Comparison of Campylobacter jejuni Slaughterhouse and Surface-Water Isolates Indicates Better Adaptation of Slaughterhouse Isolates to the Chicken Host Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates and Growth Conditions
2.2. Satureja Montana Ethanolic Extract
2.3. Antimicrobial Resistance
2.4. Chicken Juice Preparation
2.5. Adhesion of Campylobacter jejuni
2.5.1. Adhesion to Stainless Steel
2.5.2. Adhesion to Chicken Skin
2.6. Campylobacter jejuni Biofilm Treatment with SMEE and BC
2.7. Planktonic and Biofilm Cell Preparation and Their Antimicrobial Susceptibility
2.8. Ethidium Bromide Accumulation
2.9. Statistical Analysis
3. Results
3.1. Antibiotic Resistance Profiles of Campylobacter jejuni Slaughterhouse and Surface-Water Isolates
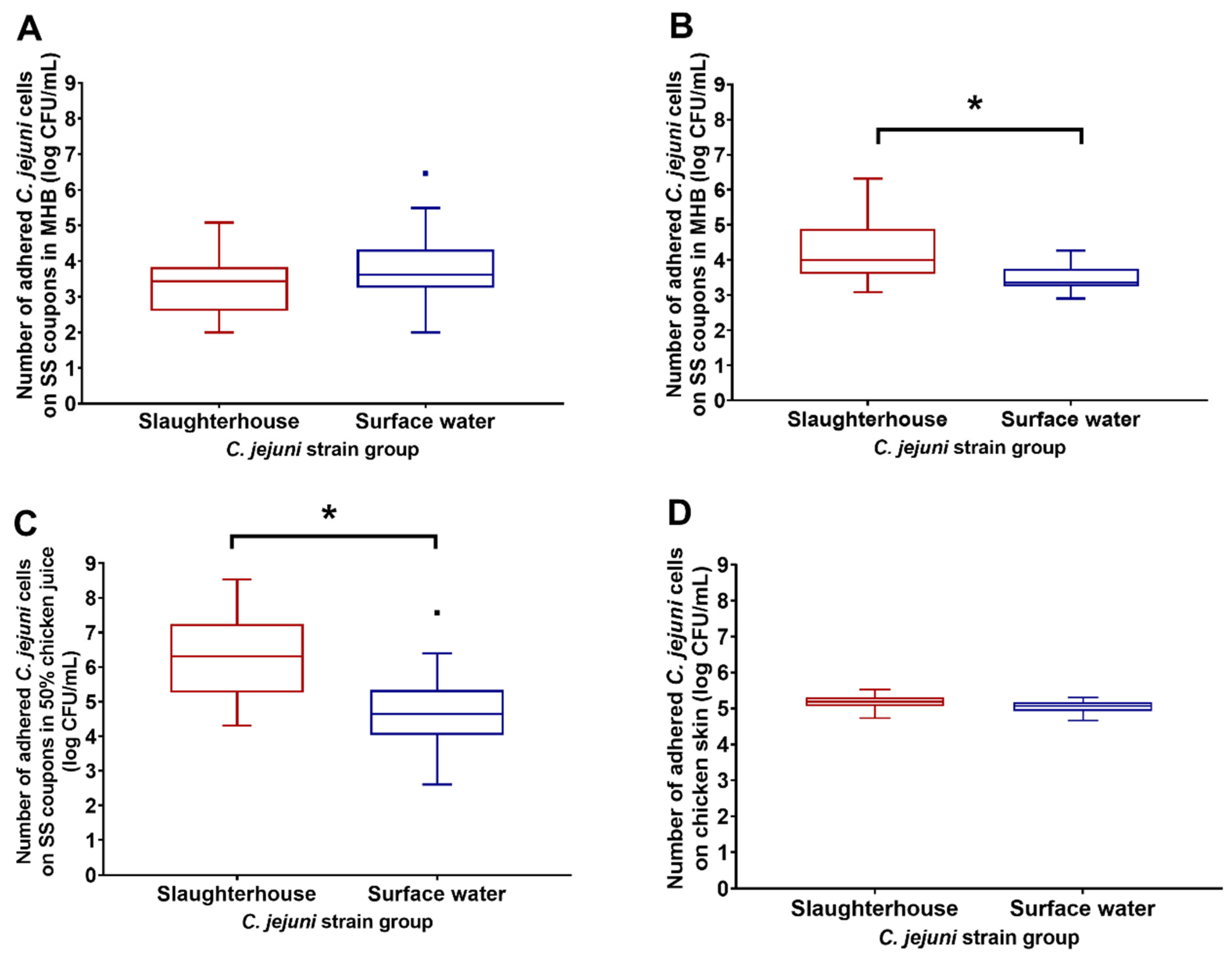
3.2. Adhesion of Slaughterhouse and Surface-Water Campylobacter jejuni Isolates
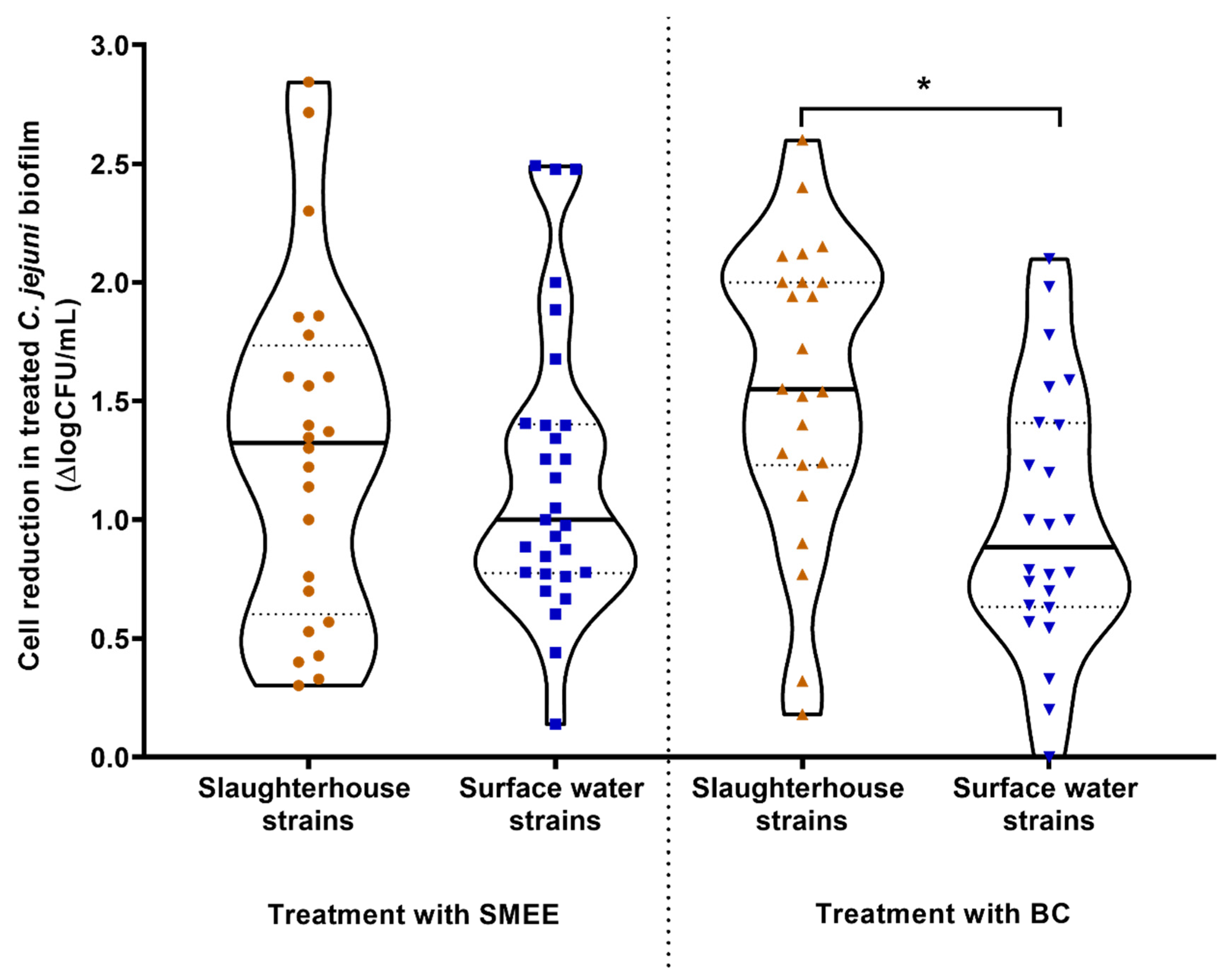
3.3. Reduction of Campylobacter jejuni Biofilms on Stainless Steel by the Satureja montana Ethanolic Extract and Benzalkonium Chloride
3.4. Resistance Profile of the Campylobacter jejuni S6 Biofilm Cells
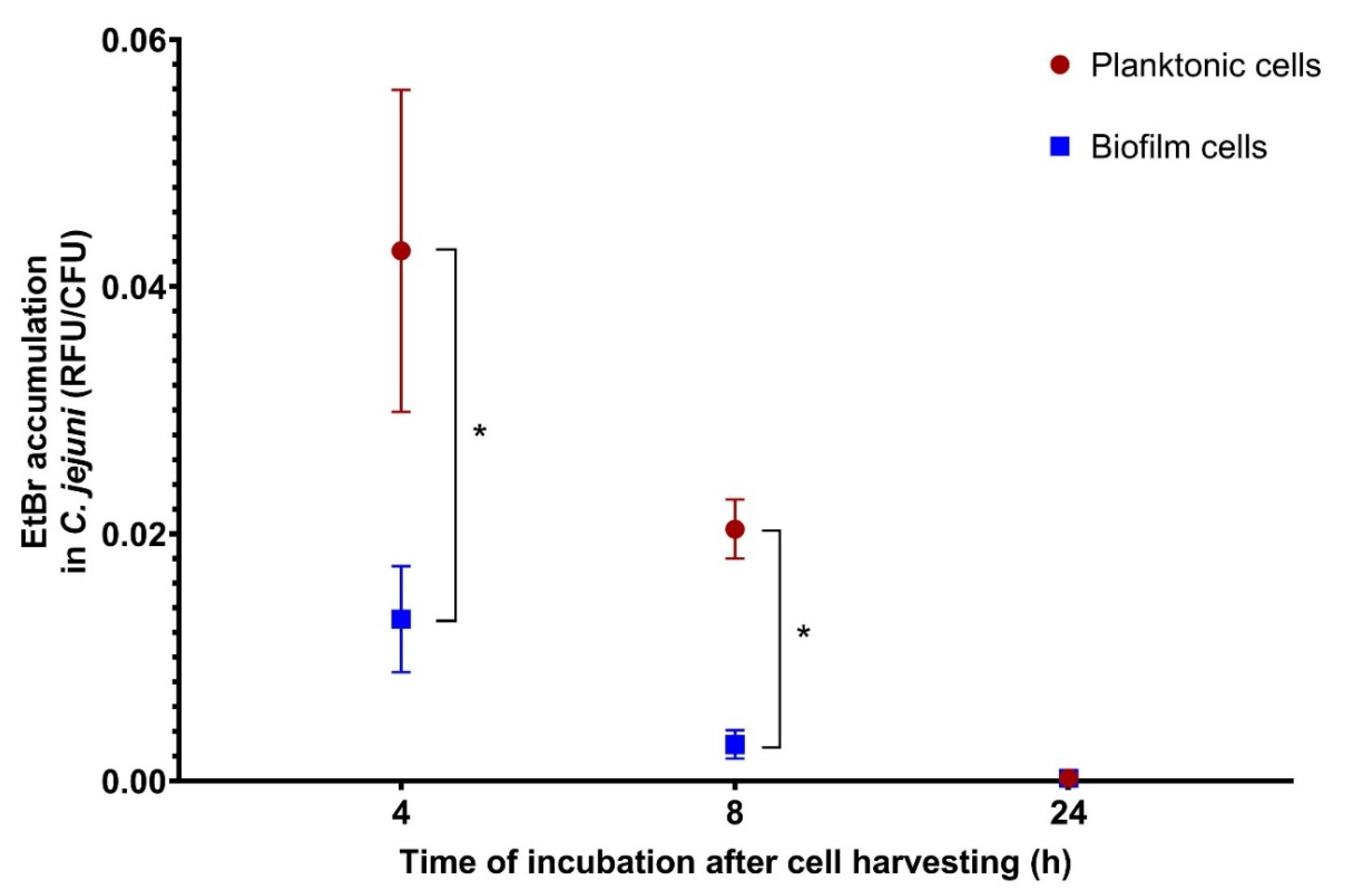
3.5. Efflux Pump Activity of Planktonic and Biofilm Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed]
