Strains belonging to the same serotype were subjected to PFGE typing following the protocols described by the PulseNet International/CDC for STEC O157 and Non-O157 (https://www.cdc.gov/pulsenet/pdf/ecoli-shigella-salmonella-pfge-protocol-508c.pdf). A CHEF-DR III apparatus (Bio-Rad) was used in all the runs, and the macrorestriction patterns obtained were analyzed with Bionumerics 7.5 (Applied Math) software using the Dice coefficient and the UPMGA method, with optimization and tolerance of 1.5%. Salmonella Braenderup was used as the reference DNA size marker.
2.4. Multi-Locus Sequence Typing (MLST)
MLST was performed by PCR amplification and Sanger sequencing of internal fragments of the housekeeping genes adk, fumC, gyrB, icdF, mdh, purA and recA [43]. Sequences were analyzed with Bionumerics 7.5 (Applied Math) software, and alleles and sequence types (ST) were assigned in accordance with the E. coli MLST database Enterobase (https://enterobase.warwick.ac.uk/species/index/ecoli). A minimal spanning tree using the eBURST algorithm was constructed to illustrate the clonal relationships between the strains analyzed.
3. Results
3.1. Serotypes and stx and eae Gene Subtypes
The 43 STEC strains studied belonged to 21 distinct O:H serotypes, and the serotypes O111:H8 and O157:H7 were the most common ones, found in 16% (7/43) and 12% (5/43) of the strains, respectively (Table 1). Overall, stx1 accounted for 26 (60%) of the isolates, while stx2 was carried by 19 (44%) strains. The majority of the strains possessed stx1 or stx2 alone, but two strains of the serotypes O111:H8 and O75:H14 harbored these two stx gene types concurrently. As can be seen in Table 1, stx1a subtype prevailed among STEC strains harboring stx1, but subtypes 1c and 1d were also detected. Regarding stx2, we found that the majority of the strains possessing this gene carried the subtypes 2a (10 strains) and 2c (eight strains), although subtypes 2d, 2e, and 2f were also present. The latter was associated exclusively with serotype O145:H34. The subtypes 2b and 2g were not detected and the strain of serotype O75:H14 had a stx2 subtype not identifiable by the subtyping scheme used.

Table 1.
Serotypes, stx types and subtypes and occurrence of eae gene in the 43 Shiga toxin-producing E. coli (STEC) of clinical origin isolated in Brazil from 2007 to 2017.
Thirty (70%) of the STEC isolates studied harbored the eae gene. Among them, the most common eae allelic type was β1 (beta 1), found in 14 strains of eight distinct serotypes. Allelic types γ2 (gamma 2) and γ1 (gamma 1) were present in all seven and five O111:H8 and O157:H7 strains, respectively. Subtypes θ (theta) and ι (iota) occurred equally in two strains: the former in a strain of serotype 077:H8 and in another strain of serotype O71:H8, and the latter in association specifically with the two strains of O145:H34 serotype.
3.2. Distribution of Other Virulence Markers
In addition to stx and eae, 28 genes related to the production of known and putative virulence factors in DEC strains, some considered to be highly specific to the STEC/EHEC pathotype, were investigated. These markers are associated with distinct genetic contexts, such as pathogenicity islands, plasmids, or phage genomes. The results obtained are shown in Figure 1 and Table 2. As can be noted, among the toxin genes searched, the sequence ehxA predominated and its occurrence was more frequently observed among the eae-harboring STEC strains, as this marker was present in 70% (21/30) of these strains, versus only 38% (5/13) for eae-negative STEC. On the other hand, the genes estA and subAB were carried only by eae-negative STEC at the same frequency of 8% (1/13) while gene astA was associated with 38% (5/13) of them. The gene related to the production of Cdt-V toxin was observed in 3% (1/30) and 8% (1/13) of the eae-positive and the eae-negative STEC strains, respectively.
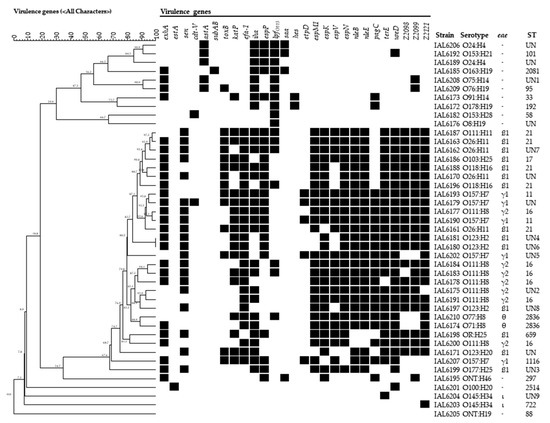
Figure 1.
Virulence profiles associated with the distinct STEC serotypes found in strains from human sources isolated in Brazil from 2007 to 2017. The dendrogram which was constructed using the software Bionumerics 7.5 (Applied Math) was based on the presence (black squares) or absence of the virulence genes investigated.

Table 2.
Occurrence of 27 distinct genes associated with several virulence mechanisms among eae-harboring and eae-lacking STEC strains of clinical origin isolated in Brazil.
Concerning genes associated with adhesion and biofilm formation, we found that in the strains harboring eae markers, iha and espP were detected in 70% (21/30) and 60% (18/30) of the strains, respectively, compared to 62% (8/13) and 46% (6/13), respectively, among the strains lacking eae, which also showed the genes lpfO113, saa, hes and toxB at frequencies of 78% (10/13), 31% (4/13), 15% (2/13) 8% (1/13), respectively. While markers saa and hes were exclusive to eae-negative STEC, toxB and lfpO113 were also found in 43% (13/30) and 23% (7/30) of the eae-positive STEC strains, respectively. The sab gene was absent in all STEC isolates studied.