- Nachamkin, I.; Allos, B.M.; Ho, T. Campylobacter species and Guillain-Barré syndrome. Clin. Microbiol. Rev. 1998, 11, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Helms, M.; Simonsen, J.; Olsen, K.E.P.; Mølbak, K. Adverse health events associated with antimicrobial drug resistance in Campylobacter species: A registry-based cohort study. J. Infect. Dis. 2005, 191, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar]
- European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA J. 2019, 17, e05598. [Google Scholar]
- Whiley, H.; van den Akker, B.; Giglio, S.; Bentham, R. The role of environmental reservoirs in human campylobacteriosis. Int. J. Environ. Res. Public Health 2013, 10, 5886–5907. [Google Scholar] [CrossRef]
- Thépault, A.; Rose, V.; Quesne, S.; Poëzevara, T.; Beven, V.; Hirchaud, E.; Touzain, F.; Lucas, P.; Méric, G.; Mageiros, L.; et al. Ruminant and chicken: Important sources of campylobacteriosis in France despite a variation of source attribution in 2009 and 2015. Sci. Rep. 2018, 8, 3905. [Google Scholar] [CrossRef]
- Kovač, J.; Stessl, B.; Čadež, N.; Gruntar, I.; Cimerman, M.; Stingl, K.; Lušicky, M.; Ocepek, M.; Wagner, M.; Smole Možina, S. Population structure and attribution of human clinical Campylobacter jejuni isolates from central Europe to livestock and environmental sources. Zoonoses Publ. Health 2018, 65, 51–58. [Google Scholar] [CrossRef]
- Engberg, J.; Gerner-Smidt, P.; Scheutz, F.; Møller Nielsen, E.; On, S.L.W.; Mølbak, K. Water-borne Campylobacter jejuni infection in a Danish town: A 6-week continuous source outbreak. Clin. Microbiol. Infect. 1998, 4, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Berthenet, E.; Thépault, A.; Chemaly, M.; Rivoal, K.; Ducournau, A.; Buissonnière, A.; Bénéjat, L.; Bessède, E.; Mégraud, F.; Sheppard, S.K.; et al. Source attribution of Campylobacter jejuni shows variable importance of chicken and ruminants reservoirs in non-invasive and invasive French clinical isolates. Sci. Rep. 2019, 9, 8098. [Google Scholar] [CrossRef]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.-C.; Naïtali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Joshua, G.W.P.; Guthrie-Irons, C.; Karlyshev, A.V.; Wren, B.W. Biofilm formation in Campylobacter jejuni. Microbiology 2006, 152, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Arnold, J.W.; Bailey, G.W. Surface finishes on stainless steel reduce bacterial attachment and early biofilm formation: Scanning electron and atomic force microscopy study. Poult. Sci. 2000, 79, 1839–1845. [Google Scholar] [CrossRef]