The investigation of the occurrence of gene markers specific to pathogenicity islands associated with severe diarrhea and outbreaks revealed different distribution patterns concerning the presence or not of the eae gene among our STEC strains. The genes of PAI OI-122 nleB, nleE, efa-1 and sen were detected only in eae-harboring STEC, at frequencies of 87% (26/30) for nleB, nleE and efa-1, and 67% (20/30) for sen. In the same way, genes Z2098 (OI-57), Z2099 (OI-57), espM1 (OI-71), espN (OI-50), and espV (OI-44) occurred only in eae-positive STEC, in the following percentages, respectively: 73% (22/30), 87% (26/30), 83% (25/30), 87% (26/30), and 70% (21/30). However, genes pagC (OI-122) and Z2099 (OI-57) could also be found among STEC lacking eae, and at the same frequency of 23% (3/13) for both. The gene espK (OI-50) occurred mostly in eae-positive STEC, in 87% (26/30) of them, but one single eae-negative STEC isolate of serotype ONT:H46 also had this marker.
The genes katP, ureD and terE, which have been demonstrated to enhance the capacity of persistence of STEC pathogens in different environmental niches, were also included in our analysis. The frequencies observed for eae-positive strains were: 93% (28/30) for ureD, 90% (27/30) for terE and 60% (18/30) for katP. In the eae-negative group, the frequencies found for such genes were 8% (1/13) for katP and 15% for ureD (2/13). None of the eae-negative STEC in our study had terE.
The etpD gene, a marker from the pO157 of the prototype STEC O157:H7 strain EDL933 occurred in this study only in the STEC isolates of this serotype, in the five of them.
Figure 1 illustrates the several virulence profiles obtained for each of the 21 serotypes of the STEC strains of this study. None of the strains had the same profile except for two strains of serotype O123:H2. Regarding the number of genes composing each of the profiles, it was observed that in the eae-harboring strains, 11 to 19 different genes were present, except for the two strains of serotype O145:H34, which showed the presence of one single gene each. In the isolates lacking eae, the number of genes composing their virulence profiles varied from a minimum of one to a maximum of seven different virulence markers.
3.3. PFGE Analysis
PFGE was performed in the strains of serotypes O24:H4, O26:H11, O111:H8, O118:H16, O123:H2, O145:H34, and O157:H7. Figure 2 illustrates the XbaI restriction patterns obtained. As it can be noted in the non-O157 serogroups (2B) PFGE typing clearly segregated serotypes O111:H8, O24:H4 and O145:H34. However, some strains of serotypes O123:H2 O118:H16 and O26:H11 tended to cluster together. The highest genetic relatedness was observed for serotypes O24:H4 and O145:H34, where the similarity indices found were 97.7 and 92.3%, respectively. Strains of serotype O111:H8 exhibited genetic similarities ranging from 75.57% to 89.5%. Two of these strains coupled together forming a small cluster (considering a similarity index >85%).
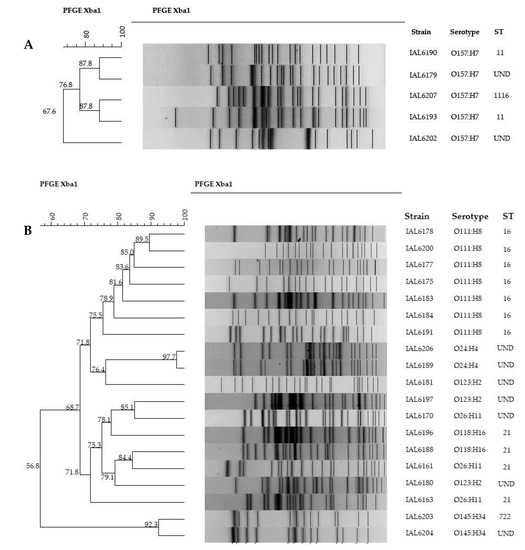
Figure 2.
Dendrogram illustrating the genetic relatedness of STEC strains of serotypes O157:H7 (A) and O24:H4, O26:H11, O111:H8, O118:H16, O123:H2, O145:H34 (B), associated with human infections in Brazil from 2007 to 2017.
Among the five O157:H7 (2A) strains, two distinct small clusters formed by two strains each with the same genetic similarity, 87.8%, was observed. A single isolate showed a low degree of relatedness with the other four, with only 67.6% similarity.
3.4. Multi-Locus Sequence Typing (MLST)
MLST allowed the assignment of 17 distinct sequence types (ST) among 28 (65%) of the studied strains. All the ST found are exhibited in Figure 3. ST 16 and 21 prevailed, where ST 16 was associated exclusively with strains of serotype O111:H8, all of them, and ST 21 with serotypes O26:H11 (two strains), O118:H16 (two strains) and one strain of serotype O111:H11. Of the five STEC strains O157:H7, three showed ST 11 and one showed ST 1116. ST 2836 was associated with serotypes O77:H8 and O71:H8; all the other ST occurred only once and in distinct serotypes. Fifteen strains could not have an ST assigned according to the E. coli MLST scheme used in our study. This occurred due to the specific combination of the seven alleles identified which resulted in the absence of a valid ST in the Enterobase database (in nine strains), or due to the impossibility to achieve an allele number for one or two of the genes in the MLST scheme (in six strains). The profiles of alleles of the strains without a valid ST number are shown in Table 3.
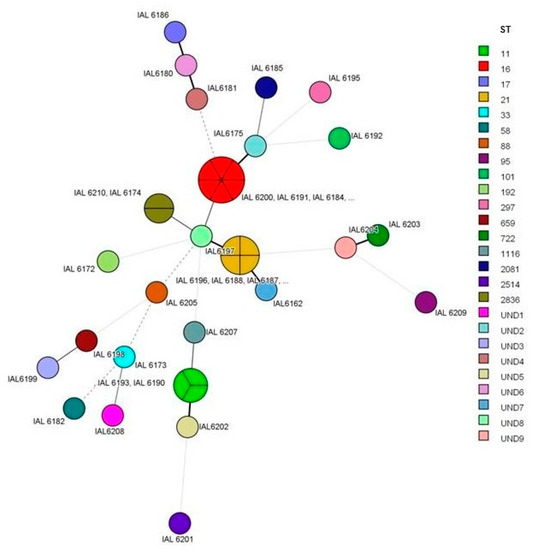
Figure 3.
Minimum spanning tree showing the clonal relationships between STEC strains from human infections as assessed by multi-locus sequence typing (MLST) in this study. Each circle represents a distinct ST and the connecting lines indicate allele differences between the different sequence types. UND, undetermined; ST according to MLST database Enterobase. A random number was assigned.

Table 3.
Allelic profiles for genes adk, fumC, gyrB, icdF, mdh, purA, recA, shown by STEC strains with unassigned ST in the Acthman MLST scheme.
Considering the isolates for which an ST could be determined, 22 of them were further assigned to seven distinct clonal complexes (CC). It was observed that CC 29 prevailed and encompassed 13 strains belonging to serotypes O26:H11 (ST 21), O71:H8 (ST 2836), O77:H8 (ST 2836), O111:H8 (ST 16), O111:H11 (ST 21) and O118:H16 (ST 21). Moreover, CC 11 harbored three of the O157:H7 strains with ST 11 (two strains) and 1116 (one strain). The remaining strains were associated with CC 10, 20, 23, 95, 101 and 115, or formed singletons.
4. Discussion
STEC pathogens pose a serious risk to public health, and this group of bacteria has been causing large outbreaks worldwide since the early 1980s, with many cases of HUS and varying lethality rates [44]. Although HUS is, from the medical point of view, the most severe clinical condition in the STEC infections, the diseases associated with these pathogens can have diverse presentations, including mild forms of diarrhea and asymptomatic carriers of the bacteria [2]. Much evidence indicates that the clinical prognosis in the STEC infections can be influenced by the presence of additional virulence factors beyond the Shiga toxin subtype produced by a given strain [10]. Thus, the characterization of STEC clones associated with human diseases regarding their virulence genes content, including stx genes subtypes, is currently a strategy of great value for the adequate management of human infections and for the prediction of the clinical evolution. Taking these points into consideration and also the fact that there are no recent studies about STEC pathogens from humans in Brazil, this study aimed to perform a comprehensive characterization regarding the presence of several virulence genotypic markers and the molecular typing of strains of clinical sources, recovered from surveillance programs conducted in Brazil, in a range of time spanning the years from 2007 to 2017.
By comparing the results of serotyping in the present study with studies conducted in Brazil up to 2006 [26], we noticed that STEC O111:H8 remains the most common serotype in association with human infections. However, in this study, we were able to describe the occurrence of serotypes that have never been detected before in our country, although they have already been implicated in sporadic human illness in other places. Examples include serotypes such as O8:H19, O178:H19, and O100:H20 which have been reported in European countries [45,46,47]. The diversity of STEC serotypes currently observed in Brazil is in agreement with reports from other countries [48,49] where due to the broader use of molecular tools such as PCR and whole genome sequencing in the characterization of STEC isolates a wider spectrum of serotypes is being detected. This is important because since the large outbreak caused by EHEC/EAEC O104:H4 in 2011, there is growing concern regarding non-O157 STEC serotypes, since their significance in human diseases is not yet well established, contrasting STEC O157 or a few non-O157 serogroups such as those forming the “big six,” which are undoubtedly major human threats. Thus, surveillance should not underestimate the pathogenic potential of any STEC serotype and should always consider that STEC pathogens may change over time to more virulent forms.
The majority of the strains analyzed carried stx1 as the sole stx type, and most of them showed the genotype 1a. This finding may partly explain the reason for which STEC infections in Brazil are mostly sporadic and of non-complicated nature, diverging from other countries where many of the reports about STEC pathogens involved in outbreaks and HUS are caused by strains carrying only stx2, with the predominance of two genotypes, 2a and 2c [50,51]. However, it should be noted that we describe here for the first time in Brazil an O26:H11 STEC carrying stx2a only. According to previous studies analyzing STEC pathogens in our country, all the isolates from human origin belonging to serotype O26:H11 had stx1 only [26]. Stx2a-producing O26:H11 STEC have emerged on the European continent in the middle of the last decade and since then became the most common cause of HUS involving non-O157 STEC serogroups, especially in Germany and France [22,52]. Our current finding of the circulation of STEC O26:H11 stx2a+ is a matter of concern and highlights the need for constant surveillance to detect changes in the virulence patterns of pathogens. Additional surveys must be performed in the near future to determine if the occurrence of O26:H11 stx2a+ STEC represents the establishment of this type of clone in our settings as observed in Europe or is an isolated finding.
A strain belonging to serotype O100:H20 (IAL6201) was found to be a hybrid clone harboring markers of both STEC (stx2e) and ETEC (estIA) pathotypes. To the best of our knowledge, this is the first description in Brazil of a hybrid STEC recovered from a human infection. Although the major focus on hybrid pathotypes is currently related to clones carrying STEC/EAEC markers, some studies have reported the occurrence of STEC/ETEC hybrids [47], and the clinical significance of these isolates needs to be established. It is noteworthy that ETEC was considered the most important bacterial enteropathogen in cases of severe and moderate diarrhea with risk of death in children up to five years old [53], so a given clone harboring STEC and ETEC virulence properties is a matter of great concern. In addition, in a strain of serotype O75:H14 (IAL6208), the stx2 subtype could not be determined with the PCR assay used in this study which has been widely used in several other studies for stx subtyping. It is plausible that this particular strain has an unknown variant for one of the stx2 subtypes, or even a new stx subtype, since new stx2 subtypes have been reported more recently [5,6,7]. Taken together, these data demonstrate how diverse the STEC pathotype has been occurring in Brazil, a fact that has been constantly demanding adequate diagnostic strategies from public health laboratories.