- Brown, H.L.; Reuter, M.; Salt, L.J.; Cross, K.L.; Betts, R.P.; van Vliet, A.H.M. Chicken juice enhances surface attachment and biofilm formation of Campylobacter jejuni. Appl. Environ. Microbiol. 2014, 80, 7053–7060. [Google Scholar] [CrossRef]
- Li, J.; Feng, J.; Ma, L.; de la Fuente Núñez, C.; Gölz, G.; Lu, X. Effects of meat juice on biofilm formation of Campylobacter and Salmonella. Int. J. Food Microbiol. 2017, 253, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.T.; Mendonça, E.P.; Monteiro, G.P.; Siqueira, M.C.; Pereira, C.B.; Peres, P.A.B.M.; Fernandez, H.; Rossi, D.A. Intrinsic and extrinsic aspects on Campylobacter jejuni biofilms. Front. Microbiol. 2017, 8, 1332. [Google Scholar] [CrossRef]
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef]
- Klančnik, A.; Možina, S.S.; Zhang, Q. Anti-Campylobacter activities and resistance mechanisms of natural phenolic compounds in Campylobacter. PLoS ONE 2012, 7, e51800. [Google Scholar] [CrossRef]
- EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 10.0. 2020. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint_Tables.pdf (accessed on 13 October 2020).