About 70% of the STEC strains analyzed carried the eae gene. These included strains belonging to serogroups of major epidemiologic importance such as O157, O111, and O26, where the presence of this marker is expected [2], and also serogroups such as O71, O77, O118, and O123, which are less frequently reported in human infections. Accordingly, two points should be taken into consideration; first, the presence of the eae gene in STEC isolates of serotypes such as O157:H7, O111:H8 and O26:H11 is a well-established risk factor for HUS development [54], but as in the case of the strains belonging to the less common serogroups mentioned above, one should wonder if it is possible to make the same assumption. Second, according to previous studies conducted in our country, most of the STEC strains isolated from animals linked to the food production chain lack the eae gene. Therefore, the source of some of the serotypes such as O123:H2 and O145:H34, which have never been described in association with the animal reservoir in Brazil, is currently unknown. A distinct situation may be drawn in relation to the eae-lacking serotypes, such as O91:H14, O178:H19 and O8:H19, also detected in this study and for which there are previous reports about their occurrence in the Brazilian animal reservoir [55,56].
Because there is no specific pattern of genetic markers capable of inferring the pathogenic potential of a given STEC isolate, the search for a broad set of virulence-associated genes has become the best strategy for measuring the microbiological and clinical risks that these pathogens may pose. In our study, a diversity of virulence profiles could be detected in the set of strains analyzed, and regarding the genes that composed such profiles, we observed a clear distinction between the strains according to the presence or not of the eae gene. While in the strains bearing eae, the profiles found were mostly composed of a large number of genes associated with pO157 and pathogenicity islands, such as OI-122, OI-44, OI-48, OI-50, OI-71, and OI-57, in the strains lacking eae, the number of genes forming the virulence profile was limited, and only in some serotypes was there a correlation with the presence of pO113, as in the case of the strains that harbored the saa gene. Our results are in agreement with reports from other countries describing the high pathogenic potential associated with serogroups such as O157, O111, and O26 [2]. It is worth mentioning that although most of the STEC infections in Brazil represent mild and uncomplicated gastroenteritis, the severe cases with evolution to HUS in our country are indeed caused by serotypes O157:H7, O26:H11 and O111:H8 [IAL unpublished data] [29,57]. Among the strains carrying eae and showing high pathogenicity profiles we also observed the presence of serotypes O71:H8, O77:H8, O118:H16 and O123:H2. The last serotype has been of particular interest and should receive attention in future studies, because in addition to the fact that it has been isolated as STEC, it has also been occurring in Brazil as aEPEC [IAL unpublished data]. Interestingly, the three strains of serotype O123:H2 analyzed in this study showed evidence of carrying a complete form of OI-122, which is a hallmark in terms of virulence and outbreak potential [42]. The exception in the group of eae-positive strains with a large number of virulence markers was observed in the two stx2f O145:H34 strains, which had very few markers indicating a low potential to cause more serious disease, as suggested previously [58].
Contrasting other studies reporting the fact that genes such as saa and subAB are frequent in eae-lacking STEC strains with high pathogenic potential [59], in our study these markers were observed at low frequencies. Our present results also diverge from those of previous analyses conducted in Brazil involving the characterization of eae-negative STEC isolated from different animal reservoirs, where it was found that saa and subAB occurred at high frequencies, and furthermore, most of the strains harboring subAB were able to express it [14,56]. This suggests that eae-negative STEC circulating in Brazil may have distinct virulence potentials depending on their source of isolation. More comparative studies between human and animal strains are needed to resolve this issue and to better understand the existing relationships between STEC pathogens and their different hosts. Two of the eae-lacking strains carried the hes gene, which was recently described in eae-negative STEC and associated with adhesion capacity and biofilm formation [15]. This is the first report on the occurrence of this gene in clinical STEC strains isolated in Brazil.
Since we found no evidence of a possible epidemiological link between the patients from whom the STEC strains involved in our study were isolated, our PFGE analysis was performed with the main purpose of investigating the genetic relatedness and the dissemination of specific clones belonging to the serotypes that were recovered more than once in our study period. The two serotypes in which the highest genetic relatedness (>90%) was observed, thus suggesting the existence of a clone, were the serotypes O24:H4 and O145:H34. For the other serotypes, PFGE typing confirmed the fact that they were sporadic cases of infection, corroborating previous surveys conducted in Brazil where it was demonstrated that the occurrence of large outbreaks is not an epidemiological feature associated with human infections, although a previous study provided molecular evidence of the occurrence of a small outbreak involving the serotype O157:H7 [26]. In Brazil, STEC infections are currently subjected to active surveillance in a sentinel surveillance system. In addition, since 2016, reporting HUS has become nationally compulsory, and therefore, the detection of outbreaks and HUS cases are expected to increase in the coming years.
Studies employing MLST to compare STEC strains isolated in Brazil with a broad range of isolates from other countries have not been performed yet, except for one study involving animal strains belonging specifically to the serotype O113:H21 [59]. In general, our MLST analyses demonstrated that STEC isolates in Brazil are phylogenetically diverse. However, for serotypes of major epidemiological importance such as O157:H7, O111:H8, and O26:H11, the ST and clonal complexes found were the same as reported in other countries [60], and about 35% of the strains analyzed in this study did not have an ST assigned, suggesting that they may represent new ones. It was not possible to assign an ST number to these isolates because the curation of the Enterobase database no longer accepts data generated by Sanger sequencing. Among these putative new STs, there were strains of serotypes such as O157:H7, O111:H8, and O26:H11. Two O157:H7 strains in this study had undefined ST and were single-locus variants (SLVs) of ST11 which was associated with serotype O157:H7. One of the strains was an SLV for the gene fumC and the other was an SLV for purA. However, as these two isolates had similar virulence gene contents compared with the other ST11-O157:H7 strains analyzed here, it is possible to infer that they are part of the same clonal complex that harbors ST11. The same was observed for one strain of serotype O111:H8, an SLV of ST16 for the fumC gene. The fact that all of the O111:H8 STEC isolates in our study, except this particular one, showed ST16 indicates that ST16 predominates in the O111:H8 STEC population in Brazil, with only a minority of clones diverging.
ST21 was the second most common ST found in the present study. This ST belongs to CC29 which encompasses highly virulent STEC clones of serotypes such as O26:H11 and O118:H16. In fact, two of the four O26:H11 strains and the two O118:H16 strains in this study fell into the ST21. Moreover, our PFGE analysis demonstrated more than 80% similarity between the strains of these two serotypes, thus corroborating the MLST results. Interestingly, one strain of serotype O111:H11 also resulted in the ST21, suggesting that a certain correlation between the ST and the flagellar antigens may exist in serogroup O111, since all the other O111 strains had H8 flagellar antigen and were typed as ST16. Two O26:H11 strains in this study had an undefined ST. One of them showing stx1a was an SLV of ST21 (mdh 23 instead of 9) and the other which showed stx2a, diverged from ST21 in four genes, but was closer to ST29, which is often associated with STEC O26:H11 [22,61]. On the basis of the ST number, stx genotypes and the plasmid gene profiles, there are currently distinct circulating clones of O26:H11 STEC [53,61]. The ST29 include those harboring stx2a or stx2d alone with specific plasmidial genes profiles: ehx+, espP+, katP+, etpD- for strains with stx2a or ehx+, espP-, katP-, etpD+ for strains with stx2d [22]. In our study, the O26:H11 strain with stx2a did not match the plasmid profile expected for stx2a O26:H11 STEC clones, so it might represent a distinct lineage of O26:H11 STEC, thus reflecting how diverse this serotype is in terms of clonal population.
Among the strains lacking eae, we found STs rarely reported in human infections, whose associated serotypes are more commonly observed in animals. But there were two exceptions involving serotypes O91:H14 and O178:H19, each isolated once in this study. The strain of serotype O91:H14 rendered ST33, which is described in the literature as being responsible for mild STEC diseases when compared with STEC O91:H21, which has already been recovered from HUS [62]. In fact, this strain was isolated from a teenage boy with uncomplicated acute diarrhea. The strain of serotype O178:H19 had ST192 in accordance with a study from Switzerland where the same ST was associated with this serotype [60]. It is worth mentioning that STEC O178:H19 is one of the most common STEC serotypes recovered from beef and dairy cattle in South America [46].
In conclusion, STEC strains from clinical sources isolated in Brazil from 2007 to 2017 were found to belong to a diversity of serotypes, but serotype O111:H8 was the most common one. The majority of the strains carried eae and stx1a, but also an array of virulence genes related to STECs causing HC and HUS, implying that these strains are potentially highly pathogenic. PFGE and MLST approaches enabled comparisons across the isolates to establish genetic diversity and phylogeny. This was important to demonstrate that STEC infections in Brazil are mostly sporadic and to uncover possible new clonal lineages. As we are heading to a genomic surveillance era by using more broadly whole genome sequencing techniques, further studies on the mechanism behind the emergence of these new clones will be possible. Given the fact that the clinical importance of some of the serotypes described in this study remains poorly appreciated, STEC epidemiology and human infections in our settings should continue to be carefully surveyed.
Author Contributions
Conceptualization, R.T.H., E.H.T., B.E.C.G., T.S.d.A., M.C.C.-N., and L.F.d.S.; Methodology, A.M.F.C., E.H.T., E.d.L.O., S.R.S.P., S.L.O., G.R.F.; Writing—original draft preparation, L.F.d.S.; Writing—review and editing, R.T.H., E.H.T., B.E.C.G., T.S.d.A., M.C.C.N and G.R.F; Supervision, L.F.d.S.; Project administration, L.F.d.S.; Funding acquisition, L.F.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by São Paulo Research Foundation (FAPESP), grant number 2017/00411-1. S.L.O. received a FAPESP fellowship (grant number: 2014/20354-4).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the following: design of the study; collection, analyses, or interpretation of data; and writing of the manuscript, or decision to publish the results.
References
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef] [PubMed]
- Paton, J.C.; Paton, A.W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 1998, 11, 450–479. [Google Scholar] [CrossRef] [PubMed]
- Karmali, M.; Petric, M.; Lim, C.; Fleming, P.C.; Arbus, G.S.; Lior, H. The association between idiophatic hemolytic uremic syndrome and infection by Verotoxin-producing Escherichia coli. J. Infect. Dis. 1985, 151, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Majowicz, S.E.; Scallan, E.; Jones-bitton, A.; Jan, M.; Stapleton, J.; Angulo, F.J.; Yeung, D.H.; Kirk, M.D. Global incidence of human Shiga toxin-producing Escherichia coli infections and deaths: a systematic review and knowledge systhesis. Foodborne Pathog Dis. 2015, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Lacher, D.W.; Gangiredla, J.; Patel, I.; Elkins, C.A.; Feng, P.C.H. Use of the Escherichia coli identification microarray for characterizing the health risks of Shiga toxin-producing Escherichia coli isolated from foods. J. Food Prot. 2016, 79, 1656–1662. [Google Scholar] [CrossRef]