- Kahlmeter, G.; Brown, D.F.J.; Goldstein, F.W.; MacGowan, A.P.; Mouton, J.W.; Österlund, A.; Rodloff, A.; Steinbakk, M.; Urbaskova, P.; Vatopoulos, A. European harmonization of MIC breakpoints for antimicrobial susceptibility testing of bacteria. J. Antimicrob. Chemother. 2003, 52, 145–148. [Google Scholar] [CrossRef]
- Kovač, J.; Šimunović, K.; Wu, Z.; Klančnik, A.; Bucar, F.; Zhang, Q.; Možina, S.S. Antibiotic resistance modulation and modes of action of (-)-α-pinene in Campylobacter jejuni. PLoS ONE 2015, 10, e0122871. [Google Scholar] [CrossRef]
- Klančnik, A.; Piskernik, S.; Bucar, F.; Vučković, D.; Možina, S.S.; Jeršek, B. Reduction of microbiological risk in minced meat by a combination of natural antimicrobials. J. Sci. Food Agricult. 2014, 94, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Bohinc, K.; Dražić, G.; Fink, R.; Oder, M.; Jevšnik, M.; Nipič, D.; Godič-Torkar, K.; Raspor, P. Available surface dictates microbial adhesion capacity. Int. J. Adhes. Adhesiv. 2014, 50, 265–272. [Google Scholar] [CrossRef]
- Valtierra-Rodríguez, D.; Heredia, N.L.; García, S.; Sánchez, E. Reduction of Campylobacter jejuni and Campylobacter coli in poultry skin by fruit extracts. J. Food Protect. 2010, 73, 477–482. [Google Scholar] [CrossRef]
- Oh, E.; McMullen, L.M.; Chui, L.; Jeon, B. Differential survival of hyper-aerotolerant Campylobacter jejuni under different gas conditions. Front. Microbiol. 2017, 8, 954. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Šikić Pogačar, M.; Trošt, K.; Tušek Žnidarič, M.; Mozetič Vodopivec, B.; Smole Možina, S. Anti-Campylobacter activity of resveratrol and an extract from waste Pinot noir grape skins and seeds, and resistance of Campylobacter jejuni planktonic and biofilm cells, mediated via the CmeABC efflux pump. J. Appl. Microbiol. 2017, 122, 65–77. [Google Scholar] [CrossRef]
- Reddy, S.; Zishiri, O.T. Detection and prevalence of antimicrobial resistance genes in Campylobacter spp. isolated from chickens and humans. Onderstepoort J. Vet. Res. 2017, 84, 1–6. [Google Scholar] [CrossRef]
- Szczepanska, B.; Andrzejewska, M.; Spica, D.; Klawe, J.J. Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from children and environmental sources in urban and suburban areas. BMC Microbiol. 2017, 17, 80. [Google Scholar] [CrossRef]
- Luo, N.; Pereira, S.; Sahin, O.; Lin, J.; Huang, S.; Michel, L.; Zhang, Q. Enhanced in-vivo fitness of fluoroquinolone-resistant Campylobacter jejuni in the absence of antibiotic selection pressure. Proc. Natl. Acad. Sci. USA 2005, 102, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Torralbo, A.; Borge, C.; García-Bocanegra, I.; Méric, G.; Perea, A.; Carbonero, A. Higher resistance of Campylobacter coli compared to Campylobacter jejuni at chicken slaughterhouse. Comp. Immunol. Microbiol. Infect. Dis. 2015, 39, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.J.; Gabriel, E.; Leatherbarrow, A.J.H.; Cheesbrough, J.; Gee, S.; Bolton, E.; Fox, A.; Fearnhead, P.; Hart, C.A.; Diggle, P.J. Tracing the source of campylobacteriosis. PLoS Genet. 2008, 4, e1000203. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.; Mallett, A.; Pearson, B.M.; van Vliet, A.H.M. Biofilm formation by Campylobacter jejuni is increased under aerobic conditions. Appl. Environ. Microbiol. 2010, 76, 2122–2128. [Google Scholar] [CrossRef] [PubMed]