- Bai, X.; Fu, S.; Zhang, J.; Fan, R.; Xu, Y.; Sun, H.; He, X.; Xu, J.; Xiong, Y. Identification and pathogenomic analysis of an Escherichia coli strain producing a novel Shiga toxin 2 subtype. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Hughes, A.C.; Zhang, Y.; Bai, X.; Xiong, Y.; Wang, Y.; Yang, X.; Xu, Q.; He, X. Structural and functional characterization of stx2k, a new subtype of Shiga toxin 2. Microorganisms 2020, 8, 4. [Google Scholar] [CrossRef]
- Caprioli, A.; Morabito, S.; Brugere, H.; Oswald, E. Enterohemorrhagic Escherichia coli: emerging issues on virulence and modes of transmission. Vet. Res. 2005, 36, 289–311. [Google Scholar] [CrossRef]
- Beutin, L.; Montenegro, M.A.; Orskov, I.; Orskov, F.; Prada, J.; Zimmermann, S.; Stephan, R. Close association of Verotoxin (Shiga-like toxin) production with enterohemolysin production in strains of Escherichia coli. J. Clin. Microbiol. 1989, 27, 2559–2564. [Google Scholar] [CrossRef]
- Ethelberg, S.; Olsen, K.E.P.; Scheutz, F.; Jensen, C.; Schiellerup, P.; Engberg, J.; Petersen, A.M.; Olesen, B.; Gerner-Smidt, P.; Mølbak, K. Virulence factors for hemolytic uremic syndrome, Denmark. Emerg. Infect. Dis. 2004, 10, 842–847. [Google Scholar] [CrossRef]
- Boerlin, P.; McEwen, S.A.; Boerlin-Petzold, F.; Wilson, J.B.; Johnson, R.P.; Gyles, C.L. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 1999, 37, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.; Espinosa, E.M.; Song, T.; Miliwebsky, E.; Chinen, I.; Iyoda, S.; Iwanaga, M.; Rivas, M.; Malbra, A.C.G.; Icrobiol, J.C.L.I.N.M. Distribution of putative adhesins in different seropathotypes of Shiga toxin-producing Escherichia coli. J. Clin. Microbiol. 2004, 42, 4937–4946. [Google Scholar] [CrossRef] [PubMed]
- Brunder, W.; Schmidt, H.; Frosch, M.; Karch, H. The large plasmids of Shiga-toxin-producing Escherichia coli (STEC) are highly variable genetic elements. Microbiology 1999, 145, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Irino, K.; Midolli Vieira, M.A.; Tardelli Gomes, T.A.; Cabilio Guth, B.E.; Furtado Naves, Z.V.; Oliveira, M.G.; Dos Santos, L.F.; Guirro, M.; Timm, C.D.; Pigatto, C.P.; et al. Subtilase cytotoxin-encoding subAB operon found exclusively among Shiga toxin-producing Escherichia coli strains. J. Clin. Microbiol. 2010, 48, 988–990. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.A.; Velasco, J.; Del Canto, F.; Puente, J.L.; Padola, N.L.; Rasko, D.A.; Farfán, M.; Salazar, J.C.; Vidal, R. Locus of adhesion and autoaggregation (LAA), a pathogenicity island present in emerging Shiga toxin–producing Escherichia coli strains. Sci. Rep. 2017, 7, 7011. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.J.; Miller, G.; Breuer, T.; Kennedy, M.; Higgins, C.; Walford, J.; McKee, G.; Fox, K.; Bibb, W.; Mead, P. A waterborne outbreak of Escherichia coli O157:H7 infections and hemolytic uremic syndrome: Implications for rural water systems. Emerg. Infect. Dis. 2002, 8, 370–375. [Google Scholar] [CrossRef]
- Ihekweazu, C.; Carroll, K.; Adak, B.; Smith, G.; Pritchard, G.C.; Gillespie, I.A.; Verlander, N.Q.; Harvey-Vince, L.; Reacher, M.; Edeghere, O.; et al. Large outbreak of Verocytotoxin-producing Escherichia coli O157 infection in visitors to a petting farm in south east England, 2009. Epidemiol. Infect. 2012, 140, 1400–1413. [Google Scholar] [CrossRef]
- González-Escalona, N.; Kase, J.A. Virulence gene profiles and phylogeny of Shiga toxin-positive Escherichia coli strains isolated from FDA regulated foods during 2010–2017. PLoS ONE 2019, 14, 1–26. [Google Scholar] [CrossRef]
- Nesse, L.L.; Sekse, C.; Berg, K.; Johannesen, K.C.S.; Solheim, H.; Vestby, L.K.; Urdahl, A.M. Potentially pathogenic Escherichia coli can form a biofilm under conditions relevant to the food production chain. Appl. Env. Microbiol. 2014, 80, 2042–2049. [Google Scholar] [CrossRef]
- Byrne, L.; Vanstone, G.L.; Perry, N.T.; Launders, N.; Adak, G.K.; Godbole, G.; Grant, K.A.; Smith, R.; Jenkins, C. Epidemiology and microbiology of Shiga toxin-producing Escherichia coli other than serogroup O157 in England, 2009–2013. J. Med. Microbiol. 2014, 63, 1181–1188. [Google Scholar] [CrossRef]
- Hughes, J.M.; Wilson, M.E.; Johnson, K.E.; Thorpe, C.M.; Sears, C.L. The emerging clinical importance of non-O157 Shiga toxin-producing Escherichia coli. Clin. Infect. Dis. 2006, 43, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Delannoy, S.; Mariani-Kurkdjian, P.; Bonacorsi, S.; Liguori, S.; Fach, P. Characteristics of emerging human-pathogenic Escherichia coli O26:H11 strains isolated in France between 2010 and 2013 and carrying the stx2d gene only. J. Clin. Microbiol. 2015, 53, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.T.; Sowers, E.G.; Wells, J.G.; Greene, K.D.; Griffin, P.M.; Hoekstra, R.M.; Strockbine, N.A. Non-O157 Shiga toxin–producing Escherichia coli infections in the United States, 1983–2002. J. Infect. Dis. 2005, 192, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Paton, A.W.; Woodrow, M.C.; Doyle, R.M.; Lanser, J.A.; Paton, J.C. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic- uremic syndrome. J. Clin. Microbiol. 1999, 37, 3357–3361. [Google Scholar] [CrossRef]