- Sulaeman, S.; Bihan, G.L.; Rossero, A.; Federighi, M.; Dé, E.; Tresse, O. Comparison between the biofilm initiation of Campylobacter jejuni and Campylobacter coli isolates to an inert surface using BioFilm Ring Test®. J. Appl. Microbiol. 2010, 108, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Chantarapanont, W.; Berrang, M.; Frank, J.F. Direct microscopic observation and viability determination of Campylobacter jejuni on chicken skin. J. Food Prot. 2003, 66, 2222–2230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jang, K.-I.; Kim, M.-G.; Ha, S.-D.; Kim, K.-S.; Lee, K.-H.; Chung, D.-H.; Kim, C.-H.; Kim, K.-Y. Morphology and adhesion of Campylobacter jejuni to chicken skin under varying conditions. J. Microbiol. Biotechnol. 2007, 17, 202–206. [Google Scholar]
- Wagle, B.R.; Upadhyay, A.; Upadhyaya, I.; Shrestha, S.; Arsi, K.; Liyanage, R.; Venkitanarayanan, K.; Donoghue, D.J.; Donoghue, A.M. Trans-cinnamaldehyde, eugenol and carvacrol reduce Campylobacter jejuni biofilms and modulate expression of select genes and proteins. Front. Microbiol. 2019, 10, 1837. [Google Scholar] [CrossRef]
- Roila, R.; Ranucci, D.; Valiani, A.; Galarini, R.; Servili, M.; Branciari, R. Antimicrobial and anti-biofilm activity of olive oil by-products against Campylobacter spp. isolated from chicken meat. Acta Sci. Pol. Technol. Aliment. 2019, 18, 43–52. [Google Scholar]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Simões, L.C.; Simões, M.; Vieira, M.J. Influence of the diversity of bacterial isolates from drinking water on resistance of biofilms to disinfection. Appl. Environ. Microbiol. 2010, 76, 6673–6679. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, M.; Guerrero, R. Living together in biofilms: The microbial cell factory and its biotechnological implications. Microb. Cell Fact. 2016, 15, 165. [Google Scholar] [CrossRef] [PubMed]
- Mavri, A.; Mozina, S.S. Involvement of efflux mechanisms in biocide resistance of Campylobacter jejuni and Campylobacter coli. J. Med. Microbiol. 2012, 61, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.; Ramesh, A.; Seddon, A.M.; Karlyshev, A.V. CmeABC multidrug efflux pump contributes to antibiotic resistance and promotes Campylobacter jejuni survival and multiplication in Acanthamoeba polyphaga. Appl. Environ. Microbiol. 2017, 83, e01600-17. [Google Scholar] [CrossRef] [PubMed]
| Isolate Designation | Erythromycin | Ciprofloxacin | Tetracycline | Gentamicin | Nalidixic Acid | Streptomycin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) | S/R | MIC (mg/L) | S/R | MIC (mg/L) | S/R | MIC (mg/L) | S/R | MIC (mg/L) | S/R | MIC (mg/L) | S/R | |
| Slaughterhouse | ||||||||||||
| S1 | 2 | S | 16 | R | <0.5 | S | 0.25 | S | 64 | R | 0.5 | S |
| S2 | <1 | S | 0.25 | S | 4 | R | 0.25 | S | 2 | S | 1 | S |
| S3 | <1 | S | 8 | R | 8 | R | <0.12 | S | 8 | S | 0.5 | S |
| S4 | <1 | S | 8 | R | 0.5 | S | 0.5 | S | 32 | R | 1 | S |
| S5 | <1 | S | 8 | R | 4 | R | 0.25 | S | 1 | S | 0.5 | S |