- de Souza, R.L.; Nishimura, L.S.; Guth, B.E.C. Uncommon Shiga toxin-producing Escherichia coli serotype O165:HNM as cause of hemolytic uremic syndrome in São Paulo, Brazil. Diagn. Microbiol. Infect. Dis. 2007, 59, 223–225. [Google Scholar] [CrossRef]
- Vaz, T.M.I.; Irino, K.; Nishimura, L.S.; Cecı, M.; Guth, B.E.C. Genetic heterogeneity of Shiga toxin-producing Escherichia coli strains isolated in São Paulo, Brazil, from 1976 through 2003 as revealed by pulsed-field gel electrophoresis. J. Clin. Microbiol. 2006, 44, 798–804. [Google Scholar] [CrossRef]
- Irino, K.; Vaz, T.M.; Kato, M.A.; Naves, Z.V.; Lara, R.R.; Marco, M.E.; Rocha, M.M.; Moreira, T.P.; Gomes, T.A.; Guth, B.E. O157:H7 Shiga toxin-producing Escherichia coli strains associated with sporadic cases of diarrhea in São Paulo, Brazil. Emerg. Infect. Dis. 2002, 8, 446–447. [Google Scholar] [CrossRef]
- Guth, B.E.C.; Ramos, S.R.T.S.; Cerqueira, A.M.F.; Andrade, J.R.C.; Gomes, T.A.T. Phenotypic and genotypic characteristics of Shiga toxin-producing Escherichia coli strains isolated from children in São Paulo, Brazil. Mem. Inst. Oswaldo Cruz 2002, 97, 1085–1089. [Google Scholar] [CrossRef][Green Version]
- Ori, E.L.; Takagi, E.H.; Andrade, T.S.; Miguel, B.T. Diarrhoeagenic Escherichia coli and Escherichia albertii in Brazil: pathotypes and serotypes over a 6-year period of surveillance. Epidemiol. Infect. 2018, 147, e10. [Google Scholar] [CrossRef]
- Peresi, J.T.M.; de Almeida, I.A.Z.C.; Vaz, T.M.I.; Hernandes, R.T.; de Carvalho Teixeira, I.S.; de Lima e Silva, S.I.; Graciano, R.A.S.; Pinheiro, S.R.; dos Santos, L.F. Search for diarrheagenic Escherichia coli in raw kibbe samples reveals the presence of Shiga toxin-producing strains. Food Control 2016, 63, 165–170. [Google Scholar] [CrossRef][Green Version]
- Edwards, P.R.; Ewing, W.H. Edwards and Ewing’s Identification of Enterobacteriaceae, 4th ed.; Elsevier: New York, NY, USA, 1986; 536p. [Google Scholar]
- Scheutz, F.; Teel, L.D.; Beutin, L.; Piérard, D.; Buvens, G.; Karch, H.; Mellmann, A.; Caprioli, A.; Tozzoli, R.; Morabito, S.; et al. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J. Clin. Microbiol. 2012, 50, 2951–2963. [Google Scholar] [CrossRef] [PubMed]
- Banjo, M.; Iguchi, A.; Seto, K.; Kikuchi, T.; Harada, T.; Scheutz, F.; Iyoda, S. Escherichia coli H-genotyping PCR: A complete and practical platform for molecular H typing. J. Clin. Microbiol. 2018, 56, e00190-18. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Blanco, J.E.; Mora, A.; Dahbi, G.; Alonso, M.P.; Gonza, E.A.; Berna, M.I.; Blanco, J. Serotypes, virulence genes, and intimin types of Shiga toxin (Verotoxin)-producing Escherichia coli. J. Clin. Microbiol. 2004, 42, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Cergole-Novella, M.C.; Nishimura, L.S.; Dos Santos, L.F.; Irino, K.; Vaz, T.M.I.; Bergamini, A.M.M.; Guth, B.E.C. Distribution of virulence profiles related to new toxins and putative adhesins in Shiga toxin-producing Escherichia coli isolated from diverse sources in Brazil. Fems Microbiol. Lett. 2007, 274, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.F.; Irino, K.; Vaz, T.M.I.; Guth, B.E.C. Set of virulence genes and genetic relatedness of O113:H21 Escherichia coli strains isolated from the animal reservoir and human infections in Brazil. J. Med. Microbiol. 2010, 59, 634–640. [Google Scholar] [CrossRef]
- Herold, S.; Paton, J.C.; Paton, A.W. Sab, a novel autotransporter of locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli O113:H21, contributes to adherence and biofilm formation. Infect. Immun. 2009, 77, 3234–3243. [Google Scholar] [CrossRef]
- Colello, R.; Vélez, M.V.; González, J.; Montero, D.A.; Bustamante, A.V.; Del Canto, F.; Etcheverría, A.I.; Vidal, R.; Padola, N.L. First report of the distribution of locus of adhesion and autoaggregation (LAA) pathogenicity island in LEE-negative Shiga toxin-producing Escherichia coli isolates from Argentina. Microb. Pathog. 2018, 123, 259–263. [Google Scholar] [CrossRef]
- Delannoy, S.; Beutin, L.; Fach, P. Discrimination of enterohemorrhagic Escherichia coli (EHEC) from Non-EHEC strains based on detection of various combinations of Type III effector genes. J. Clin. Microbiol. 2013, 51, 3257–3262. [Google Scholar] [CrossRef]
- Delannoy, S.; Beutin, L.; Fach, P. Towards a molecular definition of enterohemorrhagic Escherichia coli (EHEC): detection of genes located on O island 57 as markers to distinguish EHEC from closely related enteropathogenic E. coli strains. J. Clin. Microbiol. 2013, 51, 1083–1088. [Google Scholar] [CrossRef][Green Version]
- Vieira, M.A.; Dos Santos, L.F.; Dias, R.C.B.; Camargo, C.H.; Pinheiro, S.R.S.; Gomes, T.A.T.; Hernandes, R.T. Atypical enteropathogenic Escherichia coli as etiologic agents of sporadic and outbreak-associated diarrhea in Brazil. J. Med. Microbiol. 2016, 998–1006. [Google Scholar] [CrossRef]