| S6 | <1 | S | 8 | R | 8 | R | 0.25 | S | 1 | S | 1 | S |
| S7 | <1 | S | 8 | R | <0.5 | S | 0.5 | S | 64 | R | 1 | S |
| S8 | <1 | S | 8 | R | <0.5 | S | 0.25 | S | 32 | R | 0.5 | S |
| Surface water | ||||||||||||
| W1 | <1 | S | <0.25 | S | <0.5 | S | 0.25 | S | 4 | S | 1 | S |
| W2 | <1 | S | 4 | S | 16 | R | 0.25 | S | 16 | S | 1 | S |
| W3 | <1 | S | 8 | R | <0.5 | S | 0.25 | S | 64 | R | 1 | S |
| W4 | <1 | S | <0.12 | S | <0.5 | S | 0.5 | S | 4 | S | 1 | S |
| W5 | <1 | S | 0.12 | S | <0.5 | S | 0.25 | S | 8 | S | <1 | S |
| W6 | <1 | S | 0.12 | S | <0.5 | S | 0.5 | S | 8 | S | <1 | S |
| W7 | <1 | S | 16 | R | 0.5 | S | 0.5 | S | >64 | R | 2 | S |
| W8 | <1 | S | <0.12 | S | <0.5 | S | 0.5 | S | 4 | S | 1 | S |
| Main Source | Isolate | MIC (mg/L) | |
|---|---|---|---|
| S. montana Ethanolic Extract | Benzalkonium Chloride | ||
| Slaughterhouse | S1 | 250 | 0.96 |
| S2 | 250 | 0.96 | |
| S3 | 500 | 0.96 | |
| S4 | 250 | 0.24 | |
| S5 | 250 | 0.48 | |
| S6 | 500 | 0.96 | |
| S7 | 125 | 0.48 | |
| S8 | 250 | 1.92 | |
| Surface water | W1 | 500 | 0.96 |
| W2 | 250 | 0.96 | |
| W3 | 250 | 0.48 | |
| W4 | 125 | 0.96 | |
| W5 | 250 | 0.96 | |
| W6 | 250 | 0.96 | |
| W7 | 500 | 1.92 | |
| W8 | 61.5 | 0.96 | |
| Incubation Time (h) | MIC (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Erythromycin | S. montana Ethanolic Extract | Benzalkonium Chloride | ||||||||
| P | B | FC | P | B | FC | P | B | FC | ||
| 0 | 0.125 | 0.25 | 2 | 125 | 250 | 2 | 0.48 | 0.24 | 0.5 | |
| 4 | 0.125 | 0.25 | 2 | 250 | 250 | 1 | 0.48 | 0.24 | 0.5 | |
| 8 | 0.125 | 0.5 | 4 | 250 | 125 | 0.5 | 0.48 | 0.24 | 0.5 | |
| 24 | 0.125 | 0.125 | 1 | 500 | 500 | 1 | 0.96 | 0.96 | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimunović, K.; Zajkoska, S.; Bezek, K.; Klančnik, A.; Barlič Maganja, D.; Smole Možina, S. Comparison of Campylobacter jejuni Slaughterhouse and Surface-Water Isolates Indicates Better Adaptation of Slaughterhouse Isolates to the Chicken Host Environment. Microorganisms 2020, 8, 1693. https://doi.org/10.3390/microorganisms8111693
Šimunović K, Zajkoska S, Bezek K, Klančnik A, Barlič Maganja D, Smole Možina S. Comparison of Campylobacter jejuni Slaughterhouse and Surface-Water Isolates Indicates Better Adaptation of Slaughterhouse Isolates to the Chicken Host Environment. Microorganisms. 2020; 8(11):1693. https://doi.org/10.3390/microorganisms8111693
Chicago/Turabian StyleŠimunović, Katarina, Sandra Zajkoska, Katja Bezek, Anja Klančnik, Darja Barlič Maganja, and Sonja Smole Možina. 2020. "Comparison of Campylobacter jejuni Slaughterhouse and Surface-Water Isolates Indicates Better Adaptation of Slaughterhouse Isolates to the Chicken Host Environment" Microorganisms 8, no. 11: 1693. https://doi.org/10.3390/microorganisms8111693
APA StyleŠimunović, K., Zajkoska, S., Bezek, K., Klančnik, A., Barlič Maganja, D., & Smole Možina, S. (2020). Comparison of Campylobacter jejuni Slaughterhouse and Surface-Water Isolates Indicates Better Adaptation of Slaughterhouse Isolates to the Chicken Host Environment. Microorganisms, 8(11), 1693. https://doi.org/10.3390/microorganisms8111693