- Mercado, E.H.; Piscoche, C.; Contreras, C.; Durand, D.; Riveros, M.; Ruiz, J.; Ochoa, T.J. Pathogenicity Island O-122 in enteropathogenic Escherichia coli strains is associated with diarrhea severity in children from Lima Peru. Int. J. Med. Microbiol. 2016, 306, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Falush, D.; Lan, R.; Colles, F.; Mensa, P.; Wieler, L.H.; Karch, H.; Reeves, P.R.; Maiden, M.C.J.; Ochman, H.; et al. Sex and virulence in Escherichia coli: An evolutionary perspective. Mol. Microbiol. 2006, 60, 1136–1151. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wang, P.; Lan, R.; Ye, C.; Wang, H.; Ren, J.; Jing, H.; Wang, Y.; Zhou, Z.; Bai, X.; et al. A novel Escherichia coli O157:H7 clone causing a major hemolytic uremic syndrome outbreak in China. PLoS ONE 2012, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Saupe, A.; Edel, B.; Pfister, W.; Löffler, B.; Ehricht, R.; Rödel, J. Acute diarrhoea due to a Shiga toxin 2e-producing Escherichia coli O8:H19. JMM Case Rep. 2017, 4, 4–6. [Google Scholar] [CrossRef]
- Miko, A.; Rivas, M.; Bentancor, A.; Delannoy, S.; Fach, P.; Beutin, L. Emerging types of Shiga toxin-producing E. coli (STEC) O178 present in cattle, deer, and humans from Argentina and Germany. Front. Cell. Infect. Microbiol. 2014, 4, 1–14. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, J.; Ambikan, A.; Jernberg, C.; Ehricht, R.; Scheutz, F.; Xiong, Y.; Matussek, A. molecular characterization and comparative genomics of clinical hybrid Shiga toxin-producing and enterotoxigenic Escherichia coli (STEC/ETEC) strains in Sweden. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Nüesch-Inderbinen, M.; Morach, M.; Cernela, N.; Althaus, D.; Jost, M.; Mäusezahl, M.; Bloomberg, G.; Stephan, R. Serotypes and virulence profiles of Shiga toxin-producing Escherichia coli strains isolated during 2017 from human infections in Switzerland. Int. J. Med. Microbiol. 2018, 308, 933–939. [Google Scholar] [CrossRef]
- Rice, T.; Quinn, N.; Sleator, R.D.; Lucey, B. Changing diagnostic methods and increased detection of Verotoxigenic Escherichia coli, Ireland. Emerg. Infect. Dis. 2016, 22, 1656–1657. [Google Scholar] [CrossRef]
- Germinario, C.; Caprioli, A.; Giordano, M.; Chironna, M.; Gallone, M.S.; Tafuri, S.; Minelli, F.; Maugliani, A.; Michelacci, V. Community-wide outbreak of haemolytic uraemic syndrome associated with Shiga toxin 2-producing Escherichia coli O26:H11 in southern Italy, summer 2013. Euro Surveill. 2016, 21, 1–9. [Google Scholar] [CrossRef]
- Marejková, M.; Bláhová, K.; Janda, J.; Fruth, A.; Petráš, P. Enterohemorrhagic Escherichia coli as causes of hemolytic uremic syndrome in the Czech Republic. PLoS ONE 2013, 8, e73927. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Mellmann, A.; Bletz, S.; Zhang, W.; Köck, R.; Kossow, A.; Prager, R.; Fruth, A.; Orth-Höller, D.; Marejková, M.; et al. Enterohemorrhagic Escherichia coli O26:H11/H-: A new virulent clone emerges in Europe. Clin. Infect. Dis. 2013, 56, 1373–1381. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Wong, C.S.; Mooney, J.C.; Brandt, J.R.; Staples, A.O.; Jelacic, S.; Boster, D.R.; Watkins, S.L.; Tarr, P.I. Risk factors for the hemolytic uremic syndrome in children infected with Escherichia coli O157:H7: A multivariable analysis. Clin. Infect. Dis. 2012, 55, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.G.; Brito, J.R.F.; Gomes, T.A.T.; Guth, B.E.C.; Vieira, M.A.M.; Naves, Z.V.F.; Vaz, T.M.I.; Irino, K. Diversity of virulence profiles of Shiga toxin-producing Escherichia coli serotypes in food-producing animals in Brazil. Int. J. Food Microbiol. 2008, 127, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Vettorato, M.P.; De Castro, A.F.P.; Cergole-Novella, M.C.; Camargo, F.L.L.; Irino, K.; Guth, B.E.C. Shiga toxin-producing Escherichia coli and atypical enteropathogenic Escherichia coli strains isolated from healthy sheep of different populations in São Paulo, Brazil. Lett. Appl. Microbiol. 2009, 49, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Guth, B.E.C.; Lopes De Souza, R.; Vaz, T.M.I.; Irino, K. First Shiga toxin-producing Escherichia coli isolated from a patient with hemolytic uremic Syndrome, Brazil. Emerg. Infect. Dis. 2002, 8, 535–536. [Google Scholar] [CrossRef]
- Prager, R.; Fruth, A.; Siewert, U.; Strutz, U.; Tschäpe, H. Escherichia coli encoding Shiga toxin 2f as an emerging human pathogen. Int. J. Med. Microbiol. 2009, 299, 343–353. [Google Scholar] [CrossRef]
- Feng, P.C.H.; Delannoy, S.; Lacher, D.W.; dos Santos, L.F.; Beutin, L.; Fach, P.; Rivas, M.; Hartland, E.L.; Paton, A.W.; Guth, B.E.C. Genetic diversity and virulence potential of Shiga toxin-producing Escherichia coli O113:H21 strains isolated from clinical, environmental, and food sources. Appl. Env. Microbiol. 2014, 80, 4757–4763. [Google Scholar] [CrossRef]
- Fierz, L.; Cernela, N.; Hauser, E.; Nüesch-Inderbinen, M.; Stephan, R. Characteristics of Shigatoxin-producing Escherichia coli strains isolated during 2010–2014 from human infections in Switzerland. Front. Microbiol. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Karnisova, L.; Marejkova, M.; Hrbackova, H.; Mellmann, A.; Karch, H.; Fruth, A.; Drevinek, P.; Blahova, K.; Bielaszewska, M.; Nunvar, J. Attack of the clones: Whole genome-based characterization of two closely related enterohemorrhagic Escherichia coli O26 epidemic lineages. BMC Genom. 2018, 19, 1–12. [Google Scholar] [CrossRef]
- Mellmann, A.; Fruth, A.; Friedrich, A.W.; Wieler, L.H.; Harmsen, D.; Werber, D.; Middendorf, B.; Bielaszewska, M.; Karch, H. Phylogeny and disease association of Shiga toxin-producing Escherichia coli O91. Emerg. Infect. Dis. 2009, 15, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).